The expression of the extracellular matrix protein Laminin-332 is regulated transcriptionally by TGF-β1 as a function of cell confluence in MDCK epithelial cells. Latent TGF-β1 is secreted apically, sequestered from its receptors and activation machinery, dependent on integrin αVβ3, localized on the basolateral side of the epithelial barrier.
Abstract
Laminin (LM)-332 is an extracellular matrix protein that plays a structural role in normal tissues and is also important in facilitating recovery of epithelia from injury. We have shown that expression of LM-332 is up-regulated during renal epithelial regeneration after ischemic injury, but the molecular signals that control expression are unknown. Here, we demonstrate that in Madin-Darby canine kidney (MDCK) epithelial cells LM-332 expression occurs only in subconfluent cultures and is turned-off after a polarized epithelium has formed. Addition of active transforming growth factor (TGF)-β1 to confluent MDCK monolayers is sufficient to induce transcription of the LM α3 gene and LM-332 protein expression via the TGF-β type I receptor (TβR-I) and the Smad2–Smad4 complex. Significantly, we show that expression of LM-332 in MDCK cells is an autocrine response to endogenous TGF-β1 secretion and activation mediated by integrin αVβ3 because neutralizing antibodies block LM-332 production in subconfluent cells. In confluent cells, latent TGF-β1 is secreted apically, whereas TβR-I and integrin αVβ3 are localized basolaterally. Disruption of the epithelial barrier by mechanical injury activates TGF-β1, leading to LM-332 expression. Together, our data suggest a novel mechanism for triggering the production of LM-332 after epithelial injury.
INTRODUCTION
Epithelial cells form selective barriers between the environment and the internal milieu of organs, including kidney, breast, skin, and intestine (Schock and Perrimon, 2002; Bryant and Mostov, 2008). The most important differentiated characteristic of epithelial cells is their apical–basal polarity. Within an epithelium, each cell is organized along an axis orthogonal to the surface of the epithelium such that the protein and lipid compositions of the apical, lateral, and basal plasma membrane domains are unique and organelles are distributed asymmetrically throughout the cytoplasm. Polarization is driven by the intrinsic activity of three polarization complexes as well as extrinsic spatial cues provided by adhesive interactions between adjacent cells and the underlying extracellular matrix (Jamora and Fuchs, 2002; Nelson, 2003, 2009). In normal epithelia, the matrix underlying the epithelium is organized into a basal lamina composed of interlocking networks of laminins and collagen type IV, along with contributions from proteoglycans such as perlecan and other molecules (Yurchenco and Patton, 2009; Bruckner, 2010). Indeed, several studies have suggested that signals from assembled laminin are critical for correct orientation of the apical–basal axis and polarization of the cells (Eaton and Simons, 1995; O'Brien et al., 2001; Li et al., 2003; Yu et al., 2005).
Laminins (LM) are a family of large heterotrimeric extracellular matrix proteins consisting of at least 15 members composed of combinations of five α, three β, and three γ subunits (Miner and Yurchenco, 2004). Most laminins assemble into a prototypical cruciform structure in which the amino-terminal portion of each subunit forms the arms of the cross and a coiled-coil of all three chains forms the stem (Jones et al., 2000; Miner and Yurchenco, 2004; Aumailley et al., 2005). However, a subset of laminin molecules do not form the typical cruciform structure because their individual subunits are “headless,” lacking the amino-terminal polymerization domains (Jones et al., 2000; Miner and Yurchenco, 2004; Aumailley et al., 2005). Chief among these is LM-332 (α3β3γ2, formerly called laminin 5, kalinin, epiligrin, and nicein) (Miner and Yurchenco, 2004; Marinkovich, 2007). LM-332 consists of two splice variants distinguished by the length of their α subunits. The αA subunit has a molecular mass of approximately 190 kDa and lacks an amino-terminal polymerization domain, whereas α3B has a size and structure closer to that of other α subunits. LM-3A32 (hereinafter referred to as simply LM-332) is more common than LM-3B32 and its functions are better understood (Marinkovich, 2007). The β3 subunit does contain a globular amino terminus resembling the polymerization domains of other more typical laminins, but there is conflicting evidence about its capacity to participate in intermolecular associations (Cheng et al., 1997; Odenthal et al., 2004; Yurchenco and Patton, 2009; Yurchenco and Wadsworth, 2004). The γ2 subunit completely lacks a polymerization domain and is significantly shorter than the γ1 laminin subunit found in most typical laminins. The α3, γ2, and possibly β3 subunits are sometimes proteolytically processed after secretion, influencing their functions (Ghosh and Stack, 2000; Goldfinger et al., 2000; Aumailley et al., 2003; Marinkovich, 2007).
