Abstract
Background
Two current leading malaria blood-stage vaccine candidate antigens for Plasmodium falciparum, the C-terminal region of merozoite surface protein 1 (MSP119) and apical membrane antigen 1 (AMA1), have been prioritized because of outstanding protective efficacies achieved in a rodent malaria Plasmodium yoelii model. However, P. falciparum vaccines based on these antigens have had disappointing outcomes in clinical trials. Discrepancies in the vaccine efficacies observed between the P. yoelii model and human clinical trials still remain problematic.
Methodology and Results
In this study, we assessed the protective efficacies of a series of MSP119- and AMA1-based vaccines using the P. berghei rodent malarial parasite and its transgenic models. Immunization of mice with a baculoviral-based vaccine (BBV) expressing P. falciparum MSP119 induced high titers of PfMSP119-specific antibodies that strongly reacted with P. falciparum blood-stage parasites. However, no protection was achieved following lethal challenge with transgenic P. berghei expressing PfMSP119 in place of native PbMSP119. Similarly, neither P. berghei MSP119- nor AMA1-BBV was effective against P. berghei. In contrast, immunization with P. yoelii MSP119- and AMA1-BBVs provided 100% and 40% protection, respectively, against P. yoelii lethal challenge. Mice that naturally acquired sterile immunity against P. berghei became cross-resistant to P. yoelii, but not vice versa.
Conclusion
This is the first study to address blood-stage vaccine efficacies using both P. berghei and P. yoelii models at the same time. P. berghei completely circumvents immune responses induced by MSP119- and AMA1-based vaccines, suggesting that P. berghei possesses additional molecules and/or mechanisms that circumvent the host's immune responses to MSP119 and AMA1, which are lacking in P. yoelii. Although it is not known whether P. falciparum shares these escape mechanisms with P. berghei, P. berghei and its transgenic models may have potential as useful tools for identifying and evaluating new blood-stage vaccine candidate antigens for P. falciparum.
Introduction
Malaria is an enormous public health problem worldwide and kills one to two million people every year, mostly children residing in Africa. Clearly, an effective vaccine for the control of malaria is urgently needed. The selection of protein antigens for malaria vaccine development has been hampered by the lack of a reliable and readily accessible challenge system for Plasmodium falciparum. Accordingly, much attention has focused on the study of laboratory rodents infected by murine malaria parasite species, most notably P. yoelii and P. berghei. Although not perfect models for human infection, these systems have proved useful, and important advances in our understanding of the principles of vaccine design have followed their use. For blood-stage vaccine development, in particular, the P. yoelii-murine model has greatly contributed to the evaluation of protective efficacies of blood-stage antigens prior to human clinical trials. Based on the P. yoelii model, many asexual blood-stage candidate antigens have been identified for malaria vaccine development. Of these, two leading malaria blood-stage vaccine candidates, merozoite surface protein 1 (MSP1) and apical membrane antigen 1 (AMA1), have been intensively studied as promising vaccine candidates. These two antigens are well conserved across all species of Plasmodium and play important roles in erythrocyte invasion and blood-stage growth. Several passive and active immunization studies have indicated that both antigens elicit protective immune responses and serve as targets for invasion-blocking antibodies [1], [2], [3].
MSP1 is synthesized as an approximately 200-kDa precursor protein at the schizont stage and is further proteolytically cleaved into a number of discrete products residing on the surface of the merozoite that invades the erythrocyte [4]. After processing, the C-terminal 19-kDa fragment (MSP119) remains on the merozoite surface during erythrocyte invasion and therefore is an ideal target for blocking parasite invasion into the erythrocyte [5]. Several studies have shown that immunization with the bacterially-produced recombinant MSP119 with an adjuvant completely protects mice against P. yoelii challenge [6], [7], [8]. The P. falciparum MSP119 has been implicated as a target for protective immunity in a large number of studies, including seroepidemiological studies of naturally-acquired immunity, vaccination studies in non-human primates and in vitro cultures [9]. In particular, antibodies to MSP119, either affinity purified from immune human sera or monoclonal or polyclonal experimental sera, are capable of inhibiting parasite growth in vitro [10], [11], [12]. Recently, however, the value of these in vitro assays has come into question because cytophilic MSP119-specific antibodies appear to be more important for controlling infection than previously thought [13], [14] and the protective efficacy of MSP119-based vaccines do not correlate with anti-MSP119 antibody titers or in vitro parasite-inhibitory activity in animal models [15].
AMA1, synthesized as a 60–80-kDa protein during schizogony, is a microneme protein involved in merozoite invasion of erythrocytes. AMA1 possesses a large N-terminal cysteine-rich ectodomain, followed by a single transmembrane domain and a short C-terminal cytoplasmic tail. The ectodomain has been divided into three domains (I, II, and III) based on the disulfide bond position [16], [17] and the recent crystal structure [18]. Domain III binds to human erythrocytes [19] and serves as a target for growth-inhibitory antibodies [20]. Immunization with parasite-derived AMA1 and recombinant AMA1 induced significant levels of protection against P. yoelii challenge in mice [21] and against P. falciparum challenge in Aotus monkeys [22], respectively. Despite its promising potential, neither PfMSP119- nor PfAMA1-based vaccine candidates have yet shown satisfactory outcomes in human clinical trials. Discrepancies in the vaccine efficacies observed between the P. yoelii model and human clinical trials still remain problematic, although poor immunogenicity and genetic polymorphisms are thought to be major obstacles for vaccine development using these molecules [23], [24], [25], [26].
We have recently developed a baculoviral-based vaccine (BBV) expressing PyMSP119 on the surface of the viral envelope [27]. Adjuvant-free intranasal immunization with this vaccine induced not only strong systemic humoral immune responses with high titers of PyMSP119-specific antibody but also natural boosting of PyMSP119-specific antibody responses shortly after challenge, and conferred complete protection. As a next step, we have generated a PfMSP119-BBV vaccine to address the possibility of its use in a clinical setting. In the present study, we evaluated the protective efficacies of a series of MSP119- and AMA1-BBVs against challenge with transgenic P. berghei expressing PfMSP119 as well as P. berghei in mice. Our results show that although immunization with these BBVs induced high levels of antigen-specific antibody titers, none of the immunized mice were protected against challenge. In contrast, immunization with PyMSP119- and PyAMA1-BBVs provided 100% and 40% protection against lethal challenge with P. yoelii, respectively. These data suggest that P. berghei possesses additional molecules and/or mechanisms that circumvent the host's immune responses to MSP119 and AMA1. The present study provides important insights for malaria blood-stage vaccine development using P. yoelii and P. berghei.
Materials and Methods
Ethics Statement
All care and handling of the animals was in accordance with the Guidelines for Animal Care and Use prepared by Jichi Medical University, following approval (ID: 09193) by the Jichi Medical University Ethical Review Board.
Mice and parasites
Female BALB/c and C57/BL6 mice, 7 to 8 weeks of age at the start of the experiments, were purchased from Nippon Clea (Tokyo, Japan). P. berghei ANKA were used for challenge infection. P. yoelii 17XL, a lethal murine malaria parasite, was kindly provided by T. Tsuboi (Ehime University, Matsuyama, Japan). P. falciparum 3D7 was kindly provided by K. Kita (The University of Tokyo, Tokyo, Japan). Pb-PfM19 [28], transgenic P. berghei ANKA expressing PfMSP119 in place of native PbMSP119, was kindly provided by T. Koning-Ward (The Walter and Eliza Hall Institute of Medical research, Parkville, Australia).
