Abstract
This content discusses the individual components of multi-modal CT and multi-modal MRI, present the current status of neuroimaging for the evaluation of the acute ischemic stroke, and address the potential role of a combined multimodal stroke protocol.
Keywords: Stroke, MRI, CT, DWI, Perfusion, Thrombolysis
Introduction
Stroke is the third leading cause of death in the United States, Canada, Europe and Japan. The American Heart Association and the American Stroke Association estimate that approximately 800,000 new strokes occur each year, resulting in more than 130,000 annual deaths in the U.S. alone [1]. Direct and indirect costs related to stroke are estimated to be $70 billion annually, and will likely increase in the next decades [2]. Stroke is the leading cause of adult disability in North America [1].
Ischemic stroke is caused by the reduction of the blood supply to the brain (usually a clot occluding a cerebral artery), which subsequently disrupts the supply of oxygen and nutrients to brain tissue. Ischemic strokes account for more than 80 % of strokes [1] and can be further subdivided into cardiogenic, atherosclerotic, lacunar, hemodynamic, or cryptogenic sources [3]. Another 15% of strokes are related to the disruption of a cerebral artery resulting in intracerebral hemorrhage (ICH) [1]. Other rare causes of stroke-like symptoms include subarachnoid hemorrhage, cerebral venous sinus thrombosis, chronic subdural hematoma, neoplasms, inflammatory disease, migraine, reversible cerebral vasoconstriction syndrome (RCVS), seizure, and hypoglycemia. Many of these latter entities are discussed in this publication in the topics: Hemorrhagic Stroke and Non-traumatic Intracranial Hemorrhage; Acute Neuro-Interventional Therapies; Traumatic Brain Injury; Central Nervous System Infections; and Seizures. [COMP and E-production: Please link to the 5 article titles listed. Articles 2, 3, 5, 6 and 9]
Imaging has revolutionized acute ischemic stroke diagnosis and management. Previously, structural imaging modalities, typically noncontrast computed tomography (CT), were used to assess the presence and extent of acute ischemic stroke and exclude stroke mimics. With the development of functional imaging modalities such as perfusion-CT (PCT) and perfusion magnetic resonance imaging (MRI), stroke has been redefined from an all-or-none process to a dynamic and evolving process. In particular, the advent of effective thrombolytic therapies, such as intravenous tissue plasminogen activator (tPA) [4], has motivated us to better define the so-called ischemic “penumbra” and improve patient selection for anticoagulation therapy. Indeed, studies have indicated favorable clinical outcomes with thrombolytic therapies administered to patients selected on the basis of imaging criteria in an extended time window [5].
This content discusses the individual components of multi-modal CT and multi-modal MRI, present the current status of neuroimaging for the evaluation of the acute ischemic stroke, and address the potential role of a combined multimodal stroke protocol.
Physiopathology: the concept of penumbra
When a cerebral artery is occluded, a core of brain tissue dies rapidly. Surrounding this infarct core is an area of brain that is hypoperfused, but still viable due to collateral blood flow. This area of at risk, but potentially salvageable tissue is called the ischemic penumbra [6–8].
Studies in primates and positron emission tomography studies in humans [9–12] have shown that brain parenchyma can compensate for hypoperfusion through an increase in oxygen extraction down to a cerebral blood flow (CBF) threshold of approximately 20 to 23 mL/100 g tissue/min. If the CBF falls below this threshold, neuronal function is impaired. The affected neurons will remain viable and recover without injury after normalization of the CBF as long as it remains above approximately 10 to 15 mL/100 g tissue/min. If the CBF falls below this point, a shortage of metabolites occurs, causing a Na+/K+ channel failure in the ischemic cells. This membrane channel failure results in an uncontrolled net shift of extracellular water in the intracellular space. The consequence is “cytotoxic edema” and irreversible damage to the neuronal cells [6]. The extent of the CBF reduction and the neuronal integrity determine the size of the core and the penumbra [9–12].
The rate of change in the size of the core and penumbra is a dynamic process that depends mainly on the reperfusion of the ischemic brain. If the occlusion is not removed, the infarct core will likely grow and progressively replace the penumbra. In the case of early recanalization, either spontaneously or resulting from thrombolysis, the penumbra may be salvaged from infarction [13].
Why image an acute stroke patient?
The central premise of acute stroke treatment is to rescue the ischemic penumbra. The current guidelines [14] neglect the fact that the portion of potentially salvageable ischemic tissue is not only dependent on the time window, but also on the individual patient's collateral blood flow. The presence and extent of the ischemic penumbra is certainly time-dependent, but is especially patient-dependent. Indeed, from patient to patient, survival of the penumbra can vary from less than 3 hours to well beyond 48 hours. 90 to 100% of patients with supratentorial arterial occlusion show ischemic penumbra in the first 3 hours of a stroke, but, interestingly, 75 to 80% of patients still have penumbral tissue at 6 hours after stroke onset [13, 15, 16].
To date, the only FDA-approved drug treatment for acute stroke is intravenous thrombolysis with recombinant tPA. It is limited to the first 3 to 4.5 hours after symptom onset, and after intracranial hemorrhage has been ruled out by noncontrast CT or gradient echo MRI [17]. Unfortunately, only 3 to 8.5% of potentially eligible patients are treated because very few are medically evaluated at an early enough stage [18], and there is the widespread concern of hemorrhagic conversion resulting from overly aggressive reperfusion therapy. It has been suggested [19, 20], however, that intravenous thrombolytic therapy can be safely administered beyond 3 to 4.5 hours in selected patients with a sufficient amount of salvageable brain tissue, thereby affording a safe treatment to a much larger percentage of stroke patients. This puts a strong emphasis on the need for an improved delineation of the salvageable ischemic penumbra by imaging in acute stroke patients [21].
The relatively negative results of thrombolysis trials between 3 to 6 hours [16] are likely related to the fact that no method of penumbral imaging was used to select patients for therapy, despite penumbra being the target for treatment and despite of the high percentage of patients with penumbra within this time window. Please see the topic, Acute Neuro-Interventional Therapies, for additional information regarding neurointerventional therapy for acute ischemic infarction. [COMP and ePRODUCTION: Please hyperlink to this article]
In conclusion, a “tissue clock”, where the presence and relative extent of both infarct and penumbra are determined, would be an ideal guide to patient selection for thrombolysis, rather than a rigid time window as is recommended by the current guidelines [14]. During the last decade, a large number of models for ischemic penumbra delineation using MRI, and increasingly, CT, have been developed to predict the outcome of ischemic brain tissue.
