Abstract
Background
In vitro, urinary catheter colonization by avirulent Escherichia coli 83972 impedes subsequent catheter colonization by a variety of uropathogenic organisms. However, E. coli 83972 shows a low efficacy of adherence to silicone urinary catheter material, possibly because the fim operon encoding adhesive type 1 fimbriae is incomplete. We hypothesized that improving the catheter adherence of E. coli 83972 would improve its bacterial interference properties.
Methods
We created adhesive mutants by transforming wild-type E. coli 83972 with fim+ plasmids. Adherence to urinary catheters and ability to prevent uropathogenic E. coli from colonizing urinary catheters were studied by use of a sonication assay.
Results
The addition of a single-copy fim+ plasmid increased adherence to urinary catheters 10-fold, and addition of an 18-copy fim+ plasmid increased adherence 100-fold. The more adherent 18-copy fim+ plasmid strain was more effective at blocking catheter colonization by pathogenic E. coli than was the wild-type parental strain. Neither Δfim nor fim+ E. coli 83972 adhered to shed urinary epithelial cells.
Conclusions
Our results indicate that improving urinary catheter adherence augments the bacterial interference capabilities of benign E. coli 83972. Increased expression of type-1 fimbriae may enhance bacterial interference without conferring virulence on E. coli 83972.
Each year, more than 30 million urinary catheters are used for patients in the United States [1]. Urinary tract infection (UTI) accounts for over 30% of all nosocomial infection in US intensive care units, and 97% of these UTIs occur in catheterized patients [2]. More than 1 million catheter-associated UTIs (CA-UTI) are reported annually by infection control surveillance [3]. Persons with a catheterized urinary tract acquire bacteriuria at the rate of about 3%–10% per day, and prolonged catheterization is inevitably accompanied by bacteriuria [4]. Complications of chronic bacteriuria include prostatitis, epididymitis, scrotal abscesses, catheter blockage from encrustation, pyelonephritis, and bacteremia [5].
The catheter-associated biofilm of adherent organisms and secreted polysaccharide matrix is central to the pathogenesis of CA-UTI [6]. Compared to their planktonic (i.e., free-floating) counterparts, organisms in bio-films are less susceptible to antimicrobial agents and host defenses [7]. The use of standard antimicrobial agents to sterilize the urine of individuals undergoing prolonged catheterization leads to bladder colonization with antimicrobial-resistant organisms [8–10]. No strategy effectively prevents biofilm formation on long-term, indwelling urinary catheters [11].
Bacterial interference, or the use of benign bacteria to prevent infection by virulent pathogens [12, 13], may offer a solution to the problem of CA-UTI. Three small trials involving persons with spinal cord injury and deliberate bladder colonization with nonpathogenic Escherichia coli (E. coli 83972) have demonstrated an association between colonization with this nonpathogenic organism and decreased incidence of UTI [14–16]. In vitro, precoating urinary catheters with a biofilm of E. coli 83972 resulted in a decrease in catheter colonization by uropathogens [17, 18]. These promising results support our interest in exploring the mechanism of bacterial interference employed by E. coli 83972 so that we can we can optimize and exploit this interaction.
The adherence of bacterial cells to a surface is an essential early step in biofilm formation [19]. Type-1 fimbriae, encoded by the fim operon, are considered a major mediating factor in E. coli surface adherence [20]. Wild-type E. coli 83972 was recently discovered to have a major deletion of 4.5 kb between fimB and fimD that removes the gene encoding the major structural component of type-1 fimbriae, fimA [21]. Although the fimH gene encoding the adhesive component of type-1 fimbriae is intact, it is very unlikely that wild-type E. coli 83972 expresses functional type-1 fimbriae. We hypothesized that introducing a complete fim operon into E. coli 83972 might increase its ability to adhere to urinary catheters, which might, in turn, improve its capacity for bacterial interference.
MATERIALS AND METHODS
Adherence of E. coli isolates to silicone
Silicone is one of the predominant surface materials for the urinary catheters used in clinical settings. The capacity of clinical and laboratory isolates of E. coli to adhere to squares of siliconized latex (DaPro) after 48 h of shaking at 37°C in synthetic urine [22] was measured quantitatively by using a wash and sonication assay. Clinical uropathogenic isolates were obtained from the authors’ strain collection (R.A.H. and S.I.H.). GR12 and J96 are well characterized uropathogenic strains [23].