Recombinant baculovirus
For the construction of MSP119-expressing baculovirus transfer vectors, the DNA sequence corresponding to amino acids Asn1607–Asn1702 of PfMSP119 was amplified from P. falciparum 3D7 genomic DNA using the primer pair pPfMSP119-F1 (5′-GAATTCAACATTTCACAACACCAATGCGTAAAAAAAC-3′)/pPfMSP119-R1 (5′-CCCGGGCGTTAGAGGAACTGCAGAAAATACCATCG-3′). Similarly, the DNA sequence corresponding to amino acids Gly1672-Ser1767 of PbMSP119 was amplified from P. berghei ANKA genomic DNA using the primer pair pPbMSP119-F1 (5′-GAATTCGGTATAGACCCTAAGCATGTATGT-3′)/pPbMSP119-R1 (5′-CCCGGGAGCTACAGAATACACCATCATAATATGC-3′). Each of the resulting PCR products was ligated into the EcoRI/SmaI sites of pBACsurf-PyMSP119 [27] to construct baculovirus transfer vectors.
For the construction of AMA1-expressing baculovirus transfer vectors, the DNA sequences corresponding to amino acids Asn53-Glu478 (domains I, II and III) and Glu380-Glu478 (domain III) of PbAMA1 (PbAMA1-D123 and PbAMA1-D3, respectively) were amplified from P. berghei ANKA genomic DNA using the primer pairs pPbAMA1-F1 (5′-GAATTCAATCCATGGGAAAAGTATACGGAAAAATAT-3′)/pPbAMA1-R1 (5′-CCCGGGCTTCTCTGGTTTGATGGGCTTTCATATGCAC-3′) and pPbAMA1-F2 (5′-GAATTCGAAGAGTTCGAAGAACAATTTCCTTGTGAT-3′)/pPbAMA1-R1, respectively. Similarly, the DNA sequences corresponding to amino acids Ile52-Lys479 (domains I, II and III) and Glu380-Lys479 (domain III) of PyAMA1 (PyAMA1-D123 and PyAMA1-D3, respectively) were amplified from P. yoelii 17XL genomic DNA using the primer pairs pPyAMA1-F1 (5′-GAATTCAATCCATGGGATAAATATATGGAAAAATATGAT-3′)/pPyAMA1-R1 (5′-CCCGGGTTTTCTGGTTTGGGTTTTCATAGTCACCTAT-3′) and pPyAMA1-F2 (5′-GAATTCGAAGAAAATTTTCCTTGTGAAATATAT-3′)/pPyAMA1-R1, respectively. Each of the resulting PCR products was ligated into the EcoRI/SmaI sites of pBACsurf-PyMSP119 [27] to construct baculovirus transfer vectors. Recombinant baculoviruses, AcNPV-PfMSP119surf, AcNPV-PbMSP119surf, AcNPV-PyAMA1-D123surf, AcNPV-PyAMA1-D3surf, AcNPV-PbAMA1-D123surf, and AcNPV-PbAMA1-D3surf were generated in Spodoptera frugiperda (Sf9) cells by co-transfection of the corresponding baculovirus transfer vector with BacVector-2000 DNA (Novagen) according to the manufacturer's protocol. AcNPV-PyMSP119surf has been described previously [27]. Purification of baculovirus virions was performed as described previously [29]. The purified baculovirus particles were free of endotoxin (<0.01 endotoxin units/109 pfu), as determined by an Endospecy® endotoxin measurement kit (Seikagaku Co., Tokyo, Japan).
Recombinant proteins
The Pfmsp119, Pbmsp119, Pyama1-D3 and Pbama1-D3 genes were excised from pBACsurf-PfMSP119, pBACsurf-PbMSP119, pBACsurf-PyAMA1-D3, pBACsurf-PbAMA1-D3, respectively, by digestion with EcoRI and SmaI. Each of these DNA fragments was cloned into the EcoR I/SmaI sites of pGEX-4T-1 (GE Healthcare UK Limited, Buckinghamshire, UK). Recombinant PfMSP119, PbMSP119, PyAMA1-D3 and PbAMA1-D3 created as GST fusion proteins (termed GST-PfMSP119, GST-PbMSP119, GST-PyAMA1-D3 and GST-PbAMA1-D3 respectively), were expressed in Escherichia coli and purified using GST affinity columns (GE Healthcare UK Limited) as described previously [30]. GST-PyMSP119 was used as an immunogen for vaccination and antigen for ELISA as described previously [27]. These recombinant proteins were recognized by P. yoelii or P. berghei-hyperimmune sera, which were obtained from BALB/c mice that had recovered from repeated infections of the corresponding parasite following treatment with chloroquine as described previously [28]. A recombinant MSP119 (yPfMSP119) of P. falciparum, produced in Saccharomyces cerevisiae, was obtained from MR4 (Manassas, VA). We confirmed that the bacterially-produced GST-PfMSP119 and yPfMSP119 proteins had the similar immunogenicity available in ELISA's using sera obtained from AcNPV-PfMSP119surf-immunized mice and malaria-exposed individuals living in a hyperendemic area. These GST-fusion proteins were used as immunogens for vaccination and antigens for ELISA. A recombinant MSP119 (yPyMSP119) of P. yoelii 17XL, produced in S. cerevisiae, was obtained from MR4, and used as an antigen for ELISA.
Immunoblotting and indirect immunofluorescence assay (IFA)
For immunoblotting, protein samples were separated on a 6% SDS-PAGE gel, transferred to Immobilon™ Transfer Membrane (Millipore, Bedford, MA). The membrane was treated either with the anti-PfMSP119 mAb 5.2 (MR4, Manassas, VA), anti-gp64 mAb (BD Biosciences, Bedford, MA), or P. berghei- or P. yoelii- hyperimmune sera. Polypeptides recognized by the antibodies were visualized by color development with 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt/nitroblue tetrazolium chloride substrate (Invitrogen) following biotinylated anti-mouse IgG secondary antibody (Vector Laboratories, Burlingame, CA) as described previously [29]. Alternatively, polypeptides recognized by the antibodies were detected with ECL™ Western Blotting Detection Reagents (GE Healthcare UK Ltd.) using an HRP-conjugated goat anti-mouse IgG (H+L) secondary antibody (Bio-Rad, Hercules, CA).
For IFA, erythrocytes infected with parasites were washed, aliquoted onto multiwell slides, and fixed in 4% paraformaldehyde or methanol/acetone (4∶6) for 30 min. Sera were diluted 1∶1,000 and incubated on the slide at room temperature for 1 h following permeabilization with 1% Triton X in PBS. After washing, the slides were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG for 1 h, washed, and covered with a drop of VECTASHIELD™ with DAPI (4′ 6-diamidion-2-phenylindole) (Vector Laboratories). Bound antibodies were detected using a BZ 9000 fluorescence microscope (Keyence, Tokyo, Japan).
Immunization and challenge infections
Mice were immunized three times at 3-week intervals with 5×107 pfu of BBV either by an intramuscular (i.m.) or intranasal (i.n.) route as described previously [27]. As a comparative control, mice were immunized intraperitoneally (i.p.) with 50 µg of GST-PbMSP119, GST-PfMSP119 or GST-PyMSP119 in 2 mg of aluminum hydroxide (Imject® Alum, Pierce) three times at 3-week intervals. For each route of immunization, 2 weeks after the final immunization, sera were collected and mice were challenged with 1,000 live parasite-infected red blood cells (pRBC) by intravenous injection. The course of parasitemia was monitored by microscopic examination of Giemsa-stained thin smears of tail blood.