Acute Stroke MRI
Multimodal MRI Stroke Protocol
A typical stroke MRI protocol consists of T2/FLAIR-, T2*-, diffusion- (DWI) and perfusion- (PWI) images (Table 1) and MR angiography (MRA) [22]. This protocol can be performed in less than 30 minutes. It achieves reliable information about the site of vessel occlusion, the extent of potentially salvageable brain tissue, and the exclusion of differential diagnoses of ischemic stroke.
Table 1.
Recommended Acquisition Protocol for perfusion-weighted MR imaging (PWI).
| Sequence: | Single-shot gradient-echo echoplanar imaging |
| Image acquisition parameters | TR ≤ 1500 ms TE = 35 to 45 ms at 1.5T TE = 25 to 30 ms at 3T Flip angle = 60° to 90° at 1.5T Flip angle = 60° at 3T |
| Image acquisition duration | 90 to 120 seconds Image acquisition started 10 seconds before initiation of bolus injection to achieve at least 10 baseline images |
| Coverage and slice thickness | Field of view ≈ 24 cm Whole brain coverage using ≥ 12 slices Gap 0 to 1 mm Slice thickness 5 mm Matrix size 128 × 128 |
| Slice orientation | Parallel to hard palate |
| Contrast material | Standard Gadolinium-based contrast material |
| Contrast volume | Single dose (for half molar agent ≈ 20 mL for 100 kg person) followed by 20 to 40 mL saline flush |
| Injection rate | 4 to 6 mL per second. Same rate for saline |
| IV access | 18 to 20 gauge IV line. Right antecubital vein preferred |
The MRI protocol sequence by sequence
T2 and Fluid Attenuated Inversion Recovery (FLAIR) imaging
On T2-weighted and FLAIR images, ischemic infarction appears as a hyperintense lesion usually seen within the first 3–8 hours after stroke onset (Figure 1) [23–25]. In a recent study of acute ischemic stroke patients studied by MRI within 6 hours of symptom onset, patients without a visible hyperintense lesion on FLAIR images had greater than 90% probability of being imaged within the first 3 hours of symptom onset. Thus, a mismatch between positive DWI and negative FLAIR images appears to be useful in the identification of patients who are likely to benefit from thrombolysis [24].
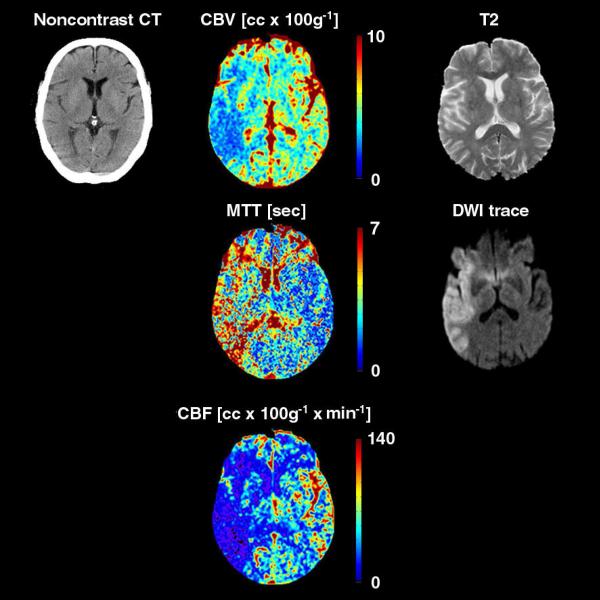
Figure 1.
75 year-old man presenting with sudden onset of a left face-arm-leg hemisyndrome. Physical examination revealed a left hemianopsia, rightward gaze deviation, dysarthria, and left hemineglect. CT and MR examination were obtained 2 and 3 hours after admission, respectively. Perfusion-CT (cerebral blood volume CBV, mean transit time MTT, cerebral blood flow CBF) and DWI-trace images clearly depict an acute stroke extending to the superficial right middle cerebral artery territory. Note how the lesion is far more subtle on the corresponding T2-weighted image, and especially on the noncontrast CT, where it features a “cortical ribbon loss” sign.
FLAIR images are also highly sensitive to subarachnoid hemorrhage [26] as well as acute cerebral venous sinus thrombosis [27, 28]. In the setting of hyperacute stroke, T2-weighted images can be useful to detect the loss of the arterial signal flow void in occluded vessels within minutes of the stroke onset [25]. T2-weighted and FLAIR images are both used to assess older cerebral infarctions and the extent of concomitant small vessel disease [25].
Diffusion-Weighted Imaging (DWI) and Apparent Diffusion Coefficient (ADC)
Diffusion magnetic resonance imaging provides image contrast that is dependent on the molecular motion of water [29]. As mentioned, cerebral ischemia leads to energy metabolism disruption with the failure of the Na+/K+ and other ionic pumps. This induces a loss of ionic gradients and a net transfer of water from the extracellular to the intracellular compartment causing a cytotoxic edema [6]. Excessive intracellular water accumulation leads to a reduced extracellular volume, where water mobility is relatively facilitated, and therefore to a reduction of water diffusion in the extracellular matrix [29]. This phenomenon is detected with DWI within minutes of vessel occlusion [13, 29] and can be measured quantitatively with the ADC.
DWI is the most sensitive method to date for the depiction of ischemia in the hyperacute stage (Figures 1 and 2) [30, 31]. However, it should be remembered that DWI lesions can be at least partially reversible in the very early phase of ischemia and the size of the DWI abnormality does not necessarily reflect irreversibly damaged tissue. Indeed, a recent series of 68 acute ischemic stroke patients [32] showed that 20% of the patients had ADC normalization in greater than 5 ml of brain tissue. The partial normalization was predominantly seen in the basal ganglia and white matter in patients with distally located vessel occlusions, and it was associated with a trend towards a better clinical outcome. This study suggests that patients with a perfusion/diffusion match within 3 hours of symptom onset may still have salvageable tissue at risk and might benefit from thrombolysis.
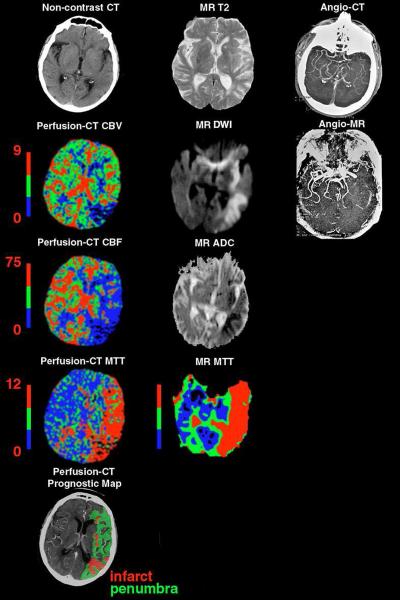
Figure 2.
