Abstract
The fact that single-layer graphene can be visualized on 300 nm SiO2/Si substrate using optical microscope has enabled the facile fabrication of single-layer graphene devices for fundamental studies and potential applications. Here we report an Al2O3/Si substrate for the fabrication of graphene devices with better contrast and higher performance. Our studies show that the contrast of single-layer graphene on 72 nm Al2O3/Si substrate is much better than that of single-layer graphene on 300 nm SiO2/Si substrate. Moreover, the transconductance of single-layer graphene transistors on Al2O3/Si substrate shows a more than 7-fold increase, due to the smaller dielectric thickness and higher dielectric constant in 72 nm Al2O3 film. These studies demonstrate a new and superior substrate for the fabrication of graphene transistors, and are of significance for both fundamental studies and technological applications.
Keywords: graphene, high-k dielectrics, contrast, field effect transistor
1. Introduction
Graphene is a one-atom-thick planar sheet of sp2-bonded carbon atoms that are arranged in a honeycomb crystal lattice. As a gapless semiconductor, charge carriers in graphene can be tuned continuously from electrons to holes, crossing the charge neutral Dirac point using an external electric field [1–7]. Graphene-based electronics is currently the subject of intense focus for nanoscale electronic applications due to its exceptional electronic properties including carrier mobilities exceeding 100,000 cm2/V·s and a micrometer-scale mean free path at room temperature [8–10].
Most efforts to date employ a silicon substrate as a global back gate and a 300 nm thick silicon dioxide (SiO2) as the gate dielectric. This substrate is used primarily because the graphene can be readily visualized using optical microscope on this particular substrate due to optical interference [11–13]. The readily viewable graphene on the 300 nm SiO2 substrate has allowed easy device fabrication and led to many interesting scientific discoveries. The requirement of 300 nm SiO2, however, can intrinsically limit the resulted device performance in several perspectives. First, optical detection technique to visualize graphene has been demonstrated and widely used only for a SiO2 thickness of 300 nm, but a 5% variation in thickness (e.g. to 315 nm) can significantly lower the contrast [11,14]. Second, the devices made on 300 nm SiO2 substrate often have too small gate capacitance due to relatively large thickness and small dielectric constant, and thus require high switching voltage.
Recently, it has been predicted that the 72 nm Al2O3 film can be much better for visualizing graphene than SiO2 and Si3N4 films by total color difference calculation [14,]. Moreover, Al2O3, with a dielectric constant of 9.1, is an important high-k materials with excellent dielectric properties, thermal and chemical stability [15]. Therefore, higher performance graphene transistors may be readily fabricated on 72 nm Al2O3/Si substrate. Here we report the integration of graphene on 72 nm Al2O3/Si substrate and investigation of the optical contrast and device performance. Compared to 300 nm SiO2/Si substrate, 72 nm Al2O3/Si substrate could enhance the normalized optical contrast of single-layer graphene (SLG) by approximately 3 times. Furthermore, higher performance of graphene transistors can be achieved on 72 nm Al2O3/Si substrate with over 7-fold increase in transconductance.
2. Experimental
Al2O3 film was deposited on top of heavily-doped p-type silicon (100) wafers by an atomic layer deposition (ALD) technique at 250 °C using trimethylaluminum and distilled water as the source. Prior to the deposition of the Al2O3 layer, the native oxide layer on the Si wafer was removed with buffered oxide etching. After 72 nm Al2O3 film was deposited, graphene layers were mechanically peeled onto the substrate. As a control, graphene was also peeled onto standard 300 nm SiO2/Si wafers. The substrates are then calcined at 300 °C to remove organic residue. The morphologies of the samples were characterized by atomic force microscope (AFM, Veeco Dimension 5000) and optical microscope (Olympus), respectively. The Raman spectra were recorded by a Renishaw inVia Raman spectroscopy. The thickness and refractive index of Al2O3 film were obtained by Sopra GES5 Ellipsometer.
Metal-insulator-semiconductor (MIS) devices were used to investigate the dielectric properties of the Al2O3 film. The MIS devices were made on Al2O3/Si substrate with circular electrodes of 100 μm in diameter, fabricated using e-beam lithography followed by e-beam metal (Ti/Au, 50/50 nm) deposition. The SLG field-effect transistors (FETs) were also fabricated by the standard e-beam lithography and e-beam evaporation (Ti/Au, 50/50 nm) process. The on-off ratio of SLG FETs is usually very low because the intrinsic SLG is a semimetal with zero band-gap. It has been demonstrated that a band gap can open up by fabricating graphene nanostructures of confined geometry such as graphene nanoribbons (GNRs)[16–21]. To this end, we have also fabricated GNR FETs on Al2O3/Si substrate with a recently reported procedure using chemically defined nanowires as the nanoscale etching mask [19]. The electrical transport properties were measured by a Lakeshore probe station with a home built data acquisition system. Capacitance vs voltage (C-V) measurements were performed using a Stanford Research Systems SR720 LCR Meter.