Enzyme-linked immunosorbent assay (ELISA) for antibody titers
Sera obtained from immunized mice were collected by tail bleeds 2 weeks after the final immunization prior to challenge. For some mice, serum was also collected periodically after challenge. For MSP119-specific antibody detection, pre-coated ELISA plates with 100 ng/well GST-PfMSP119, GST-PbMSP119, GST-PyMSP119, PyAMA1-D3, PbAMA1-D3, and yPyMSP119 were incubated with serial dilutions of sera obtained from immunized and control mice. MSP119- or AMA1D3-specific antibodies were detected using HRP-conjugated goat anti-mouse IgG (H+L) (Bio-Rad). The plates were developed with peroxidase substrate solution [H2O2 and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)]. The optical density (OD) at 414 nm of each well was measured using a plate reader. Endpoint titers were expressed as the reciprocal of the highest sample dilution for which the OD was equal or greater than the mean OD of non-immune control sera.
Infection and drug treatment
Groups of five mice were infected with P. yoelii XL or P. berghei ANKA pRBC. When the parasitemia had reached 1–3%, mice were treated i.m. on 3 consecutive days with 100 µl of 10 mg/ml Artemether Injection® (Kunming Pharmaceutical Corp., Kunming, China) dissolved in olive oil (Yoshida Pharmaceutical Corp., Tokyo, Japan). Four weeks after the completion of the infection and drug cure regimen, mice were re-infected three times with 1,000 live pRBC of homologous parasite at 4-week intervals. Self-cured mice were challenged with 1,000 live pRBC of heterologous parasites (P. yoelii XL or P. berghei ANKA). The same experiment was repeated. The course of parasitemia was monitored by microscopic examination of Giemsa-stained thin smears of tail blood.
Results
Construction of MSP119-BBV
Recently, we have developed a new PyMSP119-BBV (AcNPV-PyMSP119surf) that displays PyMSP119 on the surface of the baculoviral envelope. Adjuvant-free intranasal immunization with this vaccine induced strong systemic humoral immune responses with high titers of PyMSP119-specific antibody, naturally boosted the PyMSP119-specific antibody response a short time after infection, and allowed 100% of mice to self-cure with very low parasitemia [27]. To apply this baculoviral vaccine system to P. falciparum MSP119 vaccine development, we generated AcNPV-PfMSP119surf and AcNPV-PbMSP119surf (Figure 1A). Each construct harbored a gene cassette that consisted of the gp64 signal sequence and the MSP119 gene fused to the N-terminus of the AcNPV major envelope protein gp64. Expression of these gene cassettes was driven by the polyhedrin promoter. Thus these BBVs were designed to express MSP119 on the viral envelope as a gp64 fusion protein.
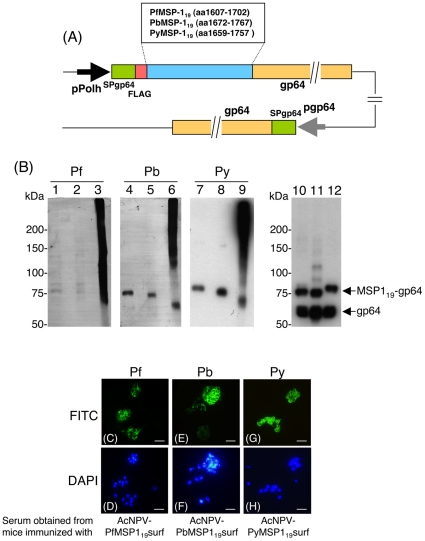
Figure 1. Construction and expression analysis of MSP119-BBVs.
(A) Schematic diagram of three MSP119-BBV genomes. MSP119 was expressed as a MSP119-gp64 fusion protein under the control of the polyhedron promoter. Numbers indicate the amino acid positions of MSP119-gp64 fusion protein and endogenous gp64 protein. pPolh, polyhedrin promoter; SP, the gp64 signal sequence; FLAG, the FLAG epitope tag; pgp64, gp64 promoter. (B) Western blot analysis of MSP119-BBVs. AcNPV-PfMSP119surf (lanes 1, 2, 3 and 10), AcNPV-PbMSP119surf (lanes 4, 5, 6 and 11) and AcNPV-PyMSP119surf (lanes 7, 8, 9 and 12) were treated with the loading buffer with 5% 2-ME (lanes 1, 4, 7, 10, 11 and 12), 0.5% 2-ME (lanes 2, 5 and 8) or without 2-ME (lanes 3, 6 and 9) and examined using the 5.2 mAb (lanes 1–3), P. berghei-hyperimmune serum (lanes 4–6), P. yoelii-hyperimmune serum (lanes 7–9) and anti-gp64 mAb (lanes 10–12). Positions of MSP119-gp64 fusion protein and endogenous gp64 are shown at the right panel of lanes 10–12. (C–H) Immunofluorescence patterns of sera obtained from mice immunized with three MSP119-BBVs on paraformaldehyde fixed erythrocyte smears infected with P. falciparum (C–D), P. berghei (E–F) and P. yoelii (G–H). The smears were incubated with serum obtained from an individual mouse immunized either with AcNPV-PfMSP119surf (C), AcNPV-PbMSP119surf (E) or AcNPV-PyMSP119surf (G), and antibody binding was detected with secondary FITC-labeled antibody. Cell nuclei were visualized by DAPI staining on the corresponding smears (D, F and H). Scale bar, 10 µm.
MSP119 fused to gp64 exhibits the three-dimensional structure of the native MSP119 with correctly formed disulfide bonds
Western blotting analysis shows that the anti-PfMSP119 mAb 5.2, which has previously been shown to recognize a conformation-dependent epitope [31], reacted with very faint doublet bands with relative molecular masses (Mr) of 75 and 85 kDa in the presence of 2-ME (Figure 1B, lanes 1–2). Much stronger smear bands with high Mr were seen in the absence of 2-ME, (lane 3), indicating formation of oligomer complexes. P. berghei-hyperimmune serum reacted with a 75-kDa band corresponding to the PbMSP119-gp64 fusion protein in the presence of 5% 2-ME. Similar to the PfMSP119-gp64 fusion protein complex, strong smear bands of PbMSP119-gp64 fusion protein with high Mr were seen in the absence of 2-ME (lane 6). These results are consistent with previous results showing that the PyMSP119-gp64 fusion protein was susceptible to treatment with 2-ME as detected using P. yoelii-hyperimmune serum (lanes 7–9) [27]. The anti-gp64 mAb reacted with three MSP119-gp64 fusion proteins and endogenous gp64 (lanes 10–12). The total intensity of endogenous gp64 plus each MSP119-gp64 fusion protein band seems to be similar to that of each smear band (lanes 3, 6 and 9) under the non-reducing conditions. These results indicate that these three MSP119-gp64 fusion proteins form oligomer complexes not only with MSP119-gp64 fusion protein but also endogenous gp64 on the virus envelope and retain the three-dimensional structures of the native MSP119 with correctly formed disulfide bonds.