Bacterial strains and plasmids
The E. coli 83972– derivative strains and plasmids used in this study are listed in table 1. The construction of pSH2 has been described elsewhere [24, 25]. In brief, pSH2 carries the chromosomal fim operon (11.2 kb) of E. coli J96 in the SalI site of the tetracycline-resistance gene on pACYC184 [26]. E. coli J96 was chosen as the source for the fim operon because it adhered to silicone catheter material better than 8 other E. coli isolates (figure 1), and because the fim operon is known to be functional in this organism [27]. Plasmid RHU2602 was created by cutting pACYC184 with HincII and religating, thus removing a major portion of the tetracycline-resistance gene. Plasmid RHU2642 was created by ligating the purified fragment of pFOS1 [26] cut with HindIII and HpaI with the purified fragment of pSH2 cut with HindIII and PshAI. Plasmid RHU2607 contains the SalI fragment from pSH2 inserted into the SalI site of pBCKS [29, 30].
Table 1.
Derivative strains of Escherichia coli 83972.
| Derivative strain | Chromosomal fim genotype | Plasmid | Predicted plasmid copy number of fim |
|---|---|---|---|
| HU2634 | ΔfimH | pRHU2602 (pACYC184ΔTc) | 0 |
| HU2603 | Wild-type | pRHU2602 (pACYC184ΔTc) | 0 |
| HU2651 | Wild-type | pRHU2642 (pFOS1, fim+) | 1 [26] |
| HU2545 | Wild-type | pSH2 [27] (pACYC184, fim+) | 18 [28] |
| HU2610 | Wild-type | pRHU2607 (pBCKS, fim+) | 75 [29, 30] |
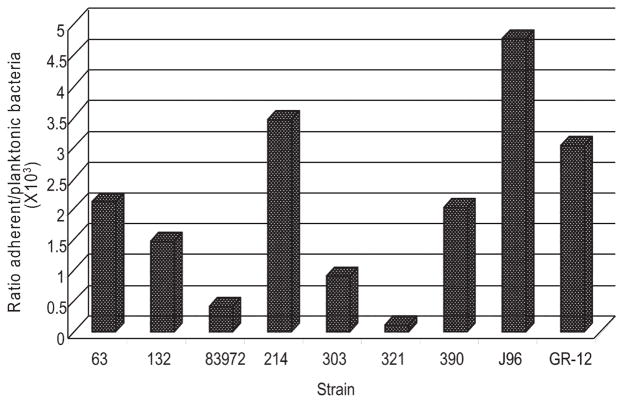
Figure 1.
Adherence of Escherichia coli 83927 to silicone, compared with the adherence of other E. coli isolates. The results are the mean of duplicate assays.
Because the chromosomally located fimH in wild-type E. coli 83972 appears to express a functional product [21], we transformed E. coli HU2222 with pRHU2602 to create HU2634. HU2222 is E. coli 83972 with a 102-bp deletion in fimH, including the start codon, and an 800-bp deletion in papG (in the P pilus operon, relevant for pyelonephritis) [31]. All other derivatives used in this study (HU2603, HU2651, HU2545, and HU2610) were created by transforming wild-type E. coli 83972. All derivative strains of 83972 were resistant to chloramphenicol and were sensitive to tetracycline. The uropathogenic challenge strain, E. coli pathogenic (ECP), was a lactose-negative E. coli isolate from a patient with symptomatic CA-UTI. This clinical isolate was modified by transformation with pACYC184, thus conferring resistance to both chloramphenicol and tetracycline
Plasmid quantitation
Plasmid DNA was extracted from an equivalent number of bacterial cells from each strain (QIA-prep Miniprep; Quiagen). Plasmids were cut once to linearize with HindIII and then electrophoresed through a 0.7% gel. AlphaDigiDoc RT 2 software (Alpha Innotec) was used to quantitate the DNA in each gel band.
Hemagglutination assay
We measured hemagglutination titers of guinea pig erythrocytes as a functional assay for expression of type-1 fimbriae [32–34]. Each strain was grown overnight in 50 mL of Luria-Bertani (LB) broth plus chloramphenicol 20 μg/mL, rocking, at 37°C. Cells were harvested by centrifugation and resuspended in PBS. Each strain was adjusted to an OD600 of 1.0. Serial 2-fold dilutions of 50 μL of bacteria were suspended in round-bottom polystyrene plates (Corning) Each well also contained 25 μL PBS and 50 μL of 5% guinea pig erythrocytes (Cambrex). Plates were incubated, rocking, at 4°C for 2 h. The hemagglutination titer was determined as the lowest dilution that prevented formation of a defined pellet of erythrocytes. In some experiments, 50 mmol/L mannose was added.