3. Results and Discussion
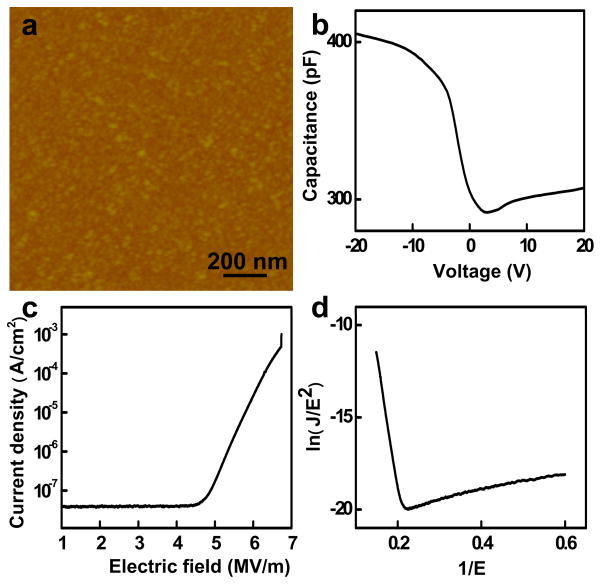
An AFM image of Al2O3 film on Si substrate indicates that the root mean square roughness for Al2O3 on Si substrate is less than 0.5 nm (Fig. 1a). To understand the dielectric properties of the Al2O3 film, metal-insulator-semiconductor (MIS) devices were used to characterize the capacitance-voltage, current tunneling and breakdown characteristics. The C-V plot for the 72 nm Al2O3 film (Figure 1b) shows an accumulation of charges in the silicon substrate in negative polarity and a depletion of charges in the positive polarity, consistent with p-type substrate used. The dielectric constant can then be calculated using: ε = Cd/ε0A, where C is the insulate capacitance (highest capacitance in the C-V curve), d is thickness of the film, ε0 is the permittivity of free space, and A is the area. The obtained dielectric constant is 7.5, consistent with previous reports on ALD Al2O3 films [15]. Current-voltage (I-V) measurements of the MIS device show typical Fowler–Nordheim (F-N) tunneling behaviour with a breakdown field of 6.7 MV/cm (Fig. 1c and 1d), comparable to the best quality ALD Al2O3 films [15]. This type of field assisted tunneling can be described by charge carriers tunneling through a triangular barrier with [15]:
| (1) |
where
| (2) |
and
| (3) |
Figure 1.
(a) AFM image of Al2O3 film by ALD. (b) Capacitance vs voltage (C-V) curve of an MIS device with 72 nm Al2O3 film on Si substrate. (c) Current-voltage (I-V) curve of the MIS device made from Al2O3 film, and (d) the corresponding Fowler–Nordheim (F-N) curve.
J is current density, Eox is the oxide electric field, m* is the effective mass of the charge carrier, which is about 0.23, and ΦB is the barrier height. Fitting the I-V characteristics with F-N tunnelling model gives a tunnel barrier of about 2.2 eV between Al2O3 and Ti, comparable to previous reports of the barrier height between ALD Al2O3 and metals of similar work function [15, 22]. These studies clearly demonstrate that the quality of our Al2O3 film is good enough as high-k gate dielectrics [15].