LM-332 plays multiple roles in tissues. In normal skin, for example, LM-332 is a structural molecule linking the epidermis to the dermis through interactions with integrin α6β4 in hemidesmosomes (Jones et al., 1998). It has similar functions in other tissues subjected to mechanical strain including other stratified squamous epithelia, the amnion, and the cornea (Marinkovich, 2007). Absence of LM-332 in the skin due to mutation leads to the severe blistering disease junctional epidermolysis bullosa (Masunaga, 2006). Under conditions of epithelial injury, LM-332 has more dynamic functions that include stimulating migration of epithelial cells into damaged areas (Nguyen et al., 2000; Frank and Carter, 2004; Marinkovich, 2007). This process has been most extensively studied in keratinocytes, where the stable adhesions mediated through integrin α6β4 are modulated by additional interactions of LM-332 with integrin α3β1 (Hamill and McLean, 2005; Sehgal et al., 2006; Margadant et al., 2009). The latter localizes to focal complexes and focal adhesions where it facilitates migration through small Rho-family GTPase signaling and interaction with the actin cytoskeleton (Hamelers et al., 2005). In the kidney, our laboratory observed the induction of LM-332 along with the integrin α3 subunit during tubular epithelial regeneration after ischemic injury, but the signals regulating expression and direct role of LM-332 in the regenerative process are not understood (Zuk and Matlin, 2002). In addition to these functions in normal epithelia, LM-332 expression is up-regulated in a variety of carcinomas, including those of the skin and stratified squamous epithelium of the oral mucosa, as well as the epithelia of the digestive tract, respiratory tract, and cervix; and down-regulated in others, including carcinomas of the breast and prostate (Marinkovich, 2007). In at least some of these cases, aberrant regulation of LM-332 expression is correlated with tumor progression (Giannelli and Antonaci, 2000; Marinkovich, 2007; Guess and Quaranta, 2009).
Our laboratory is interested in deciphering how laminins and laminin assembly affect the polar morphogenesis of epithelial cells. We recently reported that, in addition to the network-forming laminin-511, Madin-Darby canine kidney (MDCK) cells synthesize, secrete, and deposit LM-332 only when subconfluent and incompletely polarized and then cease synthesis as soon as confluence and full polarity are achieved (Mak et al., 2006). Here, we explore the control of LM-332 synthesis in MDCK cells and report that expression is transcriptionally regulated by endogenous TGF-β1 through activation and signaling mechanisms dependent upon the epithelial barrier.
MATERIALS AND METHODS
Cell Culture
Stock cultures of MDCK cells (type II, Heidelberg isolate, passages 7–35) were cultured in Dulbecco's minimal essential medium (high glucose; Invitrogen, Carlsbad, CA) supplemented with 5% (vol/vol) fetal bovine serum (FBS; Hyclone-Fisher, Rockford, IL) and 10 mM HEPES-KOH, pH 7.3, as described previously (Matlin et al., 1981). For studies under serum-free conditions, stock cultures were adapted over four passages in succeedingly higher proportions of ExCell hormone-supplemented MDCK cell medium (M3803; Sigma-Aldrich, St. Louis, MO) containing 10 mM l-glutamine, 10 mM HEPES-KOH, pH 7.3, and 1× antibiotic/antimycotic mixture (15240-104; Invitrogen). For passaging of cells in serum-free medium, trypsin was neutralized with 1 mg of soybean trypsin inhibitor per ml of trypsin (25300; Invitrogen) in ExCell. The absence of TGF-β1 in the ExCell medium was confirmed by enzyme-linked immunosorbent assay (ELISA; see Results).