Quantitative real-time polymerase chain reaction (PCR)
Quantitative real-time PCR was used to confirm differential expression of fimH by the mutant strains of E. coli 83972. Strains were grown to an A600 of 1.0. RNA was isolated using the protocol of the RNeasy Mini Kit (Qiagen) and was treated with DNase to remove any contaminating DNA. The quantity and quality of RNA was determined using an Agilent 2100 Bioanalyzer. The cDNA were synthesized by use of the SuperScript III reverse transcriptase (RT) protocol (Invitrogen) with the random primers (catalog no. 48190 – 011; Invitrogen). The cDNA was then diluted with nuclease-free water to obtain a final dilution of 1:10 for use in the real-time PCR. Diluted cDNA template (5 μL) was added to a 96-well PCR plate (Applied Biosystems). Taqman gene expression master mixtures were prepared for each primer set in accordance with the manufacturer’s protocol (Applied Biosystems). A 2-step program was used with a 7300 real-time PCR system (Applied Biosystems). The 2−ΔΔCT method was used for analysis of fimH mRNA expression by the various E. coli 83972– derivative strains in comparison with expression by the ΔfimH strain (HU2634 as the calibrator), with 16S as the normalizing gene [35]. The primer sequences were as follows: fimH-F, 5′-TGGCGATTAAAGCTGGCTCATTA-3′; fimH-R, 5′-CACAAACTGGAAATCATCGCTGTTA-3′; fimH-M2 (FAM), 5′-TCTGTCGCAAAATAAGF-3′.
Catheter adherence assay
The ability of the 5 derivative strains of E. coli 83972 to adhere to urinary catheters was assessed with the following assay. All-silicone urinary catheters (Silicone Foley Catheters; Rochester Medical) were cut into 7-cm pieces. Each catheter piece was added to a 50-mL centrifuge tube that contained 20 μg/mL chloramphenicol in LB broth (Difco Laboratories) and inoculated with 105 cfu/mL of a given strain (50 μL of a suspension with an A600 of 0.2–0.3 were added to 50 mL of LB in each tube). Catheter pieces were incubated with E. coli, rocking, for 24 h at 37°C. Segments were rinsed and flushed with PBS. Three 1-cm segments were cut from each catheter, and each was sonicated for 10 min in 1 mL PBS plus 0.01% sodium-dodecyl sulfate SDS [36]. Dilutions of the sonicates were plated, incubated overnight, and counted to determine the number of colony-forming units per 1-cm catheter segment. The median of the colony-forming unit counts for the 3 segments was used as the value for each catheter. Experiments were repeated 7 times and results were analyzed by using the Wilcoxon rank sum test. The role of type-1 fimbriae in catheter adherence was confirmed by repeating the adherence test with strain HU2545 in the presence and absence of 50 mmol/L mannose.
Bacterial interference assay
Interference trials were performed by using our established protocol for in vitro interference [17, 18]. In brief, catheters were allowed to incubate overnight with E. coli 83972 or a derivative strain in LB broth to establish a biofilm. They were then exposed to the uropathogenic challenge strain ECP in LB broth for 30 min and then were transferred to sterile urine for a further 48 h of incubation with a change of media at 24 h. Bacteria adhering to the catheters were harvested by rinsing followed by sonication. Biofilm growth occurred in the presence of chloramphenicol 20 μg/mL throughout the experiments [37]. E. coli 83972 strains (lac+) and ECP (lac−) were distinguished by their appearance on MacConkey agar and/or by their ability to grow on plates containing tetracycline. Each experiment was repeated 4–7 times, and the numbers of pathogenic E. coli recovered from the catheters were compared using the Wilcoxon rank sum test.
Biofilm imaging
E. coli in LB broth plus chloramphenicol was incubated, rocking, at 37°C for 48 h in a chambered cover-glass (Lab-Tek; Nalge Nunc International). This device consisted of 2 plastic media chambers mounted on a #1 borosilicate coverglass with a cover. Each strain was inoculated in 2 chambers, and the media was changed at 24 h. After 48 h of incubation, chambers were rinsed once with PBS and then stained with 50 μL of 20 mmol/L DRAQ5 (Biostatus Limited). Images were obtained with a Zeiss LSM 510 scanning confocal microscope using the 100× oil objective and the HeNe2 laser (633 nm). Images were processed with Zeiss LSM 5 software. Five randomly chosen fields were imaged for each strain. Bacterial cell counts were obtained with MetaMorph software (version 6.3r7; Molecular Devices) and compared by using the Kruskal-Wallis version of analysis of variance (ANOVA) to compare medians across groups (SigmaStat 3.5; Systat Software).
Uroepithelial cell adherence
For these experiments, we used bacterial strains HU2603 (wild type), HU2545 (most adherent), and E. coli CFT073 (positive control), and we modified the method of Svanborg Eden et al. [38]. The sediment from first morning urine from a female donor was washed in buffered saline gelatin (BSG) and resuspended. The number of cells per milliliter was calculated by direct-light microscopy. Pellets containing 2 × 105 uroepithelial cells/mL were resuspended in 1 mL BSG with 5 × 109 E. coli. Bacteria and cells were incubated together for 2 h at 37°C, rocking, then washed 4 times in BSG and stained with 20 μmol/L DRAQ5 (Biostatus Limited), a far-red, fluorescent DNA dye. Cells were imaged by using light and fluorescence microscopy, and the number of bacteria adhering to 80 cells was counted for each strain, up to a maximum of 100 adherent bacteria/cell.