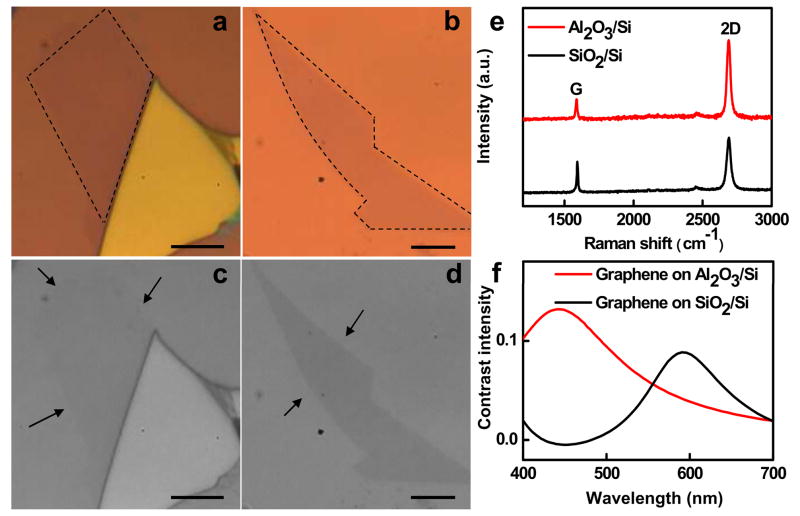
Next we discuss the optical contrast of SLG on Al2O3 films. Optical contrast is the difference in visual properties that enable us to distinguish an object from other objects and the background. Remarkably, when a proper substrate is used, single layer or few layers of graphene can be observed with sufficient contrast using an normal optical microscope [23]. The origin of the contrast can be explained by Fresnel’s equations [11]. Figure 2a and 2b show the optical image of SLG samples on Al2O3/Si and SiO2/Si substrate. The relative Raman spectra prove that samples are perfect SLG (Figure 2e). Importantly, the SLG on Al2O3/Si is much better visualized than that on SiO2/Si substrate. To quantify the difference in the contrast of the SLG samples on Al2O3/Si and SiO2/Si substrates, the color images are converted to gray-scale images with same normalized background intensity shown in Figure 2c and 2d. Indeed, the contrast intensity of SLG on Al2O3/Si is significantly stronger than that of SLG on SiO2/Si. The Weber contrast C(λ) is defined as C(λ) = (R0(λ)−R(λ))/R0(λ) [11, 23], where R0(λ) is the reflected light intensity of background and R(λ) is the reflected light intensity from graphene sheet. The contrast of SLG on Al2O3/Si is 0.070, which is 3 times larger than that of SLG on SiO2/Si with 0.023 in our system (Figure 2c and 2d). Considering the incident light from air (n = 1) onto a graphene, SiO2 or Al2O3, and Si trilayer system, the reflected light intensity from the trilayer system can be described by [11, 23]:
| (4) |
| (5) |
| (6) |
| (7) |
where r1 = (n0−n1)/(n0+n1), r2 = (n1−n2)/(n1+n2), and r3 = (n2−n3)/(n2+n3) are the reflection coefficients for different interfaces and β1 = 2πn1(d1/λ) and β2 = 2πn2(d2/λ) are the phase differences when light passes through the media which is determined by the path difference of two neighboring interfering light beams. The calculated contrast spectra of SLG are shown in Figure 2f. The best contrast of graphene on Al2O3/Si could be obtained with 450 nm light illumination. Moreover, there is moderate contrast intensity in whole visible light wavelength, in contrast to the SiO2/Si system where relatively strong contrast is only observed in the red region.
Figure 2.
(a) and (b) Optical image of graphene on 300 nm SiO2/Si and 72 nm Al2O3/Si substrate. The dashed lines highlight the edge of the single layer graphene region. (c) and (d) the gray-scale optical image from (a) and (b). The arrows highlight the edge of the single layer graphene region. (e) Raman spectra of the single-layer graphene on 300 nm SiO2/Si and 72 nm Al2O3/Si substrates. (f) The contrast spectra of single-layer graphene 300 nm SiO2/Si and 72 nm Al2O3/Si substrates.
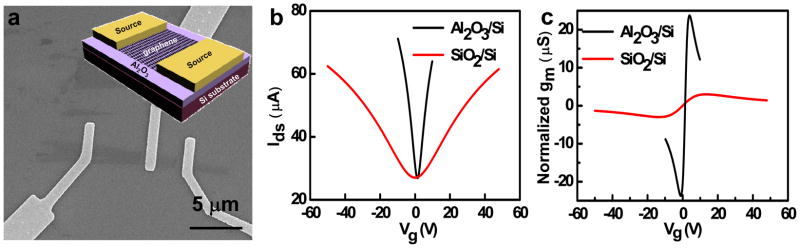
The excellent dielectric properties observed in Al2O3 film and the better optical contrast of SLG on Al2O3 film readily allows us to fabricate graphene transistors on Al2O3/Si substrate. To compare with previous studies, we adopted a similar back gated structure where Al2O3 is used the gate dielectrics and Si substrate used as the back gate. Similar devices are also fabricated on the standard SiO2/Si substrate as a control. A schematic device diagram and an SEM image of the graphene FET are shown in Figure 3a. To evaluate and compare the device performance for devices based on SiO2 and Al2O3, we have measured the transfer characteristics (Drain-source current Ids versus gate voltage Vg). The normalized transconductance can be extracted from the linear region of the Ids−Vg curve. Significantly, the Ids−Vg data show a significant improvement in transconductance when the Al2O3 is used (Fig. 3b). Figure 3c shows the measured normalized transconductance of the SLG FET as a function of gate voltage Vg. At Vds = 0.1V, the Al2O3 bottom-gated device exhibits a gm of about 22.1 μS, which is around 7 times larger than that of the SiO2 back-gated device (gm ~ 3.1 μS). This substantial improvement can be attributed to a much stronger gate coupling due to smaller thickness and higher dielectric constant material used in the this devices (72 nm Al2O3 with k = 7.5 vs. 300 nm SiO2 with k = 3.9). In order to evaluate the mobility of the devices, a device model was used [24, 25]. The extracted carrier mobility of SLG FET on Al2O3 is 7400 cm2/V·s, which is similar to 8200 cm2/V·s of the FET on SiO2.