High level PfMSP119-specifc antibody titers induced by AcNPV-PfMSP119surf did not confer protection against Pb-PfMSP1 parasites
Both i.m. and i.n. immunization of BALB/c mice with AcNPV-PfMSP119surf induced high titers of PfMSP119-specifc antibodies (72,600±19,300 and 167,000±47,300, respectively) (Table 1, EXP1). These immune sera strongly reacted with native PfMSP119 on P. falciparum schizonts with circumferential staining, characteristic of an antigen present on the parasite surface (Figure 1C). However, none of the immunized mice survived following challenge with Pb-PfM19 parasites (transgenic P. berghei expressing PfMSP119 in place of native PbMSP119). In accordance with our previous study [27], the i.m. and i.n. immunization with AcNPV-PyMSP119surf conferred 50% and 100% protection, respectively, against P. yoelii challenge infection (Table 1, EXP2,G4-5). Moreover, we and others have demonstrated that mice immunized with E. coli-producing GST-PyMSP119 formulated in Freund's or alum adjuvant were protected against P. yoelii challenge infection. While immunization with GST-PyMSP119 plus alum provided 70% protection against P. yoelii challenge infection, mice similarly immunized with the same preparation of GST-PfMSP119 did not survive following Pb-PfMSP1 parasite challenge, although the immunization induced high titers of PfMSP119-specifc antibodies (134,000±28,600). Interestingly, one of 10 naïve mice self-cured from high parasitemia following P. yoelii 17XL infection (Table 1, EXP2 G1). We observed several monocytes actively phagocytosing the parasites in the blood of the self-cured mouse (Supplementary Figure S1). This is a very rare case because P. yoelii 17XL infection of BALB/c mice was 100% lethal in our previous experiments. The parasite clearance by phagocytosis may be due to the activation of innate immunity during infection. It would be interesting to address the triggers behind the induction of protective immunity in a naïve mouse during infection.
Table 1. Protective efficacies of MSP119-BBVs against challenge infectiona.
| Vaccine (Challenge parasite) | Mouse strain | Route | Anti-MSP119 titerb mean±SE | No. of protected mice/total no. (%) |
| EXP1 (Pb-PfM19) | ||||
| G1: Non-immunized | BALB/c | - | NDc | 0/5 (0) |
| G2: GST-PfMSP1+alum | BALB/c | i.p. | 134,000±28,600 | 0/5 (0) |
| G3: AcNPV-WT | BALB/c | i.m. | ND | 0/5 (0) |
| G4: AcNPV-PfMSP119surf | BALB/c | i.m. | 72,600±19,300 | 0/5 (0) |
| G5: AcNPV-PfMSP119surf | BALB/c | i.n. | 167,000±47,300 | 0/5 (0) |
| EXP2 (P. yoelii) | ||||
| G1: Non-immunize | BALB/c | - | ND | 1/10 (10) |
| G2: GST-PyMSP119+alum | BALB/c | i.p. | 171,000±138,000 | 7/10 (70) |
| G3: AcNPV-WT | BALB/c | i.m. | ND | 0/10 (0) |
| G4: AcNPV-PyMSP119surf | BALB/c | i.m. | 159,000±55,700 | 5/10 (50) |
| G5: AcNPV-PyMSP119surf | BALB/c | i.n. | 126,000±33,900 | 10/10 (100) |
| EXP3 (P. berghei) | ||||
| G1: Non-immunize | BALB/c | - | ND | 0/5 (0) |
| G2: GST-PbMSP119+alum | BALB/c | i.p. | 256,000±55,000 | 0/10 (0) |
| G3: AcNPV-WT | BALB/c | i.m. | ND | 0/5 (0) |
| G4: AcNPV-PbMSP119surf | BALB/c | i.m. | 103,000±31,700 | 0/5 (0) |
| G5: AcNPV-PbMSP119surf | BALB/c | i.n. | 97,800±49,800 | 0/10 (0) |
| G6: Non-immunize | C57/BL6 | - | ND | 0/5 (0) |
| G7: AcNPV-WT | C57/BL6 | i.m. | ND | 0/5 (0) |
| G8: AcNPV-PbMSP119surf | C57/BL6 | i.m. | 88,100±8,590 | 0/5 (0) |
Groups of mice were immunized with MSP119-BBVs three times and challenged either with Pb-PfMSP19, P. yoelii or P. berghei following blood collection for ELISA.
Levels of PfMSP119-, PyMSP119- and PbMSP119- specific IgG for EXP1, 2 and 3, respectively, were measured by ELISA.
ND, not detectable level (<500).
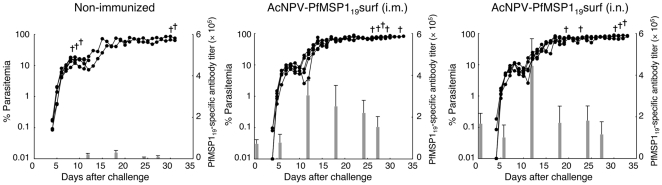
To examine whether natural boosting of PfMSP-119-specific antibodies was induced, the kinetics of the PfMSP119-specific antibody titers and parasitemia during the course of infection were determined. PfMSP119-specific antibodies induced by i.m. and i.n. immunization with AcNPV-PfMSP119surf increased 2.7- and 4.2-fold 11 days after challenge infection (Figure 2), indicating natural boosting by challenge infection. However, the immunized groups died with high levels of parasitemia and anemia but without signs of cerebral malaria, which is similar to the non-immunized group.
Figure 2. Kinetics of PfMSP119-specific antibody titers and parasitemia during the course of infection.
Groups of mice were non-immunized or immunized either i.m. or i.n. with AcNPV-PfMSP119surf, and then challenged i.v. with 103 Pb-PfM19 pRBC. Parasitemia was monitored daily 4 days after challenge and sera were collected periodically post-challenge to measure antibody titers. The bar chart indicates PfMSP119-specific antibody titers on the left vertical axis. The line graph indicates the course of parasitemia (%) on the right vertical axis. (+), death.
AcNPV-PbMSP119surf was ineffective against P. berghei
To address the possibility that P. berghei is resistant to immune responses to PbMSP119, AcNPV-PbMSP119surf was generated with a construct similar to AcNPV-PyMSP119surf and AcNPV-PfMSP119surf. As for the Pb-PfMSP1 parasites, none of the BALB/c mice immunized i.m or i.n. with AcNPV-PbMSP119surf survived following P. berghei challenge (Table 1, EXP3 G4–5), although immunization induced high titers of PbMSP119-specifc antibodies (103,000±31,700 and 97,800±49,800, respectively) with strong reactivity against P. berghei mature schizonts (Figure 1E). In addition, none of the BALB/c mice immunized with GST-PbMSP119 plus alum survived following P. berghei challenge, although the immunization induced high titers of PbMSP119-specifc antibodies (256,000±55,000). There is no difference in the course of infection and survival time between the AcNPV-PbMSP119 and non-immunized BALB/c groups (Supplementary Figure S2). Since P. berghei ANKA infection of C57BL/6 mice, but not BALB/c mice, has been shown to lead to “cerebral malaria” [32], it is important to examine whether the vaccine efficacy and the course of infection are different between BALB/c and C57BL/6 mice. All groups of C57BL/6 mice infected with P. berghei ANKA (Table 1, EXP 3 G6–8) died exhibiting low parasitemia (<15%) 8–10 days after challenge, which may be due to cerebral malaria (Supplementary Figure S2). Similar to BALB/c mice, there is no difference in the course of infection and survival time between AcNPV-PbMSP119 and non-immunized B57BL/6 groups (Supplementary Figure S2). Thus AcNPV-PbMSP119 did not contribute to any protective effect or reduction of symptoms either in BALB/c or C57BL/6 mice, indicating that P. berghei and Pb-PfM19 parasites circumvent immune responses to MSP119-BBVs, which are effective for P. yoelii.