RESULTS
Adherence of E. coli isolates to silicone
E. coli 83972 had a low efficacy of adherence to silicone urinary catheter material, compared with other E. coli strains, including uropathogenic clinical isolates and laboratory strains (figure 1). The adherence capacity of E. coli 83972 was approximately 10-fold less than that of J96.
Plasmid DNA quantitation, hemagglutination, and fimH expression
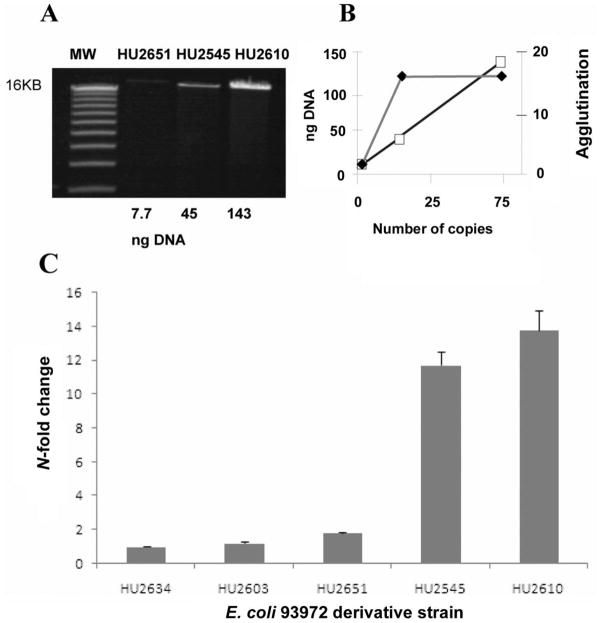
The amount of plasmid DNA per cell increased as plasmid copy number increased, as expected (figure 2A). Hemagglutination, a marker for type-1 fimbriae expression, was increased in a nonlinear fashion by adding plasmid-encoded fim (figure 2B). HU2545 and HU2610 both hemagglutinated guinea pig erythrocytes at a 1:16 dilution, while HU2651 did not hemagglutinate beyond a 1:2 dilution. HU2634 and HU2603 did not hemagglutinate in this assay. Adding 50 mmol/L mannose to the media reduced the hemagglutination titer of the 18-copy HU2545 by 8-fold, from 1:32 to 1:2. These results demonstrate that the plasmid-encoded fim was expressed in 83972, and the increased plasmid copy number correlated with increased expression of type-1 fimbriae. Quantitative real-time PCR data showed that fimH gene expression was correlated with the predicted copy number of the fimH gene (figure 2C).
Figure 2.
Plasmid copy number and fim expression. A, Plasmid quantitation. Plasmid DNA was extracted from an equivalent number of Escherichia coli cells from each strain. B, fim+ plasmids conferred increased hemagglutination. Black diamonds, hemagglutination titers; white squares, ng DNA. When reciprocal hemagglutination titers were plotted against plasmid copy number, a nonlinear relationship was seen. C, Quantitative real-time polymerase chain reaction of fimH gene expression in the 5 strains. Gene expression of fimH was measured by the 2−ΔΔCTmethod [35], in which 16S was used as the normalization gene and the fimH deletion mutant (HU2634) was used as the calibrator. The data represent the mean of 3 duplicate experiments.
Catheter adherence
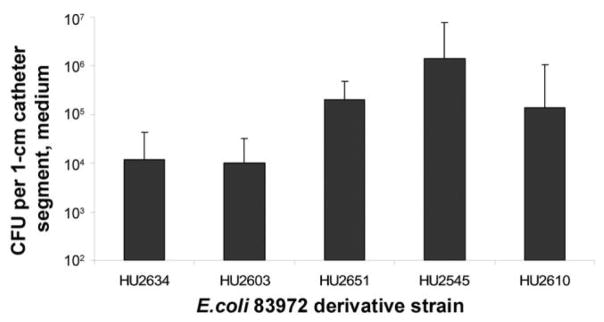
Adherence to silicone urinary catheters was increased by adding a fim-containing plasmid (figure 3). The median baseline rate of catheter adherence for the wild-type strain (HU2603) and the fimH deletion strain (HU2634) was 104 cfu per 1-cm catheter segment. The median rate of adherence was increased 10-fold by adding the single-copy fim plasmid (strain HU2651) and 100-fold by adding the 18-copy fim plasmid (strain HU2545). In the absence of mannose, 8 × 105 cfu of HU2545 per 1-cm catheter segment were recovered, whereas in the presence of 50 mmol/L mannose, we only recovered 3 × 103 cfu of HU2545 per 1-cm segment.
