Abstract
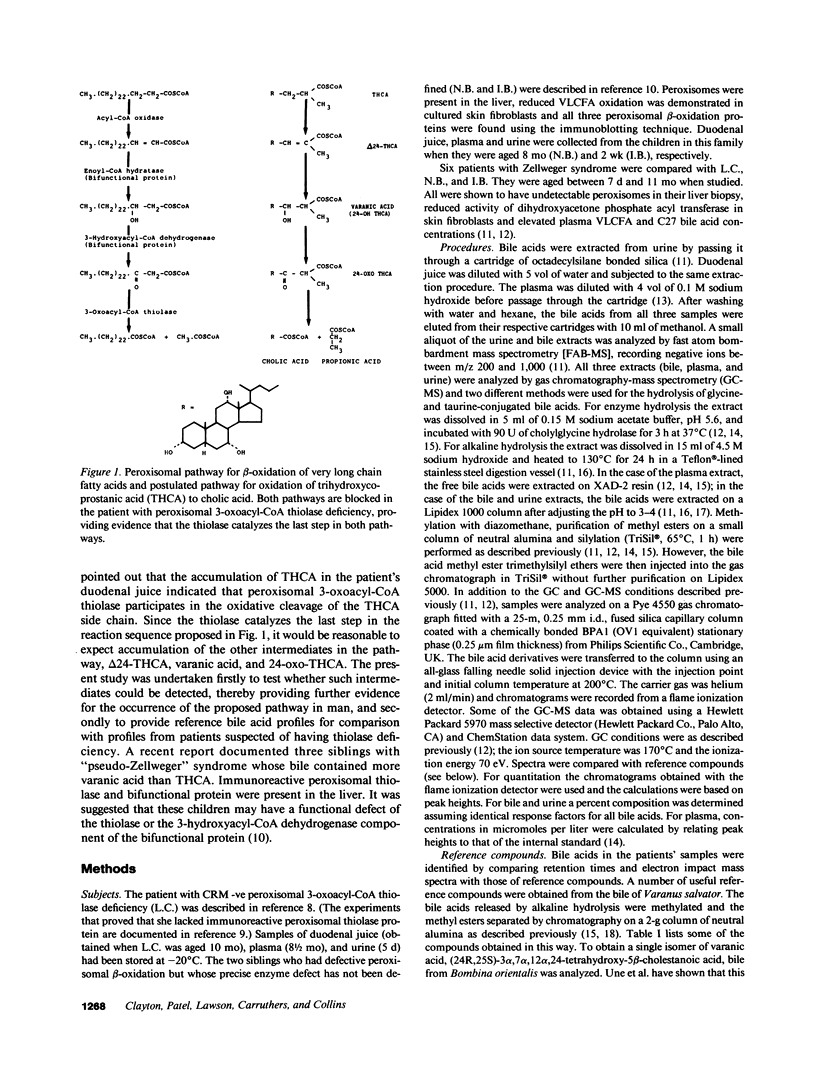
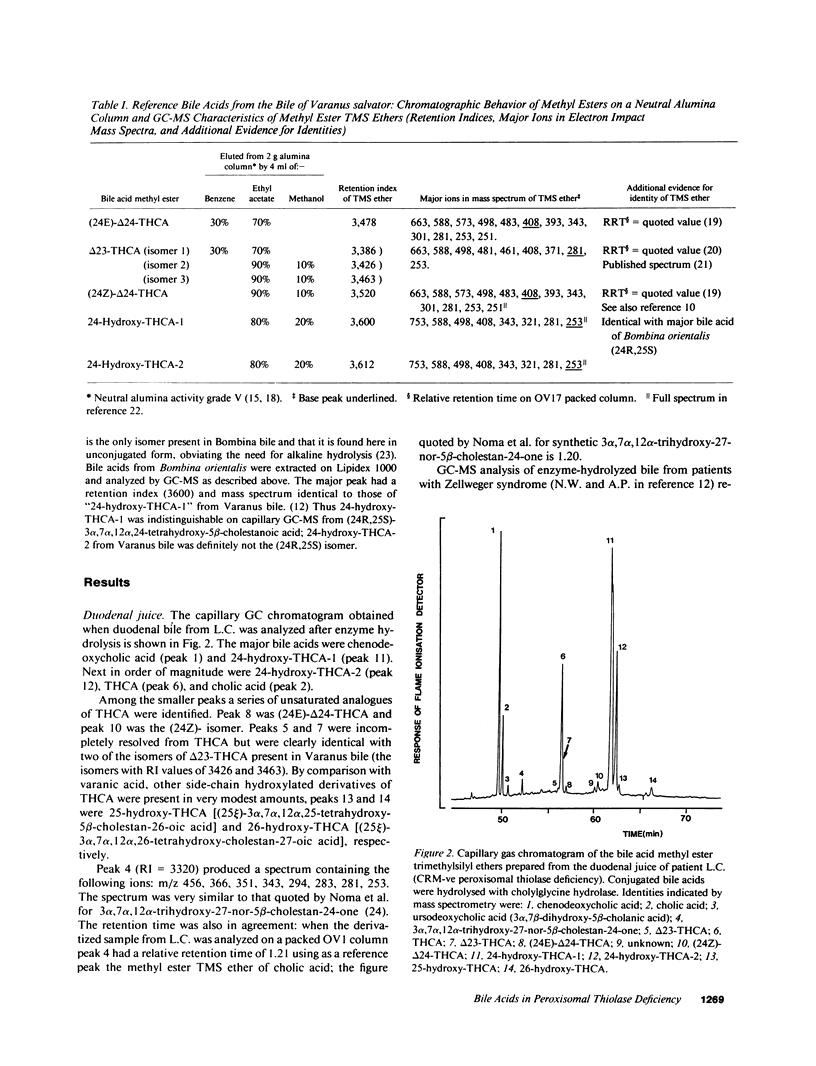
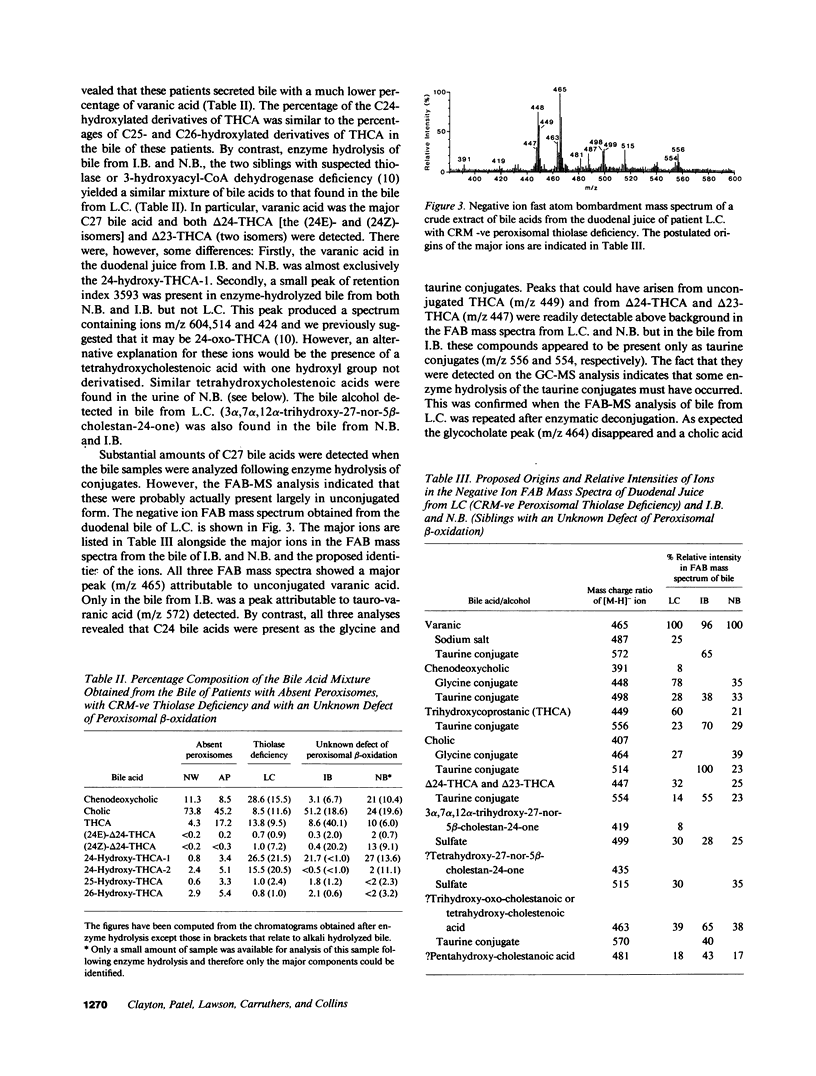
Fast atom bombardment mass spectrometry and gas chromatography-mass spectrometry were used to analyze bile acids in the body fluids of an infant (L.C.) whose liver contained no immunoreactive peroxisomal 3-oxoacyl-CoA thiolase. The profiles were compared with those of six patients with undetectable peroxisomes (Zellweger syndrome) and two siblings (N.B. and I.B.) whose defect of peroxisomal beta-oxidation could not be localized by morphological studies of peroxisomes or by immunoblotting of peroxisomal beta-oxidation proteins. 3 alpha, 7 alpha, 12 alpha-Trihydroxy-5 beta-cholestan-26-oic acid (THCA) was present in bile and plasma of all patients. However, bile from L.C., N.B. and I.B. contained unconjugated varanic acid (3 alpha, 7 alpha, 12 alpha, 24-tetrahydroxy-5 beta-cholestan-26-oic acid) as the major C27 bile acid, whereas bile from Zellweger patients contained only small amounts of varanic acid. In the bile from L.C. two isomers of varanic acid were present; in the bile from N.B. and I.B. a single isomer predominated. L.C., N.B., and I.B. all produced bile containing small amounts of (24E)-3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholest-24-en-26-oic acid [( 24E]-delta 24-THCA), its [24Z]- isomer, 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholest-23-en-26-oic acid and 3 alpha, 7 alpha, 12 alpha-trihydroxy-27-nor-5 beta-cholestan-24-one. The results provide evidence for peroxisomal pathways for cholic acid synthesis in man via THCA, delta 24-THCA and varanic acid and show that bile acid analyses can be used to diagnose peroxisomal thiolase deficiency.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. S., Stephenson E., Elliott W. H. Bile acids. LXVII. The major bile acids of Varanus monitor. J Lipid Res. 1982 Sep;23(7):947–954. [PubMed] [Google Scholar]