For most experiments, MDCK cells were grown on Transwell permeable supports (2.4 cm in diameter, 0.4-μm pore size; 3412, Corning Life Sciences, Lowell, MA) at a seeding density of 8 × 104 cells/cm2, with 1.5 ml of medium in the upper chamber and 2.5 ml in the lower chamber. Under these conditions, initial confluence was achieved after 2–3-d culture and saturation density after 4 d.
For wound-healing experiments, confluent MDCK monolayers grown on Transwell supports were scratched multiple times with a plastic pipet tip, taking care not to break the filter; cells were then rinsed with media and placed in an incubator at 37°C for an additional 6 h before analysis.
Antibodies and Reagents
Antibodies used in this study are listed in Table 1.
Table 1.
Antibodies
| Antigen | Name | Species | Conc (μg/ml) | Source/catalog no. |
|---|---|---|---|---|
| LM-332 | 9LN5 | Rabbit polyclonal | 4.0 | M. Kocha |
| LM-332 | 4104 | Rabbit polyclonal | 4.0 | M. Kocha |
| Laminin β3 | Anti-Kalinin B1 | Mouse monoclonal | 0.5–1.25 | BD Biosciences (San Diego, CA), 610423b |
| Smad 2 | Anti-Smad 2 | Rabbit polyclonal | 1.25 | Invitrogen, 51-1300 |
| Smad 3 | Anti-Smad 3 | Rabbit polyclonal | 1.25 | Invitrogen, 51-1500 |
| Phospho-Smad 2 (Ser465/467) | Anti-phosho Smad2 A5S | Rabbit monoclonal | 1:1000 (supernatant) | Millipore, 04-953 |
| Phospho-Smad 3 (P-Ser423/425) | Anti-phospho Smad 3 | Rabbit monoclonal | 0.2–0.5 | Cell Signaling Technology (Danvers, MA), 9520 |
| Smad 4 | Anti-Smad 4 (clone B.8) | Mouse monoclonal | 1–8 | Santa Cruz, 7966 |
| TβR-I | Anti-TβR-I (H-100) | Rabbit polyclonal | 0.2–1 | Santa Cruz, 9048 |
| TβR-I | Anti-TβR-I | Rabbit polyclonal | 0.1 | Cell Signaling Technology, 3712 |
| Integrin αVβ3 | LM609 | Mouse monoclonal | 10 | Millipore, mAb 1976Z |
| Tubulin (ascites) | Anti-tubulin (clone TUB2.1) | Mouse monoclonal (ascites) | 1:10,000 | Sigma-Aldrich, T4026 |
| Mouse IgG | Anti-mouse IgG-HRP | Goat polyclonal | 0.4 | Jackson Laboratories (West Grove, PA) |
| Rabbit IgG | Anti-rabbit IgG-HRP | Goat polyclonal | 0.4 | Jackson Laboratories |
| Mouse IgG | Anti-mouse IgG-Alexa 488 | Goat polyclonal | 4 | Invitrogen |
| Mouse IgG | Anti-mouse IgG-Alexa 555 | Goat polyclonal | 4 | Invitrogen |
| Rabbit IgG | Anti-rabbit IgG-Alexa 488 | Goat polyclonal | 4 | Invitrogen |
| Rabbit IgG | Anti-rabbit IgG-Alexa 555 | Goat polyclonal | 4 | Invitrogen |
a University of Cologne, Germany.
b Discontinued by manufacturer.