Figure 3.
Plasmid-encoded fim and adherence to urinary catheters. The addition of plasmid-encoded fim (3 strains on right) increased the strains’ median rate of adherence to catheters, in comparison with that of fimH deletion and wild-type strains (2 strains on left). P < .05 for the following comparisons: HU2634 to HU2651, HU2545, and HU2610; HU2603 to HU2561, HU2545, and HU2610; HU2651 to HU2545; and HU2545 to HU2610 (Wilcoxon rank sum test). The following comparisons were not statistically significant: HU2634 to HU2603 and HU2651 to HU2610 (P > .05, by the Wilcoxon rank sum test).
The median adherence rate of the 75-copy fim plasmid derivative (strain HU2610) was less than that of the 18-copy derivative (strain HU2545). We also noted that after 5 h of incubation, HU2610 was >0.5 log less abundant than the other 4 strains (5.5 × 106 vs. 1.6 × 107–2.3 × 107 cfu/mL, data not shown). To investigate whether the decreased growth rate of HU2610 could account for its decreased rate of catheter adherence in comparison with HU2545, the data from figure 3 were normalized by dividing the number of adherent organisms recovered from each catheter by the number of planktonic organisms in the media. The relationship between the relative adherence rates of HU2545 and HU2610 did not change (data not shown).
Biofilm imaging
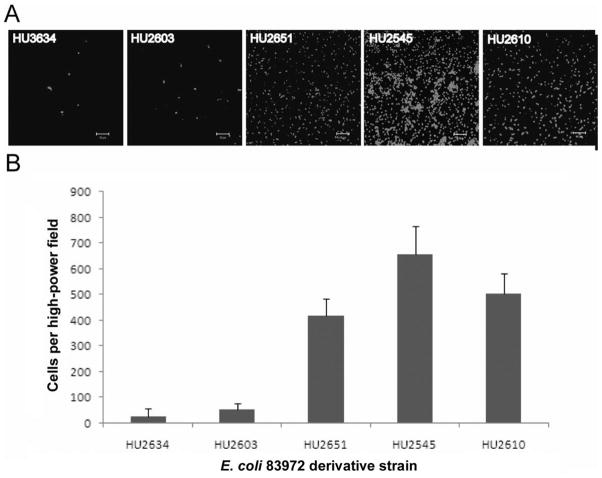
Confocal microscopy imaging of the biofilms formed by these 5 derivative strains was in agreement with the results of the catheter adherence experiments (figure 4). The median number of cells per high-power field was 28 for HU2634, 55 for HU2603, 417 for HU2651, 656 for HU2545, and 505 for HU2610.
Figure 4.
Confocal microscopy analysis of biofilm formation. A, Representative images of 24-h biofilms formed by the 5 derivative strains. B, The adherent cell counts of the 5 strains varied significantly at 48 h (P <.001, Kruskal-Wallis analysis of variance). Results are the medians of 5 microscopic fields for each strain. The main source of variance was the fact that HU2545 had higher cell counts than HU2634 or HU2603 (P <.05, Tukey test).
Bacterial interference
The pair of E. coli 83972 strains chosen for comparison was HU2603 (wild type) and HU2545 (the most adherent strain). The difference in the recovery of ECP in the presence and absence of a preexisting biofilm is a measure of bacterial interference. In all experiments, the presence of a pre-existing biofilm of a strain of E. coli 83972 (either HU2603 or HU2545) significantly decreased the median numbers of ECP recovered from the urinary catheter (figure 5). After 48 h of incubation, fewer pathogens were recovered from catheters on which the preexisting biofilm was composed of the more adhesive strain (HU2545), compared to catheters with a biofilm composed of the less adhesive strain (HU2603) (P = .021, by the Wilcoxon rank sum test).
Figure 5.