Figure 3.
(a) SEM image of a graphene field-effect transistor on a 72-nm Al2O3/Si substrate, and the inset shows schematics of the device. (b) Ids−Vg curves of graphene FET on 300 nm SiO2/Si and 72 nm Al2O3/Si substrate, (c) gm−Vg curves of graphene FET on 300 nm SiO2/Si and 72 nm Al2O3/Si substrate.
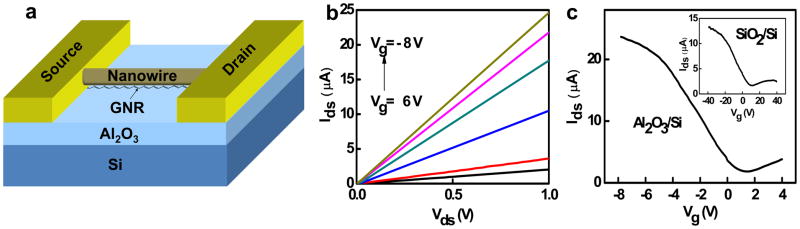
To further increase the on-off ratio of the device, we have also fabricated GNR devices on Al2O3/Si substrate and studied the device performance. Here a SiO2 nanowire was used as a nanoscale etch mask to define a graphene nanoribbon with a width in the 10–20 nm regime and the schematic device diagram of the GNR FET is shown in Figure 4a. The Figure 4b plots the Ids−Vds of the top-gated device under various gate voltages (Vg) of −8, −6, −4, −2, 0, and 2V. It shows clearly that the conductance of the nanoribbon decreases as the gate potential increases, demonstrating that the graphene nanoribbon is p-type doped, which can be attributed to edge oxidation or the physisorbed O2 from ambient or during the device fabrication process. Transfer characteristics show the GNR transistor on Al2O3 dielectric can be switched on and off with approximately 6 volts of gate swing (Fig. 4c), in contrast to ~30 V required for SiO2 back gated devices (inset, Fig. 4c) and 10–40 V for previously reported devices on SiO2 [18–20], and the transconductance gm of about 3.2 μS, also more than 9 time larger than that of the back-gated GNR transistor on SiO2/Si (~0.36 μS) (Fig. 4c). The device shows a room temperature on/off ratio of ~10 at Vds = 0.1 V, which is consistent with a graphene nanoribbon with an estimated width of 10–15 nm.
Figure 4.
(a) The schematics of the GNR FET device. (b) Ids−Vds curves of a GNR-FET on 72 nm Al2O3/Si substrate. (c) Ids−Vg curves of the GNR-FETs on the 72nm Al2O3/Si substrate and the 300 nm SiO2/Si substrate (inset).
4. Conclusions
In summary, we have demonstrated that 72 nm Al2O3/Si substrate can be a superior substrate to 300 nm SiO2/Si substrate for the visualization of graphene and fabrication of graphene transistors. Compared to 300 nm SiO2/Si substrate, 72 nm Al2O3/Si substrate could enhance the optical contrast of SLG by at least 3 times. Furthermore, using the Al2O3 film as the gate dielectrics, the back-gated graphene FETs have been fabricated to exhibit more than 7-fold increase in transconductance. This study demonstrates a new and superior substrate for the fabrication of graphene transistors, and is of significance for both fundamental studies and technological applications.