- Chu C. H., Schulz H. 3-Hydroxyacyl-CoA epimerase is a peroxisomal enzyme and therefore not involved in mitochondrial fatty acid oxidation. FEBS Lett. 1985 Jun 3;185(1):129–134. doi: 10.1016/0014-5793(85)80755-9. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Lake B. D., Hall N. A., Shortland D. B., Carruthers R. A., Lawson A. M. Plasma bile acids in patients with peroxisomal dysfunction syndromes: analysis by capillary gas chromatography-mass spectrometry. Eur J Pediatr. 1987 Mar;146(2):166–173. doi: 10.1007/BF02343226. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Lake B. D., Hjelm M., Stephenson J. B., Besley G. T., Wanders R. J., Schram A. W., Tager J. M., Schutgens R. B., Lawson A. M. Bile acid analyses in "pseudo-Zellweger" syndrome; clues to the defect in peroxisomal beta-oxidation. J Inherit Metab Dis. 1988;11 (Suppl 2):165–168. doi: 10.1007/BF01804226. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Muller D. P. A simplified gas-liquid chromatographic methods for the estimation of non-sulphated plasma bile acids. Clin Chim Acta. 1980 Aug 19;105(3):401–405. doi: 10.1016/0009-8981(80)90122-9. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Muller D. P., Lawson A. M. The bile acid composition of gastric contents from neonates with high intestinal obstruction. Biochem J. 1982 Sep 15;206(3):489–498. doi: 10.1042/bj2060489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Collins J., Rapin I., Neumann P., Neglia W., Spiro A. J., Ishii T., Roels F., Vamecq J., Van Hoof F. Pseudo-Zellweger syndrome: deficiencies in several peroxisomal oxidative activities. J Pediatr. 1986 Jan;108(1):25–32. doi: 10.1016/s0022-3476(86)80764-8. [DOI] [PubMed] [Google Scholar]
- Karlaganis G., Karlaganis V., Sjövall J. Identification of 27-nor-5 beta-cholestane-3 alpha,7 alpha,12 alpha,24 xi, 25 xi,26-hexol and partial characterization of the bile alcohol profile in urine. J Lipid Res. 1984 Jul;25(7):693–702. [PubMed] [Google Scholar]
- Kase B. F., Björkhem I., Hågå P., Pedersen J. I. Defective peroxisomal cleavage of the C27-steroid side chain in the cerebro-hepato-renal syndrome of Zellweger. J Clin Invest. 1985 Feb;75(2):427–435. doi: 10.1172/JCI111717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase F., Björkhem I., Pedersen J. I. Formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid by rat liver peroxisomes. J Lipid Res. 1983 Dec;24(12):1560–1567. [PubMed] [Google Scholar]