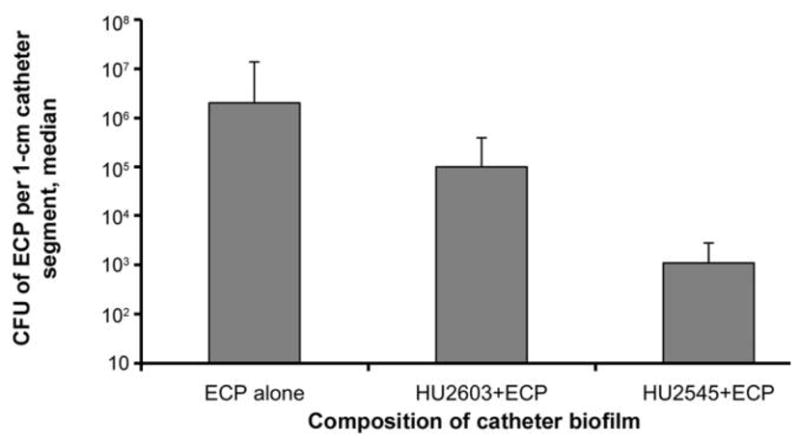
Addition of the 18-copy fim plasmid and interference with pathogenic Escherichia coli (ECP). Data are the median number of ECP recovered from the catheter surface in 5 experiments. The presence of a preexisting biofilm of E. coli HU2603 or HU2545 significantly decreased the number of ECP recovered from the catheter surface (P ≤.05 for the comparison of ECP alone and the combination of HU2603 and ECP, and for the comparison of ECP alone and the combination of HU2545 and ECP). Also, smaller numbers of ECP colonized catheters that had been precoated with the more adherent strain HU2545, compared with catheters precoated with the wild-type strain HU2603 (P = .021 for comparison of the combination of HU2603 and ECP with the combination of HU2545 and ECP).
Uroepithelial cell adherence
Adherence of HU2603 (wild-type fim strain) and HU2545 (the most adhesive fim+ strain) to bladder uroepithelial cells was low, in contrast to E. coli CFT073 (figure 6). For E. coli CFT073, the average number of adherent bacteria per cell was 59 (median, 65), in contrast to the average of 2 for HU2603 (median, 0) and 1.6 for HU2545 (median, 0) (P < .001, Kruskal-Wallis ANOVA).
Figure 6.
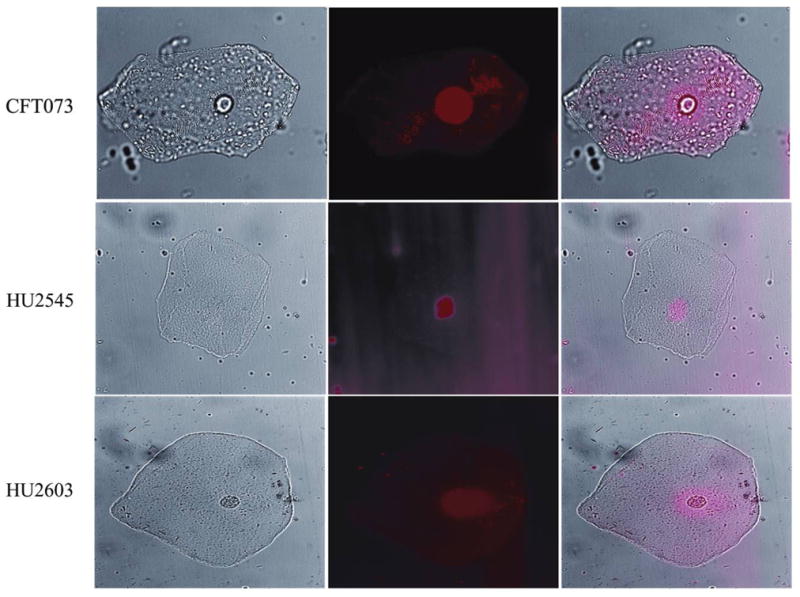
Adhesion of derivative strains of Escherichia coli 83972 to bladder uroepithelial cells. Images of uroepithelial cells and bacteria stained with DRAQ5 were acquired by using the 63× objective of a Zeiss Axioplan II upright microscope with a CoolSnap HQ CCD camera. Light images were obtained by use of differential interference contrast (DIC) prisms (left column), and fluorescent images were obtained by using Texas Red wavelength and filters (center column). DIC and fluorescent images were merged (right column) by using MetaMorph software.
DISCUSSION
The importance of type-1 fimbriae for the adherence of E. coli to abiotic surfaces such as polyvinyl chloride plates has been well documented [20, 39], but this study confirms a role for type-1 fimbriae in adherence to a clinically relevant material: silicone urinary catheters. Our results indicate that defective expression of type-1 fimbriae plays a role in the decreased rate of catheter adherence observed for E. coli 83972. This defect can be corrected by adding intact fim operon on a plasmid. The hemagglutination assays and mannose inhibition assays support the hypothesis that the observed increase in adherence rate arises from increased surface expression of type-1 fimbriae. More copies of the fim operon result in better adherence, up to a point. Type-1 fimbriae overexpression by the 75-copy plasmid in HU2610 did not result in an improved rate of adherence, in comparison to the rate for the 18-copy plasmid in HU2545. The decreased growth rate of HU2610 did not appear to account for its decreased adherence rate in comparison to that of HU2545, because the relationship of their relative adherence rates did not change when normalized by planktonic growth. Perhaps in strain HU2610 the overexpression of fim does not lead to increased surface expression of type-1 fimbriae. The similar hemagglutination titers of strains HU2545 and HU2610 support that explanation.