Acknowledgments
We acknowledge Nanoelectronics Research Facility at UCLA for support of device fabrication. Y.H. acknowledges support from the Henry Samueli School of Engineering and an Applied Science Fellowship. X.D. acknowledges support by the NIH Director’s New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1DP2OD004342-01. L.L. acknowledges the discussion with YingYing Wang in Nanyang Technological University for the calculation of the contrast.
References
- 1.Geim AK, Novoselov KS. Nature Materials. 2007;6:183. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 2.Geim AK. Science. 2009;324:1530. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 3.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Science. 2004;306:666. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 4.Bunch JS, Yaish Y, Brink M, Bolotin K, McEuen PL. Nano Letters. 2005;5:287. doi: 10.1021/nl048111+. [DOI] [PubMed] [Google Scholar]
- 5.Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA. Nature. 2005;438:197. doi: 10.1038/nature04233. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YB, TanY W, Stormer H, Land Kim P. Nature. 2005;438:201. doi: 10.1038/nature04235. [DOI] [PubMed] [Google Scholar]
- 7.Berger C, Song ZM, Li XB, Wu XS, Brown N, Naud C, Mayou D, Li TB, Hass J, Marchenkov AN, Conrad EH, First PN, de Heer WA. Science. 2006;312:1191. doi: 10.1126/science.1125925. [DOI] [PubMed] [Google Scholar]
- 8.Avouris P, Chen ZH, Perebeinos V. Nature Nanotechnology. 2007;2:605. doi: 10.1038/nnano.2007.300. [DOI] [PubMed] [Google Scholar]
- 9.Lemme MC, Echtermeyer TJ, Baus M, Kurz H. IEEE Electron Device Letters. 2007;28:282. [Google Scholar]
- 10.Lin YM, Jenkins KA, Valdes-Garcia A, Small JP, Farmer DB, Avouris P. Nano Letters. 2009;9:422. doi: 10.1021/nl803316h. [DOI] [PubMed] [Google Scholar]
- 11.Blake P, Hill EW, Neto AHC, Novoselov KS, Jiang D, Yang R, Booth TJ, Geim AK. Applied Physics Letters. 2007;91:063124. [Google Scholar]
- 12.Jung I, Pelton M, Piner R, Dikin DA, Stankovich S, Watcharotone S, Hausner M, Ruoff RS. Nano Letters. 2007;7:3569. [Google Scholar]
- 13.Abergel DSL, Russell A, Fal’ko VI. Applied Physics Letters. 2007;91:063125. [Google Scholar]
- 14.Gao LB, Ren WC, Li F, Cheng HM. ACS Nano. 2008;2:1625. doi: 10.1021/nn800307s. [DOI] [PubMed] [Google Scholar]
- 15.Groner MD, Elam JW, Fabreguette FH, George SM. Thin Solid Films. Vol. 413. 2002. p. 186. [Google Scholar]
- 16.Chen ZH, Lin YM, Rooks MJ, Avouris P. Physica E. Vol. 40. 2007. p. 228. [Google Scholar]
- 17.Han MY, Ozyilmaz B, Zhang YB, Kim P. Physical Review Letters. 2007. p. 98. [DOI] [PubMed] [Google Scholar]
- 18.Li XL, Wang XR, Zhang L, Lee SW, Dai HJ. Science. Vol. 319. 2008. p. 1229. [DOI] [PubMed] [Google Scholar]
- 19.Bai JW, Duan XF, Huang Y. Nano Letters. Vol. 9. 2009. p. 2083. [DOI] [PubMed] [Google Scholar]
- 20.Jiao LY, Zhang L, Wang XR, Diankov G, Dai HJ. Nature. Vol. 458. 2009. p. 877. [DOI] [PubMed] [Google Scholar]
- 21.Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM. Nature. 2009;458:872. doi: 10.1038/nature07872. [DOI] [PubMed] [Google Scholar]
- 22.Afanas’ev VV, Houssa M, Stesmans A, Adriaenssens GJ, Heyns MM. J Non-Cryst Solids. 2002;303:69. [Google Scholar]
- 23.Ni ZH, Wang HM, Kasim J, Fan HM, Yu T, Wu YH, Feng YP, Shen ZX. Nano Letters. 2007;7:2758. doi: 10.1021/nl071254m. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Nah J, Jo I, Shahrjerdi D, Colombo L, Yao Z, Tutuc E, Banerjee SK. Applied Physics Letters. 2009;94:062107. [Google Scholar]
- 25.Meric I, Han MY, Young AF, Ozyilmaz B, Kim P, Shepard KL. Nature Nanotechnology. 2008;3:654. doi: 10.1038/nnano.2008.268. [DOI] [PubMed] [Google Scholar]