Recombinant human TGF-β1 was purchased from PeproTech (100-21, Rocky Hill, NJ) reconstituted in 10 mM citric acid, pH 3.0, at a final stock concentration of 1 μg/ml according to the manufacturer's instructions, and stored at −20°C. Under these conditions, ELISA confirmed that it was completely activated (see Results). SB431542, a selective inhibitor of the TGF-β type I receptor (TβR-I) kinase (activin receptor-like kinases ALK4, -5, and -7) was purchased from Tocris Bioscience (1614; Ellisville, MO) and was stored at a concentration of 5 μM in dimethyl sulfoxide (DMSO) at −20°C. All other chemicals were of reagent grade.
Extraction and Analysis of Endogenous Extracellular Matrix
For analysis of endogenous extracellular matrix proteins deposited by MDCK cells, cells were cultured on Transwell supports for various times, washed twice with phosphate-buffered saline (PBS) without divalent cations (PBS−), and removed from the substratum by incubation with 20 mM NH4OH for ∼20 min at 37°C. The residual extracellular matrix attached to the support was washed extensively with PBS− and then extracted with Laemmli sample buffer [60 mM Tris-HCl, pH 6.8, 10% sucrose (wt/vol), 2% SDS, and 0.08% bromphenol blue], reduced with 50 mM dithiothreitol (DTT), heated at 95°C for 3 min, and alkylated with 5 μl of 0.5 M iodoacetamide per ∼30-μl sample for 20 min at 37°C before gel electrophoresis. Sample loading on SDS gels was normalized to culture surface area. Separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes and analyzed by Western blotting (see below).
Cell Surface Biotinylation
The polarity of the TβR-I was determined by differential biotinylation of apical and basolateral surfaces. MDCK cells grown on filters to confluence (day 4) were washed twice with PBS supplemented with 0.5 mM MgCl2 and 0.9 mM CaCl2 (PBS+) and then once with biotinylation buffer (BTB; 10 mM triethanolamine, 150 mM NaCl2, 2 mM CaCl2, and 0.5 mM MgCl2, pH 9.0) (Gottardi et al., 1995). Either the apical or the basolateral membrane was labeled by incubation with 0.5 mg of sulfo-NHS-biotin/ml BTB (21217; Pierce Chemical, Rockford, IL) in PBS+ on ice for 30 min. Cultures were then washed twice with DMEM followed by two washes with PBS+ and extracted with radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% IGEPAL-CA630, 0.5% deoxycholate, and 0.1% SDS) supplemented with protease (11836153001; Roche Diagnostics, Indianapolis, IN) and phosphatase (P5726; Sigma-Aldrich) inhibitor cocktails and 10 mM glycine. Extracts were clarified by brief microcentrifugation, and the protein concentration in the supernatants was determined by bicinchoninic acid assay (23227; Pierce Chemical). Equal amounts of protein were incubated overnight with 60 μl of a 1:1 (vol/vol) slurry of streptavidin-agarose beads (20347; Pierce Chemical) in RIPA buffer. The beads were then washed three times with RIPA buffer, and bound biotinylated proteins were solubilized by adding 2× Laemmli sample buffer, reducing with DTT, and heating at 95°C for 3 min. Samples were resolved by SDS gel electrophoresis and Western blotted with a 1:1000 dilution of anti-TβR-I antibody (see below; Table 1).
Analysis of Newly Synthesized Proteins by Metabolic Labeling
Biosynthesis of LM-332 chains was measured by metabolic labeling of cultures with 100 μCi of [35S]Met/Cys (NEG072; Perkin Elmer Life and Analytical Sciences, Boston, MA) in DMEM without l-Met/Cys (D0422; Sigma-Aldrich) for 20 min at 37°C. Cells were then extracted with RIPA buffer supplemented with protease and phosphatase inhibitor cocktails. Extracts were clarified by brief microcentrifugation, and supernatants were immunoprecipitated with a 1:100 dilution of anti-LM-332 (9LN5; Table 1) followed by incubation with protein A-Trisacryl beads (20338; Pierce Chemical). Immune complexes were extracted from the beads with 2× Laemmli sample buffer, reduced, and heated at 95°C for 3 min before gel electrophoresis and autoradiography.
