Abstract
Five hundred ninety-three cadaveric livers were used for primary liver transplantation between October 24, 1987, and May 19, 1989. The grafts were procured with a combined method, using in situ cooling with cold electrolyte solution and backtable flushing with UW solution. The mean cold-ischemia time was 12.8 (range 2.4–34.7) hr. The cases were divided into 5 groups according to the cold-ischemia time: group 1: <10 hr (n=223); group 2: 10–14 hr (n=188); group 3: 15–19 hr (n=101); group 4: 20–24 hr (n=52); and group 5: ≥25 hr (n=29). There was no difference between the 5 groups in 1-year patient survival, highest SGOT in first week after operation, and SGOT and total bilirubin during the first month after operation. However, with a logistic regression model, the retransplantation rate (P=0.001) and primary nonfunction rate (P=0.006) significantly rose as cold-ischemia time increased, meaning that the equivalency of patient survival was increasingly dependent on aggressive retransplantation.
The development of the University of Wisconsin solution was announced by Jamieson et al. (1) at an international transplantation conference held in Pittsburgh in September 1987 (1). The Madison group already had evaluated the solution for liver transplantation in dogs and had acquired an early clinical experience (1, 2). Within less than a month, we were manufacturing our own supply and were using the solution clinically (3), having confirmed the Wisconsin claims in animals (4). By the spring of 1988, the magnitude of the advance was generally appreciated. Since then, the superiority of the University of Wisconsin solution to the previously used Euro-Collins solution has been so overwhelmingly confirmed that the citation of selected trials is an inequity to many more. In contrast to maximum limit of 6 or 8 hr with EC solution, all centers have been able to relax their storage-time restrictions. In various reports, the mean and range of cold-ischemia times have been 12.6 (2–24) hr in Wisconsin (5); 11.5 (3–20) in Los Angeles (6); and 7.23 (range not given) in Dallas (7). In our center, mean cold-ischemia time with UW preservation has been 12.8 (2.4–34.7) hr.
In spite of our enthusiasm about the UW solution, we have presented evidence that deterioration of preserved canine livers is substantial by 24 hr (4). However, there has been no published evidence that human liver transplantation results are adversely affected by increasing the cold-ischemia time. Therefore, we looked at this question in 593 patients who underwent primary transplantation between October 24, 1987, and May 19, 1989.
Materials and Methods
Recipient case material
Six hundred twenty-four patients had liver transplantation for the first time using liver grafts that were preserved with UW solution. Thirty-one of these cases were excluded because of insufficient data. The remaining 593 patients were followed until June 1990, allowing 13–32-month periods of posttransplant observation; 93 (15.7%) were infants or children (<18 years), and 500 (84.3%) were adults (Table 1). The ages ranged from newborn to 73.8 years, older recipients being significantly more frequently represented in group 4. Other features are summarized in Table 1. Almost 5% of the recipients had antidonor antibodies, either with positive cytotoxic crossmatches or ABO incompatibilities. Many were critically ill as reflected by UNOS 4 or UNO-stat classification, meaning that these patients were ICU bound and usually on ventilators.
Table 1. Features of 593 recipients of primary liver grafts and their numbers in the various cold-ischemia time (CIT) groups.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| CIT (hr) | <10 | 10–14.9 | 15–19.9 | 20–24.9 | ≥25 |
| n | 223 | 188 | 101 | 52 | 29 |
| Age (years) | 38.3 | 38.3 | 40.7 | 45.5a | 45.0 |
| Sex (m/f ratio) | 1.3 | 1.4 | 1.7 | 1.1 | 0.8 |
| UNOS score 4 or UNOSTAT | 79 (35.4%) | 72 (38.2%) | 30 (29.7%) | 13 (25.0%) | 6 (20.7%) |
| Crossmatch positive | 12 (5.4%) | 8 (4.3%) | 3 (3.0%) | 0 (0.0%) | 1 (3.4%) |
| Blood type mismatch | 3 (1.3%) | 2 (1.1%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) |
P<0.5 vs. group 1, group 2; the older age in this CIT group was the only significant difference between groups in any of the parameters.
During the 13–32-month follow-up, 105 (17.7%) of the starting group underwent retransplantation, and in 57 (9.6%), the retransplantation was within 14 days. The reasons for retransplantation were classified from clinical and pathologic findings as the following: 1, technical; 2, rejection; 3, primary nonfunction; 4, infection; and 5, miscellaneous. A diagnosis of primary nonfunction was made if a graft never demonstrated evidence of initial function following transplantation. Clinical findings and examinations strongly associated with primary nonfunction included stage 4 coma, uncorrectable coagulopathy, renal failure, cardiodynamic shock, acidosis, and profound hypoglycemia. The pathology of such grafts invariably showed massive ischemic necrosis without evidence of rejection.