- Kärki T., Hakkola E., Hassinen I. E., Hiltunen J. K. Beta-oxidation of polyunsaturated fatty acids in peroxisomes. Subcellular distribution of delta 3,delta 2-enoyl-CoA isomerase activity in rat liver. FEBS Lett. 1987 May 11;215(2):228–232. doi: 10.1016/0014-5793(87)80151-5. [DOI] [PubMed] [Google Scholar]
- Lawson A. M., Madigan M. J., Shortland D., Clayton P. T. Rapid diagnosis of Zellweger syndrome and infantile Refsum's disease by fast atom bombardment--mass spectrometry of urine bile salts. Clin Chim Acta. 1986 Dec 15;161(2):221–231. doi: 10.1016/0009-8981(86)90215-9. [DOI] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Moser H. W. The peroxisome: nervous system role of a previously underrated organelle. The 1987 Robert Wartenberg lecture. Neurology. 1988 Oct;38(10):1617–1627. doi: 10.1212/wnl.38.10.1617. [DOI] [PubMed] [Google Scholar]
- Noma Y., Une M., Kihira K., Yasuda M., Kuramoto T., Hoshita T. Bile acids and bile alcohols of bullfrog. J Lipid Res. 1980 Mar;21(3):339–346. [PubMed] [Google Scholar]
- Norman A., Strandvik B. Formation and metabolism of bile acids in extrahepatic biliary atresia. J Lab Clin Med. 1971 Aug;78(2):181–193. [PubMed] [Google Scholar]
- Schram A. W., Goldfischer S., van Roermund C. W., Brouwer-Kelder E. M., Collins J., Hashimoto T., Heymans H. S., van den Bosch H., Schutgens R. B., Tager J. M. Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2494–2496. doi: 10.1073/pnas.84.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D., Lawson A. M., Tanida N., Sjövall J. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J Lipid Res. 1983 Aug;24(8):1085–1100. [PubMed] [Google Scholar]
- Setchell K. D., Matsui A. Serum bile acid analysis. Clin Chim Acta. 1983 Jan 7;127(1):1–17. doi: 10.1016/0009-8981(83)90070-0. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse-phase octadecylsilane bonded silica cartridges. Clin Chim Acta. 1982 Oct 27;125(2):135–144. doi: 10.1016/0009-8981(82)90190-5. [DOI] [PubMed] [Google Scholar]