Construction and expression of AMA1-BBV
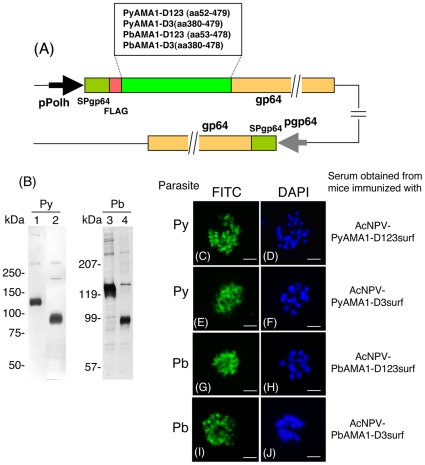
To compare the protective efficacies of another leading vaccine candidate, AMA1, against P. yoelii and P. berghei, we generated two kinds of PyAMA1- and PbAMA1-BBVs consisting of ectodomains I-III or III alone (AcNPV-PyAMA1-D123surf, AcNPV-PyAMA1-D3surf, AcNPV-PbAMA1-D123suf, and AcNPV-PbAMA1-D3surf) (Figure 3A). Similar to MSP119-BBV, each construct harbored a gene cassette that consisted of the gp64 signal sequence and the target gene (PyAMA1-D123, PyAMA1-D3, PbAMA1-D123, and PbAMA1-D3) fused to the N-terminus of the AcNPV major envelope protein gp64. Western blotting analysis shows that P. yoelii-hyperimmune serum reacted with the PyAMA1-D123- and PyAMA1-D3-gp64 fusion proteins of AcNPV-PyAMA1-D123suf and AcNPV-PyAMA1-D3surf with molecular weights of 125 kDa and 95 kDa, respectively (Figure 3B, lanes 1 and 2). Similar results were obtained with the PbAMA1-D123- and PbAMA1-D3-gp64 fusion proteins of AcNPV-PbAMA1-D123suf and AcNPV-PbAMA1-D3surf against P. berghei-hyperimmune serum (lanes 3 and 4).
Figure 3. Construction and expression analysis of AMA1-BBVs.
(A) Schematic diagram of four AMA1-BBV genomes. AMA1 was expressed as an AMA1-gp64 fusion protein under the control of the polyhedron promoter. Numbers indicate the amino acid positions of AMA1-gp64 fusion protein and endogenous gp64 protein. pPolh, polyhedrin promoter; SP, the gp64 signal sequence; FLAG, the FLAG epitope tag; pgp64, gp64 promoter. (B) Western blot analysis of AMA1-BBVs. AcNPV-PyAMA1-D123surf (lane 1), AcNPV-PyAMA1-D3surf (lane 2), AcNPV-PbAMA1-D123surf (lane 3) and AcNPV-PbAMA1-D3surf (lane 4) were treated with the loading buffer containing 1% 2-ME and examined using P. yoelii-hyperimmune serum (lanes 1 and 2), or P. berghei-hyperimmune serum (lanes 3 and 4). (C–J) Immunofluorescence patterns of sera obtained from mice immunized with four AMA1-BBVs on methanol-acetone fixed smears of erythrocytes infected with P. yoelii (C and E) and P. berghei (G and I). The smears were incubated with serum obtained from an individual mouse immunized either with AcNPV-PyAMA1-D123surf (C), AcNPV-PyAMA1-D3surf (E), AcNPV-PbAMA1-D123surf (G) or AcNPV-PbAMA1-D3surf (I), and antibody binding was detected with a secondary FITC-labeled antibody. Cell nuclei were visualized by DAPI staining on the corresponding smears (D, F, H and J). Scale bar, 10 µm.
Protective efficacy of AMA1-BBV against challenge infection
Intramuscular immunization of BALB/c mice with AcNPV-PyAMA1-D123surf induced higher titers of PyAMA1-specifc antibodies than i.n. immunization (i.m. vs. i.n. = 20,900±8,700 vs. 7,200±2,470) (Table 2, EXP4 G2–3). Both immune sera strongly reacted with native PyAMA1 on P. yoelii mature schizonts, which is consistent with AMA1 localization on the surface of merozoites (Figure 3C and E). In the i.m. AcNPV-PyAMA1-D123surf group, five of 10 mice (50%) survived P. yoelii challenge infection, whereas two of 10 mice (20%) survived challenge infection in the i.n. AcNPV-PyAMA1-D123surf group. Although both i.m. and i.n. immunization with AcNPV-PyAMA1-D3surf induced similar levels of PyAMA1-specifc antibodies, all immunized mice died following P. yoelii challenge infection, indicating that PyAMA1-D123 induced partial protective immune responses, but not PyAMA1-D3. In spite of correct folding on the surface of the baculoviral virion, PyAMA1-D3 may not have any neutralizing epitopes that protect against P. yoelii infection. Consistent with our previous study [27], the i.m. immunization (40% protection) with AcNPV-PyMSP119surf was less effective than the i.n. immunization (100% protection). Interestingly, when mice were immunized i.m. with a mixture of AcNPV-PyMSP119surf and AcNPV-PyAMA1-D123surf, all mice induced PyMSP119- and PyAMA1-specific antibodies and survived P. yoelii challenge infection with less severe infection outcomes and low peak parasitemia, indicating a synergistic effect of PyMSP119 and PyAMA1 on protection. In contrast to the P. yoelii model, two PbAMA1-BBVs (AcNPV-PbAMA1-D123surf and AcNPV-PbAMA1-D3surf) failed to protect against P. berghei challenge, although PbAMA1-specific antibodies, which can recognize P. berghei schizonts (Figure 3G and I), were induced (Table 2, EXP5).
Table 2. Protective efficacies of AMA1-BBVs against challenge infectiona.
| Vaccine (Challenge parasite) | Route | Anti-AMA1 D3 antibody titerc mean±SE | Anti-MSP119 antibody titerc mean±SE | No. of protected mice/total no. (%) |
| EXP4 (P. yoelii) | ||||
| G1: Non-immunized | - | NDd | ND | 0/5 (0) |
| G2: AcNPV-PyAMA1-D123surf | i.m. | 20,900±8,700 | ND | 5/10 (50) |
| G3: AcNPV-PyAMA1-D123surf | i.n. | 7,200±2,470 | ND | 2/10 (20) |
| G4: AcNPV-PyAMA1-D3surf | i.m. | 26,100±6,350 | ND | 0/5 (0) |
| G5: AcNPV-PyAMA1-D3surf | i.n. | 12,300±2,450 | ND | 0/5 (0) |
| G6: AcNPV-PyMSP119surf | i.m. | ND | 70,800±11,210 | 2/5 (40) |
| G7b: AcNPV-PyAMA1-D123surf + AcNPV-PyMSP119surf | i.m. | 15,200±5,800 | 94,600±15,720 | 5/5 (100) |
| EXP5 (P. berghei) | ||||
| G1: Non-immunized | - | ND | ND | 0/5 (0) |
| G2: AcNPV-PbAMA1-D123surf | i.m. | 9,800±3,010 | ND | 0/5 (0) |
| G3: AcNPV-PbAMA1-D3surf | i.m. | 16,900±5,310 | ND | 0/5 (0) |
Groups of BALB/c mice were immunized with AMA1-BBVs three times and challenged either with P. yoelii or P. berghei following blood collection for ELISA.