71 year-old woman presenting with sudden onset of a right face-arm-leg hemisyndrome and non-fluent aphasia. Noncontrast cerebral CT/perfusion-CT and DWI-/PWI-MRI were performed 2 and 2.3 hours after symptom onset, respectively. The noncontrast CT demonstrates a left “insular ribbon sign” and subtle left parietal hypodensity. The cerebral infarct and CBV abnormality on perfusion-CT ([mL × 100g−1]) show a similar size to the DWI-MRI abnormality. However, the size of the CBF/MTT abnormality on perfusion-CT ([mL × 100g−1 × min−1] / [sec]) and on the MR MTT image involves the entire left MCA territory (i.e. “unmatched” defect with penumbral tissue in the anterior MCA territory). The corresponding M1 occlusion is clearly identified on both the CTA and MRA. The patient underwent unsuccessful thrombolysis.
Noncontrast Magnetic Resonance Angiography (MRA)
The most commonly used noncontrast MRA technique is time-of-flight (TOF) imaging. This sequence depicts vascular flow by repeatedly applying a radiofrequency pulse to a volume of tissue, followed by dephasing and rephasing gradients. Stationary tissue in the volume becomes saturated by the repeated excitation pulses and has relatively low signal. Conversely, inflowing blood protons are not saturated and therefore produce relatively increased signal intensity [25, 33]. The vessel contrast is proportional to the blood velocity (i.e., “flow-related enhancement”). For selective imaging of arteries, saturation bands are applied on the venous side of imaging sections to null signal from the venous flow [33].
3D TOF MRA is the preferred technique for the examination of intracranial vessels. MRA is particularly useful in the detection of vascular occlusion and/or stenosis in patients with ischemic stroke (Figure 2) [25]. Technical improvements such as parallel imaging and higher magnetic fields allow high spatial isotropic resolution, fast acquisition times, and reduced artifacts [25].
Perfusion Magnetic Resonance Imaging (PWI)
PWI allows the measurement of capillary perfusion to the brain. The bolus passage of a paramagnetic intravascular MRI contrast agent through the cerebral capillaries causes a nonlinear signal loss on T2* images [34]. This dynamic contrast-enhanced technique tracks the tissue signal changes caused by the susceptibility effect to create a hemodynamic time-to-signal intensity curve [35].
Gradient-recalled echo (T2*) weighted imaging
Hyperacute stroke imaging demands the differentiation between ischemic stroke and hemorrhagic stroke (discussed in Hemorrhagic Stroke and Non-traumatic Intracranial Hemorrhage in this publication) [COMP and ePRODUCTION, please hyperlink to this article], which is impossible by clinical means only. Although CT is the standard method for the diagnosis of ICH, studies have shown that hyperacute ICH can be identified on MR (mainly FLAIR and gradient-recalled echo imaging) with excellent accuracy [36].
Moreover, microbleeds (small hemosiderin deposits) not apparent on CT, can be detected by T2*-weighted images [37]. These chronic lesions are associated with an increased risk of spontaneous ICH and may also be a risk factor for thrombolysis-related hemorrhage [38, 39].
Finally, in suspected acute stroke, T2*-weighted images can detect an intraluminal thrombus as a linear low signal region of magnetic susceptibility [25].
Alternative sequences for selected conditions
Susceptibility-Weighted Imaging (SWI)
Susceptibility-weighted imaging (SWI) is a high-resolution 3D gradient-echo sequence that uses magnitude and phase information to create a new source of contrast. It offers information about any tissue that has a different susceptibility than its surrounding structures, such as deoxygenated blood, hemosiderin, ferritin, and calcium [40].
SWI is exquisitely sensitive in detecting hemorrhage [40]. Studies have shown that it is much more sensitive in detecting hemorrhagic conversion and old microbleeds than CT and conventional GRE sequences [40, 41].
Fresh clots contain a high concentration of deoxyhemoglobin, and thus appear hypointense on SWI images. As a complementary sequence, SWI may be useful to depict distal branch thrombi that are not well visualized by MRA [40]. Because of a decrease in arterial flow, and subsequent increase in the proportion of deoxyhemoglobin, acute thromboembolism may result in prominent hypointensity in the draining veins located in hypoperfused areas on SWI. In suspected acute stroke, therefore, SWI could also have a potential role by assessing areas of hypoperfusion without the need of contrast [40].
Despite its many potential advantages, this technique is not available on all major MR manufacturers systems and, in spite of the technical advances, approximately 5 minutes are still needed to image the entire brain [40].
Fat-saturated T1 (T1-FS)-Weighted Imaging
Fat-saturated T1 (T1-FS)-weighted images should be considered if cervical artery dissection is suspected. Vascular dissection is an important etiology of acute infarction, causing up to 20% of cerebral infarctions in young patients. It occurs when blood extends into the wall of a vessel through an intimal tear [42].
Dissection occurs most frequently in the distal extracranial portion of the internal carotid artery and vertebral artery. It causes ischemic stroke primarily through embolization rather than through hemodynamic flow limitation [25]. The intramural blood appears hyperintense on T1-FS-weighted images when met-hemoglobin develops, typically within 2 to 3 days after dissection (Figure 3) [42].
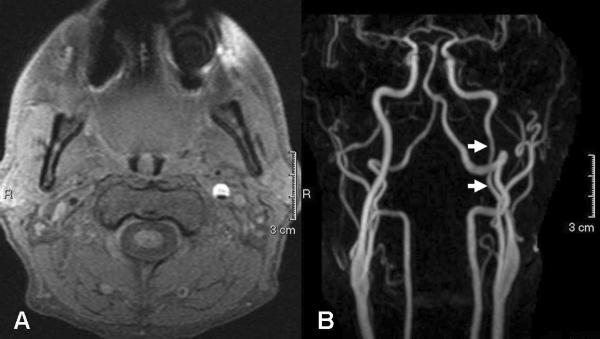
Figure 3.
40 year-old man presenting with left-sided neck pain and right-sided weakness. A. Axial T1 fat-saturated (T1-FS)-weighted MR image shows a large crescent of hyperintense signal (representing intramural met-hemoglobin) within the cervical portion of the left ICA, consistent with a carotid dissection. B. Coronal contrast-enhanced MRA MIP image demonstrates narrowing of the left ICA at the site of dissection (arrows).