Donor case material
The 593 donors were newborn to 58 years old. Their length of hospitalization, cardiopulmonary function, and hepatic status are summarized in Table 2. One donor whose total bilirubin was 18 mg% died during a sickle cell crisis; the transplanted liver functioned well. Low mean arterial pressures were common, and 2 livers were removed after the donors had cardiac arrest. The most common causes of death were motor-vehicle accidents, gunshot wounds, and cerebrovascular accidents in that order. More than half the livers were procured with kidneys and hearts. The foregoing donor factors were evenly distributed in livers with short, intermediate, and long cold-ischemia times (CIT), with no statistically significant differences (Table 2).
Table 2. Features of the donors in the various cold-ischemia time (CIT) groups.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| Demographics | |||||
| CIT (hr) | <10 | 10–14.9 | 15–19.9 | 20–24.9 | ≥25 |
| n | 223 | 188 | 101 | 52 | 29 |
| Age (years) | 22.9 | 23.0 | 23.5 | 24.1 | 20.9 |
| Sex (M/F ratio) | 1.9 | 2.2 | 1.8 | 1.8 | 2.5 |
| Hospital stay (days) | 3.0 | 5.6 | 3.2 | 4.1 | 2.4 |
| Liver functions | |||||
| T. BIL (mg/dl) | 0.96 | 1.72 | 1.23 | 0.91 | 0.91 |
| SGOT (u/L) | 87.7 | 84.3 | 78.6 | 76.9 | 63.6 |
| SGPT (u/L) | 49.5 | 52.8 | 47.2 | 52.3 | 47.1 |
| PT (sec) | 13.7 | 13.9 | 13.4 | 14.1 | 14.5 |
| Cardio/pulmonary functions | |||||
| PO2 (mmHg) | 146.3 | 154.7 | 154.7 | 158.4 | 205.3 |
| PCO2 (mmHg) | 32.9 | 30.3 | 30.1 | 29.8 | 31.8 |
| PH | 7.39 | 7.42 | 7.39 | 7.44 | 7.42 |
| Lowest blood pressure (mmHg) | 80.0 | 82.0 | 77.9 | 81.6 | 79.1 |
| Solution | |||||
| Electrolyte (ml)a | 7175 | 7130 | 7427 | 7813 | 7815 |
| UW (ml)b | 1874 | 2187 | 2097 | 2082 | 2441 |
Used for in situ cooling.
Used for backtable flushing and priming of preservation bag.
Donor hepatectomy was performed by standard techniques as part of multiple organ procurements (8) or with a modified rapid-flush method (9) for unstable donors. All procurements wee made before UW solution was commercially available, and during the first 4 months the solution was prepared in the School of Pharmacy of the University of Pittsburgh from raw materials bought from chemical suppliers. After this, it was supplied by the Department of Medical Research, DuPont Corporation, 1600 Waukegan Road, Waukegan, IL 60085. Throughout the period of case acquisition, the UW solution was a scarce resource worldwide and subject to rationing. In addition, host procurement teams at first were suspicious of the solution and usually would not permit its intraaortic infusion into the kidneys. For these reasons, a “mixed” technique was used in which core aortic cooling was with lactated Ringer's or a Collins solution according to the host-team instructions. The procurements were with standard surgical technique. The amounts used are recorded in Table 2. The liver was secondarily perfused on the back table with UW solution, primarily through the portal vein, but sometimes with an additional arterial flush. In retrieving data, the total UW solution use was recorded (Table 2) that included about 500 ml instilled into the preservation bag. The volumes were smaller in pediatric donors.
Recipient care
Recipient hepatectomy and hepatic replacement were performed by standard techniques (10). Before revascularizing the new liver, the organ was perfused with 300–500 ml lactated Ringer's solution or in a few cases plasmanate to remove air and excess potassium. Postoperative immunosuppression was with cyclosporine and low-dose steroids, with supplementary monoclonal antilymphocyte globulin (OKT3) and/or azathioprine as indicated (10).
Data analysis
All evaluation parameters were in the recipient, and included patient and graft survival, graft function, and the frequency of retransplantation. Stratification was into 5 groups defined arbitrarily by the cold-ischemia time (CIT) as follows: group 1: <10 hr (n=223); group 2: 10–14 hr (n=188); group 3: 15–19 hr (n=101); group 4: 20–24 hr (n=52); and group 5: ≥25 hr (n=29). The exact CIT distribution to the nearest hour is shown in Figure 1.
Figure 1.

Distribution of cold-ischemia time (CIT).