The two BBVs (AcNPV-PyAMA1-D123surf and AcNPV-PyMSP119surf) were mixed (2.5×107 pfu each) and used for immunization.
Levels of PyAMA1D3 and PyMSP119- specific IgGs and PbAMA1D3 and PbMSP119- specific IgGs for EXP4 and 5, respectively, were measured by ELISA.
ND, not detectable level (<500).
Naturally acquired protective immunity to P. berghei confers resistance to P. yoelii, but not vice versa
While many studies have consistently shown that the ELISA titer of MSP119- and AMA1-immunized mice correlates with protective immunity against P. yoelii challenge, the corresponding P. berghei vaccines failed to protect against P. berghei. Therefore, it was of interest to determine the degree of heterologous immunity occurring between P. yoelii and P. berghei. BALB/c mice infected either with P. yoelii or P. berghei were drug-cured by three doses of Artemether. This drug treatment regimen completely cleared parasitemia so that no recurrence or recrudescence parasite appeared. The self-cured mice were re-infected three times with the homologous parasite. At the first challenge after drug treatment, some mice developed low levels of parasitemia (<10%) in both groups, and all self-cured within 7 days (data not shown). No parasitemia appeared following the second and third homologous challenges, indicating that mice naturally acquired sterile protective immunity against homologous infection. Subsequently, these mice were challenged with the heterologous parasite. All of the self-cured mice from P. berghei completely protected against P. yoelii with undetectable levels of parasitemia (Table 3, EXP6 G1), indicating the acquisition of cross-resistance to P. yoelii infection. In contrast, all the self-cured mice from P. yoelii suffered from severe courses of P. berghei infection with high maximum parasitemia levels (>70% parasitemia) and 100% mortality (G2). Thus naturally acquired sterile immunity induced by drug treatment and repeated P. berghei infection can persistently protect mice against P. yoelii infection, but not vice versa.
Table 3. Protective efficacies against heterologous challenge following drug treatment and 3 homologous re-infectionsa.
| Group | Parasite used for drug treatment and re-infection | Parasite used for heterologous challenge | No. of protected mice/total no. (%) |
| EXP6 | |||
| G1 | Pbb | Py | 10/10 (100) |
| G2 | Pyc | Pb | 0/10 (0) |
| G3 | NTd | Pb | 0/5 (0) |
| G4 | NT | Py | 0/5 (0) |
P. berghei- or P. yoelii-infected BALB/c mice were treated with Artemether®. The drug-cured mice were re-infected three times with homologous parasites at 4-week intervals. All mice survived these re-infections. The self-cured mice were then challenged with heterologous parasites.
Pb, P. berghei ANKA.
Py, P. yoelii 17XL.
NT, neither drug-treatment nor infection.
Discussion
In the present study, we demonstrate that the two leading malaria blood-stage vaccine candidate antigens, MSP119 and AMA1, failed to protect against P. berghei and its transgenic parasite challenge infection, although immunization with these vaccines induced high levels of antigen-specific antibody titers, and the immune sera strongly reacted with the blood-stage parasites.
Our previous study showed that immunization with AcNPV-PyMSP119surf completely clears P. yoelii shortly after challenge by a quick natural boosting response [27]. On the other hand, immunization with the bacterially-produced GST-PyMSP119 plus alum impairs P. yoelii growth at the time of infection by induced PyMSP119-specific antibodies, resulting in a delay in the onset of a patent parasitemia and protracted period of parasite inhibition [6], [33], [34], [35]. Therefore, these two vaccine formulas inducing different protective immune responses are suitable immunogens to investigate whether MSP119-specific immune responses could confer protection or affect the course of infection in P. berghei ANKA and its transgenic models. Since MSP119 is highly structured on the surface of merozoites, folding into two epidermal growth factor-like domains [36], development of MSP119-based vaccines with its native three-dimensional structure would be necessary to induce protective immune responses. This is similar to PyMSP119, PfMSP119 and PbMSP119 displayed on BBVs, which are stabilized by disulfide bonds, as evidenced by the loss of conformational mAb and hyperimmune IgG binding, respectively, in immunoblots with reduced MSP119-BBVs (Figure 1B). Additional evidence of the correct structure includes the induction of IFA-reactive antibodies (Figures 2C–H). In the P. berghei transgenic parasite model, although the AcNPV-PfMSP119surf group elicited natural boosting of vaccine-induced PfMSP119-specific antibody responses during infection, there was no significant reduction of parasitemia or prolonging of survival time compared to non-immunized groups. Similarly, neither PbMSP119- nor PbAMA1-BBV was effective against P. berghei. In addition, the bacterially-produced GST-PbMSP119 protein with alum adjuvant conferred no protection. Thus P. berghei and its transgenic parasites completely circumvent immune responses induced by MSP119- and AMA1-based vaccines.
One possible mechanism by which the growth of P. berghei cannot be controlled by high levels of PbMSP119- or PbAMA1-specific antibodies is that P. berghei possesses additional molecules and/or mechanisms other than MSP119 and AMA1 for erythrocyte invasion that are lacking in P. yoelii. In support of this, we found that when mice self-cured from P. berghei infection by drug treatment and subsequent P. berghei re-infections, they naturally acquired sterile immunity against P. yoelii as well as P. berghei, but not vice versa. This is consistent with a previous report that protective cross-immunity operates only in one direction (P. berghei→P. yoelii) since mice immunized with a formalin-fixed blood-stage P. yoelii were fully susceptible to P. berghei [37]. P. falciparum has been shown to have a number of invasion pathways and to be capable of entering erythrocytes by sialic acid-dependent and -independent pathways [38]. While no such parallel invasion pathways have been described for rodent malaria parasites, it is possible that the multiplicity of merozoite surface proteins in P. berghei may reflect involvement in alternative pathways of invasion. Although a transgenic P. berghei line expressing PyMSP119 in place of native PbMSP119 would be a good model to address this possibility, we have failed to generate the transgenic line using similar methodology for the construction of Pb-PfM19 [28], suggesting that PbMSP119 and PfMSP119 share similar functions essential for growth and/or invasion, but not with PyMSP119.
Another possible mechanism is that P. berghei infection may impair antibody-dependent cellular inhibition of parasite growth. We have previously demonstrated that a genetically engineered bispecific single-chain antibody targeted to human CD3 and PfMSP119 promotes merozoite phagocytosis and growth inhibition in in vitro P. falciparum culture through cooperation with T cells and monocytes [39]. Evidence has been accumulated to suggest that antibody action via Fc interaction with monocytes/macrophages plays an important role in protective immunity against blood-stage parasites [14], [40], [41]. In the case of the P. yoelii model, MSP119- and AMA1-specific antibodies may effectively function in the parasite killing by cooperation with monocytes/macrophages. However, P. berghei infection may strongly suppress monocyte/macrophage activation through Fc receptors with MSP119- or AMA1-specific antibodies. If P. falciparum uses mechanisms similar to those utilized by P. berghei to circumvent MSP119- and AMA1-based vaccine properties, P. berghei rather than P. yoelii would be a more useful model to identify and evaluate new blood-stage vaccine candidate antigens for P. falciparum.