Contrast-Enhanced Magnetic Resonance Angiography (CE-MRA) of the Cervical Arteries
Contrast-enhanced MRA is the technique of choice for extracranial artery imaging [29]. It relies on injection of a paramagnetic agent such as gadolinium to reduce the T1 relaxation time of tissue and to generate contrast between the intravascular lumen and surrounding tissues [43]. Unlike TOF-MRA, vascular contrast is therefore relatively independent of flow dynamics, and artifacts associated with saturation effects are substantially reduced. Vessels from the aortic arch to the circle of Willis can be obtained in less than 1 minute. This sequence enables potential assessment of stenosis or vessel occlusion [43]. CE-MRA is also used to demonstrate luminal narrowing in acute dissection (Figure 3) [44].
MRI for the differentiation of infarct core and penumbra
Simplistically, it has been hypothesized that DWI reflects the irreversibly damaged infarct whereas PWI reflects the overall area of hypoperfusion [45]. The volume difference between these, termed the PWI/DWI-mismatch, represents the MRI correlate of the ischemic penumbra (see Figures 1 and 2). Conversely, if there is no difference in PWI and DWI volumes, or even a negative difference (PWI < DWI), this is termed a PWI/DWI-match. This “matched defect” is seen in the patient who does not have penumbral tissue either because of normalization of prior hypoperfusion or because of completion of the infarction and the total loss of penumbra [45–47]. It may be argued that this model does not take into account that the PWI lesion also assesses areas of oligemia that are not in danger and that DWI abnormalities do not necessarily turn into infarction [32, 48]. It is not yet clear which parameter gives the best approximation to critical hypoperfusion and allows differentiation of infarct from penumbra. Most authors, however, agree that in current clinical practice, Tmax and mean transit time (MTT) appear to give the best results.
Stroke MRI in clinical trials
Stroke MRI has been investigated in the clinical setting to evaluate its role for thrombolysis in an extended time window. The DIAS and DEDAS trials [19, 20] used a new fibrinolytic drug, desmoteplase, in acute ischemic stroke patients within a 3- to 9-hour time window after symptom onset. Patient screening was based on clinical examination, medical history and guided by stroke MRI. Only patients presenting a clear DWI/PWI mismatch were randomized. The patients who received a placebo or an ineffective dosage showed a low recanalization rate and an unfavorable outcome. Patients who achieved early vessel recanalization and reperfusion of penumbral tissue, showed a significant clinical benefit, and 60% of the patients from the most effective dose tier had an excellent clinical outcome [19].
In the DIAS 2 study [49], patients were enrolled based on a mismatch diagnosed either by MR or by perfusion computed tomography (PCT). Unfortunately, the intention-to-treat analysis found no significant difference between the groups in clinical response rates, contrasting sharply with their previous findings with desmoteplase in the DIAS and DEDAS trials. Clinical response rate was 46.0% in the placebo group, 47.4% in the 90 μg/kg group, and 36.4% in the 125 μg/kg group.
The DEFUSE trial [5] was designed to determine whether a mismatch between perfusion and diffusion could be used to predict clinical outcome in patients with early reperfusion after treatment with recombinant tPA during the 3- to 6-hour time window after symptom onset. Patients with a baseline mismatch between PWI and DWI of at least 30% and a reduction in perfusion abnormality volume of at least 10 mL had a better clinical outcome. The trial thus showed that baseline MRI findings can be used to identify groups of patients who are more likely to benefit from thrombolytic therapy and, potentially, other forms of reperfusion therapy. Data from this study suggested that a larger difference, a mismatch ratio (PWI volume-DWI volume/DWI volume) of 2.6, provided the highest sensitivity and specificity for identifying patients in whom reperfusion was associated with a favorable response. However, even in the presence of a large PWI/DWI mismatch, no benefit could be expected if early recanalization of the occluded vessel failed [50].
Other trials have studied the role of early MRI changes for the prediction of thrombolysis outcome. It is known that large ischemic lesions on DWI are predictive of poor outcome regardless of whether thrombolysis is performed [51]. A prospective study in patients with anterior circulation ischemic stroke treated with tPA within 3 hours of stroke onset compared the baseline DWI findings using the Alberta Stroke Program Early CT Score (ASPECTS) with patient clinical outcome at 7 days [51]. Clinical worsening and poor outcome was noted more frequently in patients with ASPECTS 5 or less. The authors suggested that these patients should be excluded from studies of thrombolysis beyond the 3-hour time window.
Acute Stroke CT
Multimodal CT Stroke Protocol
Modern CT imaging, including noncontrast CT (NCT), perfusion-CT (PCT) (Table 2), and CT-angiography (CTA) fulfills all the requirements for hyperacute stroke imaging [52]. NCT can exclude hemorrhage; perfusion-CT can differentiate between penumbra and irreversibly damaged brain tissue [52]; and CTA identifies intracranial thrombus and vascular narrowing. Multimodal CT offers rapid data acquisition and can be performed with conventional CT equipment.
Table 2.
Recommended Acquisition Protocol for Perfusion-CT.
| Image acquisition rate: | Image acquisition 6–7 seconds after start of injection of the contrast bolus. 2 phases: First phase: 1 image per second, duration 30 to 45 seconds Second phase: 1 image per 2 or 3 seconds, duration 30 to 45 seconds Total duration at least 70 seconds |
| Image acquisition parameters: | 80 kVp, 100 mAs |
| Coverage and slice thickness | Field of view ≈ 24 cm Maximal coverage possible based on CT scanner configuration (Minimal coverage of 20 mm slab per bolus injection preferable. Two boluses injection is possible to double coverage in scanners with under 40 mm detector length under precluded by contrast dose considerations) |
| Slice orientation | Parallel to hard palate. |
| Contrast material | High concentration (350–370 mg/mL) low/iso osmolar contrast preferred |
| Contrast volume | 35 to 50 mL, followed by 20 to 40 mL saline flush |
| Injection rate: | 4 to 6 mL per second. Same rate for saline |
| IV access | 18 to 20 gauge IV line. Right antecubital vein preferred |
The CT protocol sequence by sequence
Noncontrast CT (NCT)
With its widespread availability, short scan time, non-invasiveness and safety, NCT has been the traditional first-line imaging modality for the evaluation of acute ischemic stroke. In this setting, NCT is typically used to rule-out intracranial hemorrhage and other stroke mimics. Occasionally, NCT can provide information that supports the diagnosis of hyperacute infarction [53–55]. Early CT signs of brain ischemia include:
1) Insular ribbon sign
The insular cortex is particularly vulnerable to a proximal middle cerebral artery (MCA) occlusion because it is the region most distal from the potential anterior and posterior collateral circulation, and therefore it is a watershed arterial zone. When ischemic, the insular region shows loss of definition of the gray-white interface, or loss of the “insular ribbon” (see Figures 1 and 2) [53].