We did not observe any differences in adherence rates between strains HU2634 and HU2603. These strains are isogenic except for the deletions in fimH and papG in HU2634. Our results suggest that the presence of an intact fimH in wild-type 83972 is not sufficient for expression of a functional gene product in the absence of the remainder of the fim operon.
Improved rates of catheter adherence resulted in better bacterial interference with respect to urinary catheter colonization by a pathogen. Better adherence rates may allow the preexisting biofilm to block uropathogens’ access to the catheter surface through spatial constraints. The preexisting biofilm could also impair catheter colonization by pathogens through production of extracellular matrix, consumption of nutrients, changes in the local microenvironment, and production of quorum inhibitors. A greater number of organisms in the preexisting biofilm would be expected to enhance any of these interference mechanisms. Gene expression studies in dual species biofilms (E. coli 83972 and uropathogens) may elucidate which of these factors are important to the observed interference effect.
In the clinical setting, improved rates of catheter adherence may translate to better bacterial persistence in the bladder. Enhanced interference and persistence are desirable traits for the organism that we use in our clinical trials of bacterial interference for the prevention of CA-UTI. Ideally, we will be able to modify the adhesive component of the type-1 fimbriae, FimH, so that it adheres better to catheters without adhering well to urinary epithelial cells and thus becoming virulent. Previous work suggests that such a modification is possible, as FimH mutants have been described that have enhanced capacities to form biofilms yet are less susceptible to mannose inhibition than wild-type FimH [20]. Because the normal bladder epithelial cell ligand for FimH is mannose [40], such mutants might have lost their ability to adhere to bladder epithelial cells. Furthermore, we found that strain HU2545, despite its mannose-sensitive adherence to urinary catheter material, did not adhere significantly to bladder epithelial cells. This finding is in accord with those of other researchers, indicating that type-1 fimbriae expressed in the genetic background of E. coli 83972 do not promote mucosal inflammation in the human urinary tract [41]. These results have important implications for our clinical work, as they suggest that our goal of improving the capacity of E. coli 83972 for catheter adherence and bacterial interference can be achieved without increasing the organism’s virulence.
Acknowledgments
Financial support: Department of Veterans Affairs (Rehabilitation Research and Development Service Merit Review B4623W to B.W.T., B2125-RA to R.A.H., and B2–2552RA0 to R.A.H.); Paralyzed Veterans of America (grant 302 to R.A.H.); US Public Health Service (grants HD42014 to B.W.T. and DK077313. 3 to B.W.T.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, Canada, 12–15 October 2006 (abstract 586).
References
- 1.Darouiche R. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33:1567–72. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 2.Richards M, Edwards J, Culver D, Gaynes R. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 3.Tambyah P, Maki D. Catheter-associated urinary tract infection is rarely symptomatic. Arch Intern Med. 2000;160:678–82. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 4.Warren J, Tenney J, Hoopes J, Muncle H, Anthony W. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146:719–23. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 5.Warren J. Catheter-associated urinary tract infections. Int J Antimicrob Agents. 2001;17:299–303. doi: 10.1016/s0924-8579(00)00359-9. [DOI] [PubMed] [Google Scholar]
- 6.Morris N, Stickler D, McLean R. The development of bacterial biofilms on indwelling urethral catheters. World J Urol. 1999;17:345–50. doi: 10.1007/s003450050159. [DOI] [PubMed] [Google Scholar]
- 7.Donlan R. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid G, Howard L. Effect on uropathogens of prophylaxis for urinary tract infection in spinal cord injured patients: preliminary study. Spinal Cord. 1997;35:605–7. doi: 10.1038/sj.sc.3100456. [DOI] [PubMed] [Google Scholar]
- 9.Warren J, Anthony W, Hoopes J, Muncie H. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA. 1982;248:454–8. [PubMed] [Google Scholar]
- 10.Gribble M, Puterman M. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95:141–52. doi: 10.1016/0002-9343(93)90254-m. [DOI] [PubMed] [Google Scholar]
- 11.Prevention and management of urinary tract infections in paralyzed persons. Summary, evidence report/technology assessment: Number 6. Vol. 2006. Rockville, MD: Agency for Health Care Policy and Research; 1999. [PMC free article] [PubMed] [Google Scholar]
- 12.Sprunt K, Leidy G. The use of bacterial interference to prevent infection. Can J Microbiol. 1988;34:332–8. doi: 10.1139/m88-061. [DOI] [PubMed] [Google Scholar]
- 13.Bibel D. Bacterial interference, bacteriotherapy, and bacterioprophylaxis. In: Aly R, Shinefield H, editors. Bacterial interference. Boca Raton, FL: CRC Press; 1982. pp. 1–12. [Google Scholar]
- 14.Darouiche R, Donovan W, Del Terzo M, Thornby J, Rudy D, Hull R. Pilot trial of bacterial interference for preventing urinary tract infection. Urology. 2001;58:339–44. doi: 10.1016/s0090-4295(01)01271-7. [DOI] [PubMed] [Google Scholar]