Recently, P. berghei has been genetically engineered to express representative vaccine candidate antigens (e.g., PfMSP119, PfAMA1, PfCSP, Pfs25 and Pvs25) from the human malaria parasites, P. falciparum and P. vivax [28], [42], [43], [44], [45]. These transgenic P. berghei parasites provide great potential to investigate the protective efficacies of vaccine candidates against the human malaria parasites in vivo. Very recently, we have shown that transmission blocking vaccines using the BBV system can induce a remarkable reduction of malaria transmission to mosquitoes, directly evaluated by immunization of mice following challenge with Pfs25- or Pvs25-expressing P. berghei [46], [47]. Although P. berghei transgenic parasites expressing PfMSP119 and PfAMA1 have been used to evaluate the inhibitory effects on parasite growth in vitro and through passive immunization [15], [28], [48], in vivo challenge experiments following active immunization have not been reported. Unlike P. yoelii, P. berghei has not been used for the evaluation of vaccine efficacy against blood-stage parasites, although the parasite has been well-studied not only for pre-erythrocytic vaccine development but also a cerebral malaria model [49]. To date, the usefulness of the transgenic P. berghei model for evaluating protective efficacies of blood-stage antigens remains unclear. In the present study, we were unable to show the usefulness of PfMSP119-BBV as a promising malaria vaccine candidate using the transgenic P. berghei, although a similar construct of PyMSP119-BBV provided 100% protection in the P. yoelii model [27]. We still cannot exclude the possibility that the absence of protection induced by the PfMSP119 vaccine formulations is due to qualitative differences between the PyMSP119 and PfMSP119 vaccine formulations used here, rather than differences in parasite susceptibility to anti-MSP119 responses, although western blot and immunologiocal analyses show that the three MSP119-gp64 fusion proteins as well as GST- MSP119 proteins were expressed at quantitatively similar levels and immunization with these proteins induced high titers of MSP119-specific antibodies with strong reactivity against mature schizonts of the corresponding parasites. Further experiments using PfMSP1-based vaccines with GMP level, which have been evaluated in human clinical trials, would be needed to investigate the susceptibility of Pb-PfMSP119 to anti-PfMSP119 responses.
Our results provide important insights for malaria blood-stage vaccine development using P. yoelii and P. berghei and highlight the need to deeply investigate the relationship between rodent and human parasites. Obviously, it is important to elucidate the discrepancy in the vaccine efficacies observed between the P. yoelii model and human clinical trials. A better understanding of the precise relationship between rodent and human parasites should lead to a transfer of information from transgenic P. berghei to human vaccine development prior to human clinical settings.
Supporting Information
Photomicrographs of Giemsa-stained thin blood smears of the self-cured mouse. Ten non-immunized mice were infected with P. yoelii 17XL-pRBC by i.v. injection. The course of parasitemia was monitored daily from 4 days post-challenge by microscopic examination of Giemsa-stained thin blood smears obtained from tail bleeds. One of these mice self-cured from high parasitemia of P. yoelii infection. The mouse cleared the parasites 21 days after challenge. The photomicrographs of the self-cured mouse were taken at 19, 21 and 24 days after challenge. Arrows indicate malaria pigment in monocytes phagocytosing the parasites. Original magnification, ×1,000.
(6.18 MB TIF)
The course of parasitemia. BALB/c and C57BL/6 mice were immunized i.m. with AcNPV-PbMSP119surf or AcNPV-WT and challenged i.v. with 103 P. berghei-pRBC. Parasitemia was monitored daily from 5 days post-challenge. All groups of BALB/c and C57BL/6 mice died 18 and 10 days after challenge, respectively. Data (mean ±SD) are from the BALB/c (EXP3 G 1, 3 and 4) and C57BL/6 (EXP3 G6–8) shown in Table 1. closed triangle, non-immunized; open square, AcNPV-WT; closed circle, AcNPV-PbMSP119surf.
(6.11 MB TIF)
Acknowledgments
We would like to thank C. Seki, H. Okuya, J. Sato and K. Watano for excellent assistance with the ELISAs and handling of the mice. We also thank Robert E. Sinden for critical comments. We would like to express special thanks to H. Okuya for encouraging us.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Ministry of Education, Culture, Sports and Science of Japan (21390126). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70:6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocken CH, Withers-Martinez C, Dubbeld MA, van der Wel A, Hackett F, et al. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect Immun. 2002;70:4471–4476. doi: 10.1128/IAI.70.8.4471-4476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holder AA. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- 5.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly TM, Long CA. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 7.Ahlborg N, Ling IT, Howard W, Holder AA, Riley EM. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect Immun. 2002;70:820–825. doi: 10.1128/IAI.70.2.820-825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Near KA, Stowers AW, Jankovic D, Kaslow DC. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by the addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect Immun. 2002;70:692–701. doi: 10.1128/IAI.70.2.692-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holder AA. Preventing merozoite invasion of erythrocytes. In: Hoffman SL, editor. Malaria Vaccine Development: a multi-immune response approach. Washington, DC: ASM Press; 1996. pp. 77–104. [Google Scholar]
- 10.Blackman MJ, Scott-Finnigan TJ, Shai S, Holder AA. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan AF, Burghaus P, Druilhe P, Holder AA, Riley EM. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Reed ZH, Kieny MP, Engers H, Friede M, Chang S, et al. Comparison of immunogenicity of five MSP1-based malaria vaccine candidate antigens in rabbits. Vaccine. 2008;27:1651–1660. doi: 10.1016/j.vaccine.2008.10.093. [DOI] [PubMed] [Google Scholar]
- 13.Rotman HL, Daly TM, Clynes R, Long CA. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J Immunol. 1998;161:1908–1912. [PubMed] [Google Scholar]
- 14.McIntosh RS, Shi J, Jennings RM, Chappel JC, de Koning-Ward TF, et al. The importance of human FcγRI in mediating protection to malaria. PLoS Pathog. 2007;3:e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murhandarwati EE, Wang L, de Silva HD, Ma C, Plebanski M, et al. Growth-inhibitory antibodies are not necessary for protective immunity to malaria infection. Infect Immun. 2010;78:680–687. doi: 10.1128/IAI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodder AN, Crewther PE, Matthew ML, Reid GE, Moritz RL, et al. The disulfide bond structure of Plasmodium apical membrane antigen-1. J Biol Chem. 1996;271:29446–29452. doi: 10.1074/jbc.271.46.29446. [DOI] [PubMed] [Google Scholar]
- 17.Fraser TS, Kappe SH, Narum DL, VanBuskirk KM, Adams JH. Erythrocyte-binding activity of Plasmodium yoelii apical membrane antigen-1 expressed on the surface of transfected COS-7 cells. Mol Biochem Parasitol. 2001;117:49–59. doi: 10.1016/s0166-6851(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 18.Pizarro JC, Vulliez-Le Normand B, Chesne-Seck ML, Collins CR, Withers-Martinez C, et al. Crystal structure of the malaria vaccine candidate apical membrane antigen 1. Science. 2005;308:408–411. doi: 10.1126/science.1107449. [DOI] [PubMed] [Google Scholar]