2) Obscuration of the lentiform nucleus
Due to its blood supply via end-arteries, the basal ganglia are also particularly vulnerable to early infarction [54]. When ischemic, an obscured outline or partial disappearance of the lentiform nucleus can be seen on NCT (Figure 4).
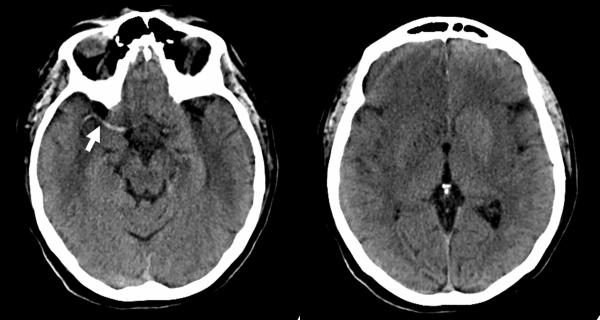
Figure 4.
Noncontrast brain CT in a 62 year-old man obtained 4 hours after the onset of symptoms shows a hyperdense right MCA (arrow), obscuration of the right lentiform nucleus, and subtle loss of gray-white differentiation within the right orbitofrontal lobe.
3) Hyperdense artery sign
The presence of an acute thrombus in the MCA creates a linear hyper-attenuation on NCT, the so-called “hyperdense artery” sign (figure 4 and 5) [55]. Contrary to the other early CT signs, this one represents not an infarction but a thrombotic event [55]. Although it is highly specific for ischemia, its sensitivity is poor [56], and false positives such as high hematocrit level or atherosclerotic calcification should be excluded. In those cases, however, the hyper-attenuation is usually bilateral [57].
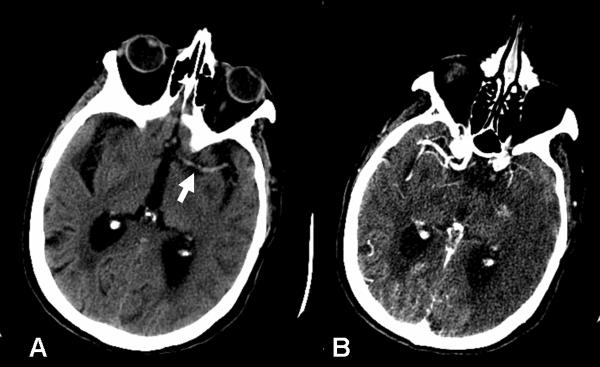
Figure 5.
86-year-old woman admitted to the emergency department after sudden onset of right face-arm-leg hemisyndrome. A. Non-contrast CT of the brain shows a hyperdense left MCA (arrow). B. Concurrent CTA shows non-filling of the left MCA, consistent with intraluminal thrombus. Note how the margins of the infarct are more conspicuous on the CTA source image.
Although these early signs can be helpful in stroke detection, they are subtle, difficult to detect, and exhibit limited sensitivity in the hyperacute stage of ischemic stroke - approximately 25% during the first 3 hours, when compared with DWI-MR [58]. Moreover, the relationship between early ischemic changes on CT and adverse outcomes after t-PA treatment is not straightforward [59, 60].
In contrast to these early ischemic changes, obvious hypo-attenuation is highly specific for irreversible tissue damage [61], and its extent is predictive of the risk of hemorrhagic transformation [62]. In the European Cooperative Acute Stroke Study trials (ECASS I and II), involvement of more than one-third of the MCA territory on NCT was used as a criterion for patient exclusion from reperfusion therapy due to the potential increased risk for hemorrhagic transformation [63]. Despite its many advantages, NCT provides solely anatomic - and not physiologic - information and it cannot reliably differentiate between irreversibly damaged brain tissue and penumbral tissue.
Perfusion-CT (PCT)
PCT imaging, using standard nonionic iodinated contrast, relies on the speed of modern helical CT scanners that can sequentially trace the entry and washout of a bolus of contrast injected into an arm vein through an intravenous line [64]. The relationship between contrast concentration and signal intensity of CT data is linear. Thereby, analysis of the signal density first increasing then decreasing during the passage of the contrast provides information about brain perfusion [65].
More specifically, the PCT evaluation of brain perfusion consists of 3 types of parametric maps: cerebral blood volume (CBV), mean transit time (MTT), and cerebral blood flow (CBF). CBV reflects the blood volume per unit of brain (4–6 mL/100g in gray matter). MTT designates the average time required by a bolus of blood to cross the capillary network (4 s in gray matter). Finally, CBF relates to the volume of blood flowing per brain mass during a time interval of 1 minute (50 to 60 mL/100 g/min in gray matter) [64, 65]. The relationship between CBF and CBV is expressed by the equation CBF=CBV/MTT [64, 65]. Recently, CBF values from PCT imaging have been shown to be highly accurate in humans when compared to the gold standard, PET [66].
CT Angiography (CTA)
Advances in multidetector row CT technology have made CTA a valid alternative to conventional catheter-based cerebral angiography [67, 68]. With current CT scanners, the region from the aortic arch up to the circle of Willis can be covered in a single data acquisition with excellent, isotropic spatial resolution, in less than 5 seconds. Multiplanar reformatted images, maximum intensity projection (MIP) images, and 3D reconstructions of source images provide images comparable to those obtained with conventional angiography [69, 70].
CT angiography allows a detailed evaluation of the intra- and extracranial vasculature [70, 71]. Its utility in acute stroke lies not only in its ability to detect large vessel thrombi within intracranial vessels (Figure 2), and to evaluate the carotid and vertebral arteries in the neck [71], but also in its potential for guiding therapy. In particular, the exact location and the extent of vascular occlusion have been shown to have prognostic value in the response to thrombolytics and the determination of collateral circulation and possible risk of subsequent recanalization [72]. For example, patients with a “top of carotid” occlusion, proximal MCA branch occlusion, or significant thrombus burden might be poor candidates for intravenous thrombolytics, and may be better candidates for intra-arterial or mechanical thrombolysis [73].