- 15.Darouiche R, Thornby J, Cerra-Stewart C, Donovan W, Hull R. Bacterial interference for preventing urinary tract infection: a prospective, randomized, placebo-controlled, double-blind pilot trial. Clin Infect Dis. 2005;41:1531–40. doi: 10.1086/497272. [DOI] [PubMed] [Google Scholar]
- 16.Trautner BW, Hull RA, Thornby JI, Darouiche RO. Coating urinary catheters with an avirulent strain of Escherichia coli as a means to establish asymptomatic colonization. Infect Control Hosp Epidemiol. 2007;28:92–4. doi: 10.1086/510872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trautner B, Darouiche R, Hull R, Hull S, Thornby J. Pre-inoculation of urinary catheters with Escherichia coli 83972 inhibits catheter colonization by Enterococcus faecalis. J Urol. 2002;167:375–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Trautner B, Hull R, Darouiche R. Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology. 2003;61:1059–62. doi: 10.1016/s0090-4295(02)02555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costerton J, Stewart P, Greenberg E. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 20.Schembri M, Klemm P. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect Immun. 2001;69:1322–8. doi: 10.1128/IAI.69.3.1322-1328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemm P, Roos V, Ulett GC, Svanborg C, Schembri MA. Molecular characterization of the Escherichia coli asymptomatic bacteriuria strain 83972: the taming of a pathogen. Infect Immun. 2006;74:781–5. doi: 10.1128/IAI.74.1.781-785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minuth J, Musher D, Thorsteinsson S. Inhibition of the antibacterial activity of gentamicin by urine. J Infect Dis. 1976;133:14. doi: 10.1093/infdis/133.1.14. [DOI] [PubMed] [Google Scholar]
- 23.Svanborg Eden C, Hull R, Falkow S, Leffler H. Target cell specificity of wild-type E. coli and mutants and clones with genetically defined adhesins. Prog Food Nutr Sci. 1983;7:75–89. [PubMed] [Google Scholar]
- 24.Hull RA, Gill RE, Hsu P, Minshew BH, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981;33:933–8. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orndorff PE, Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984;159:736–44. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shizuya H, Birren B, Kim UJ, et al. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci U S A. 1992;89:8794–7. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchanan K, Falkow S, Hull RA, Hull SI. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol. 1985;162:799–803. doi: 10.1128/jb.162.2.799-803.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang A, Cohen S. Construction and characterization of multicopy amplifiable DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–56. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin-Chao S, Chen W, Wong T. High copy number of the pUC plasmid results from a Rom/Rop-supressible point mutation in RNA II. Mol Microbiol. 1992;6:3385–93. doi: 10.1111/j.1365-2958.1992.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Yasukochi T, Nagatani H, et al. Construction of a plasmid vector for the regulatable high level expression of eukaryotic genes in Escherichia coli: an application to overproduction of chicken lysozyme. Protein Eng. 1987;1:327–32. doi: 10.1093/protein/1.4.327. [DOI] [PubMed] [Google Scholar]
- 31.Hull R, Donovan W, DelTerzo M, Stewart C, Rogers M, Darouiche R. Role of type 1 fimbria- and P fimbriae-specific adherence in colonization of the neurogenic human bladder by Escherichia coli. Infect Immun. 2002;70:6481–4. doi: 10.1128/IAI.70.11.6481-6484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hull R, Rudy D, Donovan W, Wieser I, Stewart C, Darouiche R. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect Immun. 1999;67:429–32. doi: 10.1128/iai.67.1.429-432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salit IE, Gotschlich EC. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977;146:1169–81. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holden NJ, Totsika M, Mahler E, et al. Demonstration of regulatory cross-talk between P fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology. 2006;152:1143–53. doi: 10.1099/mic.0.28677-0. [DOI] [PubMed] [Google Scholar]
- 35.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCtmethod. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Sherertz R, Raad I, Belani A, et al. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos V, Klemm P. Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect Immun. 2006;74:3565–75. doi: 10.1128/IAI.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svanborg Eden C, Eriksson B, Hanson LA. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infection and Immunity. 1977;18:767–74. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt L, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 40.Sokurenko EV, Chesnokova V, Dykhuizen DE, et al. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci U S A. 1998;95:8922–6. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergsten G, Wullt B, Schembri MA, Leijonhufvud I, Svanborg C. Do type 1 fimbriae promote inflammation in the human urinary tract? Cell Microbiol. 2007;9:1766–81. doi: 10.1111/j.1462-5822.2007.00912.x. [DOI] [PubMed] [Google Scholar]