- 19.Kato K, Mayer DC, Singh S, Reid M, Miller LH. Domain III of Plasmodium falciparum apical membrane antigen 1 binds to the erythrocyte membrane protein Kx. Proc Natl Acad Sci USA. 2005;102:5552–5557. doi: 10.1073/pnas.0501594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller MS, Renard A, Boato F, Vogel D, Naegeli M, et al. Induction of parasite growth-inhibitory antibodies by a virosomal formulation of a peptidomimetic of loop I from domain III of Plasmodium falciparum apical membrane antigen 1. Infect Immun. 2003;71:4749–4758. doi: 10.1128/IAI.71.8.4749-4758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narum DL, Ogun SA, Thomas AW, Holder AA. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect Immun. 2000;68:2899–2906. doi: 10.1128/iai.68.5.2899-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stowers AW, Kennedy MC, Keegan BP, Saul A, Long CA, et al. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect Immun. 2002;70:6961–6967. doi: 10.1128/IAI.70.12.6961-6967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saul A, Lawrence G, Allworth A, Elliott S, Anderson K, et al. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant. Vaccine. 2005;23:3076–3083. doi: 10.1016/j.vaccine.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Lyon JA, Angov E, Fay MP, Sullivan JS, Girourd AS, et al. Protection induced by Plasmodium falciparum MSP1(42) is strain-specific, antigen and adjuvant dependent, and correlates with antibody responses. PLoS ONE. 2008;3:e2830. doi: 10.1371/journal.pone.0002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanabe K, Sakihama N, Nakamura Y, Kaneko O, Kimura M, et al. Selection and genetic drift of polymorphisms within the merozoite surface protein-1 gene of Plasmodium falciparum. Gene. 2000;241:325–331. doi: 10.1016/s0378-1119(99)00472-2. [DOI] [PubMed] [Google Scholar]
- 26.Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida S, Araki H, Yokomine T. Baculovirus-based nasal drop vaccine confers complete protection against malaria by natural boosting of vaccine-induced antibodies in mice. Infect Immun. 2010;78:595–602. doi: 10.1128/IAI.00877-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Koning-Ward TF, O'Donnell RA, Drew DR, Thomson R, Speed TP, et al. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J Exp Med. 2003;198:869–875. doi: 10.1084/jem.20030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida S, Kondoh D, Arai E, Matsuoka H, Seki C, et al. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003;316:161–170. doi: 10.1016/j.virol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Daly TM, Long CA. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang SP, Gibson HL, Lee-Ng CT, Barr PJ, Hui GS. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- 32.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 33.Wykes MN, Zhou YH, Liu XQ, Good MF. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J Immunol. 2005;175:2510–2516. doi: 10.4049/jimmunol.175.4.2510. [DOI] [PubMed] [Google Scholar]
- 34.Hirunpetcharat C, Vukovic P, Liu XQ, Kaslow DC, Miller LH, et al. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J Immunol. 1999;162:7309–7314. [PubMed] [Google Scholar]
- 35.Tian JH, Miller LH, Kaslow DC, Ahlers J, Good MF, et al. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]
- 36.Morgan WD, Birdsall B, Frenkiel TA, Gradwell MG, Burghaus PA, et al. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J Mol Biol. 1999;289:113–122. doi: 10.1006/jmbi.1999.2753. [DOI] [PubMed] [Google Scholar]
- 37.McColm AA, Dalton L. Heterologous immunity in rodent malaria: comparison of the degree of cross-immunity generated by vaccination with that produced by exposure to live infection. Ann Trop Med Parasitol. 1983;77:355–377. doi: 10.1080/00034983.1983.11811724. [DOI] [PubMed] [Google Scholar]
- 38.Holder AA, Blackman MJ, Borre M, Burghaus PA, Chappel JA, et al. Malaria parasites and erythrocyte invasion. Biochem Soc Trans. 1994;22:291–295. doi: 10.1042/bst0220291. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida S, Kobayashi T, Matsuoka H, Seki C, Gosnell WL, et al. T-cell activation and cytokine production via a bispecific single-chain antibody fragment targeted to blood-stage malaria parasites. Blood. 2003;101:2300–2306. doi: 10.1182/blood-2002-03-0831. [DOI] [PubMed] [Google Scholar]
- 40.Badell E, Oeuvray C, Moreno A, Soe S, van Rooijen N, et al. Human malaria in immunocompromised mice: an in vivo model to study defense mechanisms against Plasmodium falciparum. J Exp Med. 2000;192:1653–1660. doi: 10.1084/jem.192.11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoneto T, Waki S, Takai T, Tagawa Y, Iwakura Y, et al. A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J Immunol. 2001;166:6236–6241. doi: 10.4049/jimmunol.166.10.6236. [DOI] [PubMed] [Google Scholar]
- 42.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, et al. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169:6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 43.Mlambo G, Maciel J, Kumar N. Murine Model for Assessment of Plasmodium falciparum transmission-blocking vaccine using transgenic Plasmodium berghei parasites expressing the target antigen Pfs25. Infect Immun. 2008;76:2018–2024. doi: 10.1128/IAI.01409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramjanee S, Robertson JS, Franke-Fayard B, Sinha R, Waters AP, et al. The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine. 2007;25:886–894. doi: 10.1016/j.vaccine.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Triglia T, Healer J, Caruana SR, Hodder AN, Anders RF, et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol Microbiol. 2000;38:706–718. doi: 10.1046/j.1365-2958.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- 46.Mlambo G, Kumar N, Yoshida S. Vaccine; 2010. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission blocking vaccine candidate antigen. in press. [DOI] [PubMed] [Google Scholar]
- 47.Blagborough AM, Yoshida S, Sattabongkot J, Tsuboi T, Sinden RE. Vaccine; 2010. Intranasal and intramuscular immunization with Baculovirus Dual Expression System-based Pvs25 vaccine substantially blocks Plasmodium vivax transmission. in press. [DOI] [PubMed] [Google Scholar]
- 48.Cao Y, Zhang D, Pan W. Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9. PLoS ONE. 2009;4:e6894. doi: 10.1371/journal.pone.0006894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jennings VM, Lal AA, Hunter RL. Evidence for multiple pathologic and protective mechanisms of murine cerebral malaria. Infect Immun. 1998;66:5972–5979. doi: 10.1128/iai.66.12.5972-5979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photomicrographs of Giemsa-stained thin blood smears of the self-cured mouse. Ten non-immunized mice were infected with P. yoelii 17XL-pRBC by i.v. injection. The course of parasitemia was monitored daily from 4 days post-challenge by microscopic examination of Giemsa-stained thin blood smears obtained from tail bleeds. One of these mice self-cured from high parasitemia of P. yoelii infection. The mouse cleared the parasites 21 days after challenge. The photomicrographs of the self-cured mouse were taken at 19, 21 and 24 days after challenge. Arrows indicate malaria pigment in monocytes phagocytosing the parasites. Original magnification, ×1,000.
(6.18 MB TIF)
The course of parasitemia. BALB/c and C57BL/6 mice were immunized i.m. with AcNPV-PbMSP119surf or AcNPV-WT and challenged i.v. with 103 P. berghei-pRBC. Parasitemia was monitored daily from 5 days post-challenge. All groups of BALB/c and C57BL/6 mice died 18 and 10 days after challenge, respectively. Data (mean ±SD) are from the BALB/c (EXP3 G 1, 3 and 4) and C57BL/6 (EXP3 G6–8) shown in Table 1. closed triangle, non-immunized; open square, AcNPV-WT; closed circle, AcNPV-PbMSP119surf.
(6.11 MB TIF)