CT in differentiation of infarct core and penumbra
Perfusion CT distinction of the infarct core from the penumbra is based on the concept of cerebral vascular autoregulation [65, 74, 75]. In hypoperfused areas of brain parenchyma, there are typically high MTT values due to supply via collateral circulation. Autoregulation attempts to preserve CBF values by inducing vasodilatation, which results in an increased CBV. Brain regions characterized by such PCT values are considered areas of ischemic penumbra that could benefit from reperfusion. Conversely, when ischemic injury is more severe and prolonged, autoregulation is unable to maintain the CBV above the threshold for neuronal death, and the tissue experiences irreversible hypoxic damage with a subsequent decrease in CBV; this area represents the infarct core, where tissue no longer viable will not benefit from reperfusion and is at risk for hemorrhage (see Figures 1 and 2) [65, 74].
Large prospective studies on patients with acute ischemic infarction have proved the validity of this theory and have provided evidence about the optimal approach to defining the infarct core and penumbra [76]. The PCT parameter that most accurately describes the tissue at risk of infarction is the relative MTT, with an optimal threshold of 145%. The parameter that most accurately describes the infarct core on admission is the absolute CBV, with an optimal threshold of 2.0 ml/100g. The mismatch between these two parameters affords the most accurate delineation of the tissue at risk of infarction in the absence of recanalization.
Therefore, by combining MTT and CBV results, PCT has the ability to reliably identify the reversible ischemic penumbra and the irreversible infarct core in acute stroke patients immediately after admission [76]. In the infarct core, MTT is prolonged and CBV values are lowered, whereas in the penumbra, cerebral vascular autoregulation attempts to compensate for a reduced CBF by a local vasodilatation, resulting in increased CBV values [65, 74].
The penumbra, as described by perfusion CT, has been shown to be accurate when compared to acute and delayed diffusion-/perfusion-weighted MRI (DWI/PWI) (see Figure 2) [75, 77].
CT or MR, which one to choose?
CT and MRI provide similar information. The infarct core and the ischemic penumbra, as demonstrated by DWI/PWI and by PCT are comparable [74, 77]. In addition to the similarity of their results, both CT and MRI techniques show respective advantages and drawbacks to be considered in the special setting of acute stroke.
Stroke MRI is still available only in a limited number of hospitals. Depending on each individual setting, it is hard to conduct stroke MRI without losing too much time before treatment onset. The main advantages of MRI are the direct visualization of the full extent of infarction that is seen on DWI and the whole brain coverage that allows the detection of small but clinically relevant hypoperfusion areas. Visualization of the circle of Willis can be performed in ~3 minutes with a TOF-MRA. No additional x-ray dosage or iodinated contrast agent is needed and hence no nephrotoxicity or relevant allergic reactions are expected. However, the control of vital signs and the access to the patient during the MRI study is limited by the magnet. In addition, it takes some effort to train technicians to conduct a stroke MRI in a short period of time to establish an adequate work flow during the hyperacute phase of an ischemic stroke.
CT is often criticized for its use of x-rays and iodinated contrast material. Nevertheless, when using such parameters, the effective radiation dose associated with a single slab PCT study is approximately equal to that of an unenhanced head CT, roughly 2 to 3 mSv [78] and no renal failure has yet been reported following a PCT examination [79]. Because of limited spatial resolution, however, PCT cannot detect small lacunas, and NCT is not as sensitive to microbleeds as GRE or SWI MRI. Further, PCT has a limited spatial coverage (usually 20- to 48-mm thickness). This issue is currently been solved through the development of multidetector CT scanners with greater arrays of elements. Volumetric studies using the full width of the new 320 detector CT scanners enables full brain coverage in a single rotation. The major advantages of this technology in acute stroke are the extent of coverage and the ability to obtain combined perfusion and angiographic data from the same contrast injection. The dynamic aspect of stroke can be used to assess collateral flow based on temporal resolution [80]. Despite its many advantages, recent phantom measurements indicate that imaging with this newly introduced volumetric CT results in a higher effective radiation dose compared to a multimodal stroke protocol using a conventional 64 detector row CT scanner dose [81]. More recently, flat detector (FD)-equipped angiography machines have been used to obtain not only high-quality 3D rotational angiography images but also CT- like images (FD-CT) of brain parenchyma. A recent preliminary study has shown good correlation of CBV color maps and absolute values between FD-CT and standard PCT. In the future, this technology may be able to obtain both anatomic and physiologic information without the need to transfer patients from the angiography suite to a CT facility [82].
Even at the present time, PCT demonstrates 95% accuracy in the delineation of supratentorial strokes despite its limited spatial coverage [83]. PCT has also been shown to be equal to MRI for the evaluation of vertebrobasilar ischemia [84].
The low technical requirements for performing PCT/CTA and its wide availability are key advantages. Indeed, due to the relatively low cost and utility in other areas of medicine, particularly emergency medicine and trauma, CT scanners are becoming widely available, and it is foreseeable that every major emergency center will eventually be able to perform this form of imaging within minutes of the patient entering the emergency department. Another major advantage of PCT over MRI relates to its quantitative accuracy. Whereas MRI perfusion imaging affords only semiquantitative comparison of one hemisphere with the other, quantitative accuracy of PCT makes it a potential surrogate marker to monitor the efficiency of acute reperfusion therapy.