Statistical evaluation of the results was by chi-square test, analysis of variance, simple regression analysis, and logistic regression analysis.
Results
Patient and graft survival
Over all, patient survival was 77.2% at the end of 1 year (Fig. 2). There was no difference in patient survival between the different CIT groups. However, there were differences in early graft survival (Fig. 3). At various time points within the first few weeks, the need for retransplantation in groups 4 and 5 (especially group 4) was significantly greater (Fig. 3). Thus, the equivalence of eventual patient survival shown in Figure 2 was dependent on aggressive retransplantation.
Figure 2.
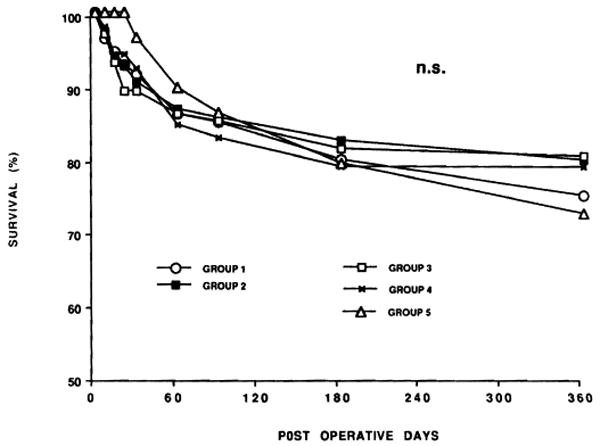
Recipient patient survival according to CIT. Actual follow-up for all cases was more than 1 year.
Figure 3.
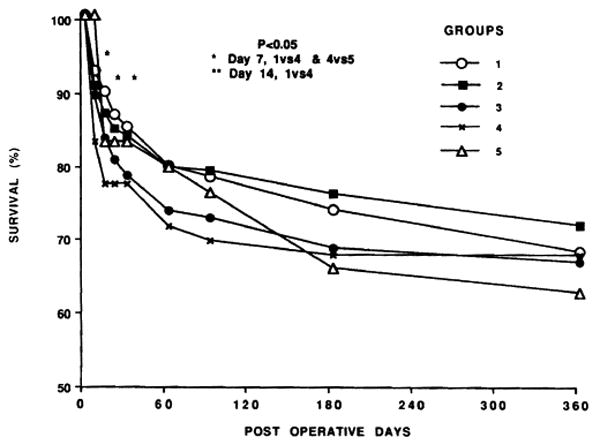
Graft survival according to CIT. Note the significantly increased early loss rate of grafts with the longer ischemia times.
Retransplantation
The incidence and reasons for retransplantation during the first 14 postoperative days are summarized in Figure 4. When the CIT was less than 10 hr, the retransplantation rate was 5.4%, whereas it was double, triple, or quadruple this rate with successively longer preservation times. Primary nonfunction was the principal course of graft loss during the first 2 weeks no matter what the CIT, and rejection was the least important factor (Fig. 4). The increase in retransplantation in groups 4 and 5 versus groups 1–3 was significant (see significance notations in Fig. 4). The trend to a higher retransplantation rate with longer preservation using the logistic regression model was unmistakable and significant (P<0.001) throughout the entire spectrum of preservation time (Fig. 5). The dominant contribution of primary nonfunction to retransplantation need was also evident (Fig. 5). The increased incidence of rejection with increased CIT (Fig. 4) was noteworthy but not significant.
Figure 4.

Incidence and cause of retransplantation within 2 weeks.
Figure 5.
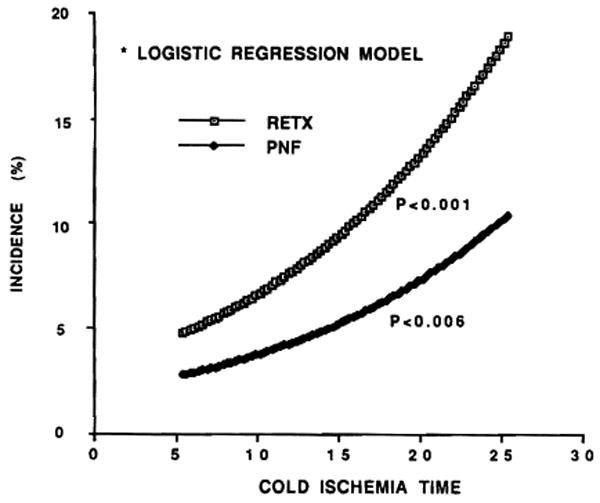
Estimated rates of retransplantation and primary nonfunction of grafts related correlated with cold-ischemia time (CIT), using a logistic regression model.