Western Blotting
For Western blotting, SDS gels were transferred to PVDF membranes (IPVH00010; Millipore, Billerica, MA) with a semidry apparatus (Bio-Rad Laboratories, Hercules, CA) at 15 V for 60 min. Membranes were blocked with 5% nonfat dry milk or 3% bovine serum albumin (BSA; for detection of phosphorylated proteins) in 10 mM Tris-Cl, pH 7.4, 150 mM NaCl, and 0.1% Tween 20 (TBS/T), and incubated with primary antibodies for 2 h at room temperature (RT). After 3 washes with TBS/T, membranes were incubated for an additional 1 h with a 1:1000 dilution with horseradish peroxidase-conjugated secondary antibodies (Table 1). Peroxidase was detected by chemiluminescence (170-5040; Bio-Rad Laboratories) on x-ray films (Denville Scientific, Metuchen, NJ). Bands were quantified by scanning and analyzed using ImageJ software (http://rsbweb.nih.gov/ij/index.html).
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Expressions of the α3, β3, and γ2 subunits of LM-332 mRNA and the α5 subunit of LM-511 mRNA were determined by quantitative real-time reverse transcriptase-PCR (qRT-PCR). Total RNA from cell lysates was extracted and purified using the RNeasy kit (QIAGEN, Valencia, CA), quantified by absorbance measurements, and checked for integrity by formaldehyde agarose gel electrophoresis. After DNase digestion using DNase I amplification grade (18068-015; Invitrogen), 900 ng of RNA was used as template for reverse-transcription PCR (RT-PCR) using random hexamer primers and the SuperScript III First-Strand kit (18080-400; Invitrogen). The resulting cDNAs were treated with RNase H (18201-071; Invitrogen), and 4 μl was used for qRT-PCR using specific primers for the canine α3, β3, and γ2 LM chain sequences (QIAGEN) and a SYBR-Green master mix (QIAGEN). Amplification was carried out with a DNA Engine Opticon 2 thermal cycler (Bio-Rad Laboratories) using the following cycling conditions: 95°C for 15 min, 35 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 40 s. Amplified PCR products corresponding to subconfluent cells were cloned into the TOPO 2.1 vector (K4500-01; Invitrogen), and entire amplicon sequences including the PCR primers determined by automated sequencing using primers flanking the cloning site. Location and identity of the amplicons in the canine genome were then verified by BLAST analysis. Serial dilutions of plasmids containing the cloned PCR products were used to establish a standard curve for quantification of unknown samples by interpolation. Samples and standards were analyzed in triplicate and in duplicate, respectively, in at least two separate experiments, and values were averaged.
Transient Expression of SMAD Constructs
Recombinant constructs of dominant-negative (DN) Smad2, Smad3, and Smad4 cloned into the pRK5 vector (BD Biosciences Pharmingen, San Diego, CA) were provided by Dr. Rik Derynck (University of California, San Francisco, CA; Addgene plasmids 12624, 12626, and 12628, respectively; Zhang et al., 1996). Subconfluent cultures of MDCK cells were trypsinized, and 1 × 106 cells were transfected in suspension with 4 μg of each plasmid by using an AMAXA nucleofector (Program A-024) according to the manufacturer's instructions (Lonza AG, Cologne, Germany). Transfection efficiency as estimated by parallel transfection of a green fluorescent protein expression construct was ∼60%. After transfection, cells where plated at standard subconfluent density on permeable supports and analyzed for expression of LM-332 subunits by qRT-PCR and Western blotting.