The debate regarding the superiority of either CT or MRI for acute stroke imaging should not obscure the ultimate goal; that is, to increase the availability and improve the efficiency of thrombolytic therapy. From this standpoint, CT and MRI must be considered equivalent tools, and whichever technique available at each individual institution should be used in the best interest and benefit of the acute stroke patient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Kolominsky-Rabas PL, Heuschmann PU, Marschall D, et al. Lifetime cost of ischemic stroke in Germany: results and national projections from a population-based stroke registry: the Erlangen Stroke Project. Stroke. 2006;37(5):1179–83. doi: 10.1161/01.STR.0000217450.21310.90. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 6.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–5. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 7.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–65. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 8.Hossmann KA. Neuronal survival and revival during and after cerebral ischemia. Am J Emerg Med. 1983;1(2):191–7. doi: 10.1016/0735-6757(83)90088-8. [DOI] [PubMed] [Google Scholar]
- 9.Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54(6):773–82. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- 10.Powers WJ, Grubb RL, Jr, Darriet D, et al. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5(4):600–8. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood JD, Lev MH, Azhari T, et al. CT perfusion scanning with deconvolution analysis: pilot study in patients with acute middle cerebral artery stroke. Radiology. 2002;222(1):227–36. doi: 10.1148/radiol.2221010471. [DOI] [PubMed] [Google Scholar]
- 12.Mayer TE, Hamann GF, Baranczyk J, et al. Dynamic CT perfusion imaging of acute stroke. AJNR Am J Neuroradiol. 2000;21(8):1441–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Read SJ, Hirano T, Abbott DF, et al. The fate of hypoxic tissue on 18F-fluoromisonidazole positron emission tomography after ischemic stroke. Ann Neurol. 2000;48(2):228–35. [PubMed] [Google Scholar]
- 14.Donnan GA, Davis SM. Neuroimaging, the ischaemic penumbra, and selection of patients for acute stroke therapy. Lancet Neurol. 2002;1(7):417–25. doi: 10.1016/s1474-4422(02)00189-8. [DOI] [PubMed] [Google Scholar]
- 15.Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30(10):2043–52. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–74. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 17.Schellinger PD, Warach S. Therapeutic time window of thrombolytic therapy following stroke. Curr Atheroscler Rep. 2004;6(4):288–94. doi: 10.1007/s11883-004-0060-3. [DOI] [PubMed] [Google Scholar]
- 18.Bambauer KZ, Johnston SC, Bambauer DE, et al. Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol. 2006;63(5):661–4. doi: 10.1001/archneur.63.5.661. [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36(1):66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 20.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37(5):1227–31. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 21.Kaste M. Reborn workhorse, CT, pulls the wagon toward thrombolysis beyond 3 hours. Stroke. 2004;35(2):357–9. doi: 10.1161/01.STR.0000115165.43847.ED. [DOI] [PubMed] [Google Scholar]
- 22.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. AJNR Am J Neuroradiol. 2008;29(5):e23–30. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr JP, Biller J, Hilal SK, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke. 1995;26(5):807–12. doi: 10.1161/01.str.26.5.807. [DOI] [PubMed] [Google Scholar]
- 24.Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65(6):724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez RG, Schaefer P. In: Conventional MRI and MR Angiography of Stroke. Gonzalez RG, Hirsch JA, Koroshetz WJ, et al., editors. Springer; Berlin: 2006. pp. 115–37. [Google Scholar]
- 26.Fiebach JB, Schellinger PD, Geletneky K, et al. MRI in acute subarachnoid haemorrhage; findings with a standardised stroke protocol. Neuroradiology. 2004;46(1):44–8. doi: 10.1007/s00234-003-1132-8. [DOI] [PubMed] [Google Scholar]
- 27.Boukobza M, Crassard I, Bousser MG, et al. MR imaging features of isolated cortical vein thrombosis: diagnosis and follow-up. AJNR Am J Neuroradiol. 2009;30(2):344–8. doi: 10.3174/ajnr.A1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovblad KO, Bassetti C, Schneider J, et al. Diffusion-weighted mr in cerebral venous thrombosis. Cerebrovasc Dis. 2001;11(3):169–76. doi: 10.1159/000047634. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan A, Goyal M, Al Azri F, et al. State-of-the-art imaging of acute stroke. Radiographics. 2006;26(Suppl 1):S75–95. doi: 10.1148/rg.26si065501. [DOI] [PubMed] [Google Scholar]
- 30.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33(9):2206–10. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 31.Saur D, Kucinski T, Grzyska U, et al. Sensitivity and interrater agreement of CT and diffusion-weighted MR imaging in hyperacute stroke. AJNR Am J Neuroradiol. 2003;24(5):878–885. [PMC free article] [PubMed] [Google Scholar]
- 32.Fiehler J, Knudsen K, Kucinski T, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35(2):514–9. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- 33.Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology. 2008;248(1):20–43. doi: 10.1148/radiol.2481071497. [DOI] [PubMed] [Google Scholar]
- 34.Grandin CB. Assessment of brain perfusion with MRI: methodology and application to acute stroke. Neuroradiology. 2003;45(11):755–66. doi: 10.1007/s00234-003-1024-y. [DOI] [PubMed] [Google Scholar]
- 35.Rosen BR, Belliveau JW, Vevea JM, et al. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14(2):249–65. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 36.Fiebach JB, Schellinger PD, Gass A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35(2):502–6. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- 37.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292(15):1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 38.Fiehler J, Albers GW, Boulanger JM, et al. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38(10):2738–44. doi: 10.1161/STROKEAHA.106.480848. [DOI] [PubMed] [Google Scholar]
- 39.Vernooij MW, van der Lugt A, Breteler MM. Risk of thrombolysis-related hemorrhage associated with microbleed presence. Stroke. 2008;39(7):e115. doi: 10.1161/STROKEAHA.108.520197. author reply e116. [DOI] [PubMed] [Google Scholar]
- 40.Mittal S, Wu Z, Neelavalli J, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30(2):232–52. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004;35(8):1989–94. doi: 10.1161/01.STR.0000133341.74387.96. [DOI] [PubMed] [Google Scholar]
- 42.Ozdoba C, Sturzenegger M, Schroth G. Internal carotid artery dissection: MR imaging features and clinical-radiologic correlation. Radiology. 1996;199(1):191–8. doi: 10.1148/radiology.199.1.8633145. [DOI] [PubMed] [Google Scholar]
- 43.Leclerc X, Gauvrit JY, Nicol L, et al. Contrast-enhanced MR angiography of the craniocervical vessels: a review. Neuroradiology. 1999;41(12):867–74. doi: 10.1007/s002340050858. [DOI] [PubMed] [Google Scholar]
- 44.Bowen BC. MR angiography versus CT angiography in the evaluation of neurovascular disease. Radiology. 2007;245(2):357, 60. doi: 10.1148/radiol.2452061706. discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 45.Jansen O, Schellinger P, Fiebach J, et al. Early recanalisation in acute ischaemic stroke saves tissue at risk defined by MRI. Lancet. 1999;353(9169):2036–7. doi: 10.1016/S0140-6736(99)01146-0. [DOI] [PubMed] [Google Scholar]