- Swell L., Gustafsson J., Danielsson H., Schwartz C. C., Vlahcevic Z. R. Biosynthesis of bile acids in man. An in vivo evaluation of the conversion of R and S 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic and 3 alpha, 7 alpha, 12 alpha-24 xi-tetrahydroxy-5 beta-cholestanoic acids to cholic acid. J Biol Chem. 1981 Jan 25;256(2):912–916. [PubMed] [Google Scholar]
- Tager J. M., Van der Beek W. A., Wanders R. J., Hashimoto T., Heymans H. S., Van den Bosch H., Schutgens R. B., Schram A. W. Peroxisomal beta-oxidation enzyme proteins in the Zellweger syndrome. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1269–1275. doi: 10.1016/0006-291x(85)90322-5. [DOI] [PubMed] [Google Scholar]
- Une M., Kuramoto T., Hoshita T. The minor bile acids of the toad, Bufo vulgaris formosus. J Lipid Res. 1983 Nov;24(11):1468–1474. [PubMed] [Google Scholar]
- Une M., Morigami I., Kihira K., Hoshita T. Stereospecific formation of (24E)-3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholest-24-en-26-oic acid and (24R,25S)-3 alpha,7 alpha,12 alpha,24-tetrahydroxy-5 beta-cholestan-26-oic acid from either (25R)- or (25S)-3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestan-26-oic acid by rat liver homogenate. J Biochem. 1984 Oct;96(4):1103–1107. doi: 10.1093/oxfordjournals.jbchem.a134927. [DOI] [PubMed] [Google Scholar]
- Une M., Nagai F., Hoshita T. High-performance liquid chromatographic separation of higher bile acids. J Chromatogr. 1983 Mar 4;257(2):411–415. doi: 10.1016/s0021-9673(01)88201-7. [DOI] [PubMed] [Google Scholar]
- Une M., Nagai F., Kihira K., Kuramoto T., Hoshita T. Synthesis of four diastereoisomers at carbons 24 and 25 of 3 alpha,7 alpha,12 alpha,24-tetrahydroxy-5 beta-cholestan-26-oic acid, intermediates of bile acid biosynthesis. J Lipid Res. 1983 Jul;24(7):924–929. [PubMed] [Google Scholar]
- Watkins P. A., Chen W. W., Harris C. J., Hoefler G., Hoefler S., Blake D. C., Jr, Balfe A., Kelley R. I., Moser A. B., Beard M. E. Peroxisomal bifunctional enzyme deficiency. J Clin Invest. 1989 Mar;83(3):771–777. doi: 10.1172/JCI113956. [DOI] [PMC free article] [PubMed] [Google Scholar]