Immunofluorescence
Cells grown on permeable supports were washed twice with PBS+ and fixed with 3% formaldehyde (FA) for 20 min at RT. Fixed cells were washed twice with PBS−, and unreacted FA was quenched with fresh 0.1% NaBH4/PBS− for 15 min at RT. After washing twice with PBS−, cells were permeabilized with cold 0.1% (wt/vol) Triton X-100/PBS− for 4 min and again washed twice with PBS−. Nonspecific binding sites were blocked with 10% (vol/vol) goat serum in PBS− for at least 1 h at RT and washed twice with PBS−. At this stage, the permeable membranes were sliced out of the plastic support and cut into four pieces. Primary antibodies (Table 1) were diluted in blocking solution and briefly centrifuged. Cells were incubated with antibody by pipeting 100-μl drops of the diluted antibody solution onto a sheet of Parafilm, placing the membrane pieces on top of the drops with the cells upright, and then adding an additional 100 μl of dilute antibody to the top. Incubation was carried out for 2 h at RT in a moist chamber. Membranes were then transferred to 24-well plates, washed four times with PBS− (2 min each), and then incubated with secondary antibodies for 1 h as described above. After three washes with PBS−, filters were mounted in Mowiol-based mounting media (81381; Fluka, Buchs, Switzerland) containing 0.6% (wt/vol) DABCO as antifade agent and DAPI (4,6-diamidino-2-phenylindole; 1 μg/ml) within a well formed on glass slides with dried nail polish. Samples were observed with a 63× 1.2W numerical aperture C-Apochromat UV-VIS objective using an LSM510 confocal microscope Carl Zeiss, Jena, Germany). Digital images were collected with AIM software (Carl Zeiss) and optimized with Photoshop (Adobe Systems, Mountain View, CA).
Flow Cytometry
MDCK cells plated under subconfluent or confluent conditions in normal growth medium were washed twice with PBS− and detached by incubation with 4 mM EDTA, 1 mM EGTA in PBS− at 37°C. Cells were centrifuged at 250 × g and 4°C and washed twice with 1% BSA/PBS−. After counting, aliquots containing 5 × 105 cells were incubated with 100 μl of dilute anti-αVβ3 integrin LM609 or nonspecific mouse antibodies for 30 min on ice. Cells were washed twice with 1% BSA/PBS−, resuspended in 100 μl of 1% BSA/PBS containing anti-mouse immunoglobulin (Ig)G-Alexa-488 (1:200), and incubated on ice for 30 min. Cells were then washed twice with 1% BSA/PBS− and resuspended in 200 μl of PBS−. The cell suspension was analyzed using a BD LSRII flow cytometer at the University of Chicago Flow Cytometry Facility (Chicago, IL).
Chromatin Immunoprecipitation (ChIP)
MDCK stock cells were plated in T-75 flasks and cultured in normal growth medium for 6 h (subconfluent) or 4 d (confluent). The medium was then removed, and cells were fixed for 15 min at RT with 10 ml of DMEM containing 1% formaldehyde, followed by quenching with 125 mM glycine for 10 min. After two washes with cold PBS−, the fixed cells were scraped from the dish in 5 ml of PBS− containing 0.01% BSA (wt/vol) and 0.1 mM phenylmethylsulfonyl fluoride (PMSF). Samples corresponding to 2 × 106 cells were centrifuged at 200 × g for 5 min at 4°C, resuspended in 500 μl of “swelling buffer” (25 mM HEPES, pH 7.8, 1.5 mM MgCl2, 10 mM KCl, 0.1% IGEPAL-CA630, 1 mM DTT, and 0.5 mM PMSF, supplemented with protease and phosphatase inhibitor cocktails), and incubated on ice for 10 min. The resulting cell suspension was centrifuged at 400 × g for 10 min at 4°C and resuspended in 500 μl of “sonication buffer” (50 mM HEPES, pH 7.9, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, and 