- 46.Schellinger PD, Fiebach JB, Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: present status. Stroke. 2003;34(2):575–83. [PubMed] [Google Scholar]
- 47.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51(1):28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 48.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34(11)):2729–35. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 49.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–50. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks MP, Olivot JM, Kemp S, et al. Patients with acute stroke treated with intravenous tPA 3–6 hours after stroke onset: correlations between MR angiography findings and perfusion-and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249(2):614–23. doi: 10.1148/radiol.2492071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura K, Iguchi Y, Shibazaki K, et al. Large ischemic lesions on diffusion-weighted imaging done before intravenous tissue plasminogen activator thrombolysis predicts a poor outcome in patients with acute stroke. Stroke. 2008;39(8):2388–91. doi: 10.1161/STROKEAHA.107.510917. [DOI] [PubMed] [Google Scholar]
- 52.Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke. 2003;34(4):1084–104. doi: 10.1161/01.STR.0000064840.99271.9E. [DOI] [PubMed] [Google Scholar]
- 53.Truwit CL, Barkovich AJ, Gean-Marton A, et al. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology. 1990;176(3):801–6. doi: 10.1148/radiology.176.3.2389039. [DOI] [PubMed] [Google Scholar]
- 54.Tomura N, Uemura K, Inugami A, et al. Early CT finding in cerebral infarction: obscuration of the lentiform nucleus. Radiology. 1988;168(2):463–7. doi: 10.1148/radiology.168.2.3393665. [DOI] [PubMed] [Google Scholar]
- 55.Tomsick TA, Brott TG, Chambers AA, et al. Hyperdense middle cerebral artery sign on CT: efficacy in detecting middle cerebral artery thrombosis. AJNR Am J Neuroradiol. 1990;11(3):473–7. [PMC free article] [PubMed] [Google Scholar]
- 56.Leys D, Pruvo JP, Godefroy O, et al. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke. 1992;23(3):317–24. doi: 10.1161/01.str.23.3.317. [DOI] [PubMed] [Google Scholar]
- 57.Rauch RA, Bazan C, 3rd, Larsson EM, et al. Hyperdense middle cerebral arteries identified on CT as a false sign of vascular occlusion. AJNR Am J Neuroradiol. 1993;14(3):669–73. [PMC free article] [PubMed] [Google Scholar]
- 58.Barber PA, Darby DG, Desmond PM, et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke. 1999;30(10):2059–65. doi: 10.1161/01.str.30.10.2059. [DOI] [PubMed] [Google Scholar]
- 59.Patel SC, Levine SR, Tilley BC, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. 2001;286(22):2830–8. doi: 10.1001/jama.286.22.2830. [DOI] [PubMed] [Google Scholar]
- 60.Roberts HC, Dillon WP, Furlan AJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33(6):1557–65. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 61.von Kummer R, Bourquain H, Bastianello S, et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology. 2001;219(1):95–100. doi: 10.1148/radiology.219.1.r01ap0695. [DOI] [PubMed] [Google Scholar]
- 62.Larrue V, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32(2):438–41. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 63.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 64.Eastwood JD, Lev MH, Provenzale JM. Perfusion CT with iodinated contrast material. AJR Am J Roentgenol. 2003;180(1):3–12. doi: 10.2214/ajr.180.1.1800003. [DOI] [PubMed] [Google Scholar]
- 65.Wintermark M, Maeder P, Thiran JP, et al. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol. 2001;11(7):1220–30. doi: 10.1007/s003300000707. [DOI] [PubMed] [Google Scholar]
- 66.Kudo K, Terae S, Katoh C, et al. Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H2(15)O positron emission tomography. AJNR Am J Neuroradiol. 2003;24(3):419–26. [PMC free article] [PubMed] [Google Scholar]
- 67.Prestigiacomo CJ. Surgical endovascular neuroradiology in the 21st century: what lies ahead? Neurosurgery. 2006;59(5 Suppl 3):S48, 55. doi: 10.1227/01.NEU.0000237340.82724.19. discussion S3–13. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Fraser K, Wang D, et al. The evolution of endovascular therapy for neurosurgical disease. Neurosurg Clin N Am. 2005;16(2):223, 9, vii. doi: 10.1016/j.nec.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Lell MM, Anders K, Uder M, et al. New techniques in CT angiography. Radiographics. 2006;26(Suppl 1):S45–62. doi: 10.1148/rg.26si065508. [DOI] [PubMed] [Google Scholar]
- 70.Prokop M. Multislice CT angiography. Eur J Radiol. 2000;36(2):86–96. doi: 10.1016/s0720-048x(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 71.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr. 2001;25(4):520–8. doi: 10.1097/00004728-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Tan JC, Dillon WP, Liu S, et al. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61(6):533–43. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 73.Zaidat OO, Suarez JI, Santillan C, et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke. 2002;33(7):1821–6. doi: 10.1161/01.str.0000020363.23725.67. [DOI] [PubMed] [Google Scholar]
- 74.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002;51(4):417–32. doi: 10.1002/ana.10136. [DOI] [PubMed] [Google Scholar]
- 75.Heiss WD, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35(11 Suppl 1):2671–4. doi: 10.1161/01.STR.0000143329.81997.8a. [DOI] [PubMed] [Google Scholar]
- 76.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37(4):979–85. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 77.Wintermark M, Reichhart M, Cuisenaire O, et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke. 2002;33(8):2025–31. doi: 10.1161/01.str.0000023579.61630.ac. [DOI] [PubMed] [Google Scholar]
- 78.Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am J Neuroradiol. 2009;30(5):885–92. doi: 10.3174/ajnr.A1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith WS, Roberts HC, Chuang NA, et al. Safety and feasibility of a CT protocol for acute stroke: combined CT, CT angiography, and CT perfusion imaging in 53 consecutive patients. AJNR Am J Neuroradiol. 2003;24(4):688–90. [PMC free article] [PubMed] [Google Scholar]
- 80.Salomon EJ, Barfett J, Willems PW, et al. Dynamic CT angiography and CT perfusion employing a 320-detector row CT: protocol and current clinical applications. Klin Neuroradiol. 2009;19(3):187–96. doi: 10.1007/s00062-009-9019-7. [DOI] [PubMed] [Google Scholar]
- 81.Diekmann S, Siebert E, Juran R, et al. Dose Exposure of Patients Undergoing Comprehensive Stroke Imaging by Multidetector-Row CT: Comparison of 320-Detector Row and 64-Detector Row CT Scanners. AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat Detector CT in the Evaluation of Brain Parenchyma, Intracranial Vasculature, and Cerebral Blood Volume: A Pilot Study in Patients with Acute Symptoms of Cerebral Ischemia. AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wintermark M, Fischbein NJ, Smith WS, et al. Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemispheric stroke. AJNR Am J Neuroradiol. 2005;26(1):104–12. [PMC free article] [PubMed] [Google Scholar]
- 84.Nagahori T, Hirashima Y, Umemura K, et al. Supratentorial dynamic computed tomography for the diagnosis of vertebrobasilar ischemic stroke. Neurol Med Chir (Tokyo) 2004;44(3):105, 10. doi: 10.2176/nmc.44.105. discussion 110–1. [DOI] [PubMed] [Google Scholar]