Liver function tests
The duration of CIT was not reflected in the SGOT and serum bilirubin values that are summarized in Figures 6 and 7 up to 1 month for all primary grafts that remained in place for this long. In all the groups, the original sample sizes were reduced with the passage of time, since data entry ceased when grafts were lost by death or retransplantation. The early postoperative culling process was more extensive with each increment of ischemia time because larger numbers of dysfunctional grafts were replaced (Figs. 4 and 5). The livers that remained had similar average SGOT (Fig. 6) and serum bilirubin values (Fig. 7). However, even when all cases were recorded during the first 7 days, there was no correlation between CIT and the highest SGOT values (Fig. 8).
Figure 6.
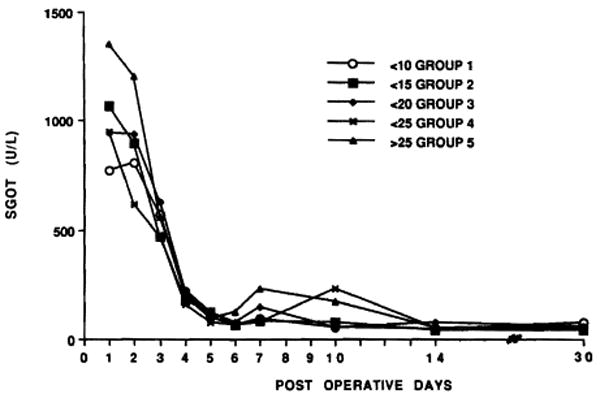
SGOT during the first month related to CIT. See text for explanation of possible culling artifact.
Figure 7.

Total bilirubin in different CIT groups during the first month. See text for explanation of possible culling artifact.
Figure 8.

Highest SGOT values during the first 7 days after transplantation of all 593 grafts and the relation of these figures to CIT. There was no correlation.
Discussion
The improved preservation made possible by the UW solution has revolutionized the field of liver transplantation (1–3). Long-distance procurement, organ sharing, back-table evaluation and reconstruction including size reduction, and more complete preoperative and intraoperative recipient preparation have all become feasible. In addition, the incidence of primary graft nonfunction and thrombosis of the graft vessels were reduced (3). However, the results herein reported caution against undue procrastination in the use of these livers. A statistically definable price could be identified that was cumulative with time but that often was not evident in individual cases. Most of the organs preserved for 20 hr or more were satisfactory, attesting the efficiency of the method used, but the necessity for life-saving retransplantation because of primary graft nonfunction or other reasons became progressively more frequent. The effective use of retransplantation prevented a commensurate increase in mortality.
It was reassuring that success often was possible beyond 20 hr. After our longest preservation (>34 hr), the result was perfect. However, planned acceptance of an extra risk is not justifiable. Our present policy is to make every attempt to revascularize liver grafts within 20 hr since the early graft failure rate was increasingly nonlinear beyond this time.
Although the folly of complete indifference to CIT is evident, the time constraints delineated in this study may understate the value of UW because of the conditions of testing (11). The potentially suboptimal policy was in effect throughout the study of preliminary flushing with electrolyte solutions. Since completion of this study, we have used the now freely available UW solution as the sole infusate from the outset. This is the policy recommended by Belzer (11). However, the trend in the new cases is similar to that in the earlier experience.
Footnotes
This work was supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Jamieson NV, Sundberg R, Lindell S, et al. Successful 24- to 30- hour preservation of the canine liver: a preliminary report. Transplant Proc. 1988;20(Suppl 1):945. [Google Scholar]
- 2.Kalayoglu M, Sollinger WH, Stratta RJ, et al. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 3.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711. [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Podesta L, Ueda Y, et al. A comparison of UW with other solutions for liver preservation in dogs. Clin Transplant. 1989;3:253. [PMC free article] [PubMed] [Google Scholar]
- 5.D'Alessandro AM, Kalayoglu M, Sollinger HW, et al. Experience with Belzer UW cold storage solution in human liver transplantation. Transplant Proc. 1990;22:474. [PubMed] [Google Scholar]
- 6.Olthoff KM, Millis JM, Imagawa DK, et al. Comparison of UW and Euro-Collins solutions for cold preservation of human liver grafts. Transplantation. 1990;49:284. doi: 10.1097/00007890-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Cofer JB, Klintmalm GB, Howard TK, et al. A comparison of UW with Eurocollins preservation solution in liver transplantation. Transplantation. 1990;49:1088. doi: 10.1097/00007890-199006000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ. Liver transplantation. Chicago, IL: Year Book; 1990. [Google Scholar]
- 11.Belzer FO. Clinical organ preservation with UW solution. Transplantation. 1989;47:1098. doi: 10.1097/00007890-198906000-00045. [DOI] [PubMed] [Google Scholar]