0.5 mM PMSF, supplemented with protease and phosphatase inhibitor cocktails), and sonicated using a Misonix S4000 ultrasonic processor set at 30% amplitude (microtip) and 10 cycles of 20 s “on”/40 s “off.” Subsequent steps were based on standard EZ-ChIP kit (17-371; Millipore) procedures, with the following modifications. Precleared chromatin:protein cross-linked complexes were incubated with 4 μg of anti-Smad4-X (Santa Cruz Biotechnology, Santa Cruz, CA; Table 1), anti-RNA polymerase II, or control IgG overnight at 4°C on a rotary shaker. Cross-linking of eluted chromatin:protein complexes was reversed by incubation for 4.5 h at 65°C in a water bath and treated with proteinase K. The resulting free, fragmented chromatin was column purified, and 8 μl of the eluted DNA was amplified by touchdown-PCR (Korbie and Mattick, 2008) with a manual hot-start (using the primers described in Table 2) as follows: 95°C for 1.5 min followed by addition of 1 μl of Amplitaq polymerase (Invitrogen); 1.5 min at 95°C, amplification phase 1 consisting of 10 cycles: 95°C for 30 s, 64°C for 45 s (decreasing the annealing temperature 1°C every cycle; final temperature 54°C), and 72°C for 60 s; amplification phase 2 consisting of 25 cycles of 95°C for 30 s, 54 and 72°C for 60 s; and finally a termination phase at 72°C for 5 min. Ten microliters of the PCR products was mixed with 6× loading sample buffer, resolved by 2% agarose gel electrophoresis and analyzed using a GelDoc (Bio-Rad Laboratories). Positive samples were sequenced at the University of Chicago Cancer Research Center DNA Sequencing Facility.
Table 2.
Oligonucleotides used for PCR and qRT-PCR
| LM α3-Fwda & Revb | QuantiTect Primer Assay QT01465975* |
| LM β3-Fwd & Rev | QuantiTect Primer Assay QT01116472* |
| LM γ2-Fwd & Rev | QuantiTect Primer Assay QT00898541* |
| LM α5-Fwd & Rev | QuantiTect Primer Assay QT01450638* |
| SBE-Fwd | 5′-ATG TAG GGA AAC TTC AGA CAT GC-3′ |
| SBE-Rev | 5′-ACT TCC TAC CTG ACA TGA GTC ACC-3′ |
| GAPDHc-Fwd | 5′-ACA GTC AAG GCT GAG AAC G-3′ |
| GAPDH-Rev | 5′-GGC ATT GCT GAC AAT CTT G-3′ |
* Qiagen.
a Forward.
b Reverse.
c Glyceraldehyde-3-phosphate dehydrogenase.
Treatment with Blocking Antibodies and Peptides
Cells grown in ExCell were detached and 1 × 106 cell aliquots were preincubated with 1 μg of LM609 (anti-αVβ3 integrin), nTGF-β1 (neutralizing active TGF-β1), or nonspecific mouse IgG in 300 μl of ExCell medium for 30 min at RT on a rotary shaker. Cells were plated on Transwell permeable supports as described above in the presence of an additional 1 μg of antibody in both the upper and lower chambers and incubated at 37°C. For peptide treatment, cells were preincubated as described above in the presence of 1 mM GRGDS peptide (RGD; 03-34-0027; EMD Chemicals, Gibbstown, NJ) or RGES (RGE; A5686; Sigma-Aldrich) and then for the entire experiment.
Measurement of TGF-β1 Levels
The concentration of active and total TGF-β1 in culture media was determined by sandwich ELISA using the ELISA Emax kit purchased from Promega (G7590; Madison, WI) in 96-well enzyme immunoassay/radioimmunoassay high-binding plates (3590; Corning Life Sciences) according to the manufacturer's instructions with the following modifications. Samples of culture media were collected in sterile tubes, briefly microcentrifuged, passed through a 0.45-μm filter to remove cell debris, and snap-frozen until analysis. The day of the assay, samples were divided in two, and one half was acidified with HCl to pH ∼3.0 for 30 min at RT to activate TGF-β1 and then neutralized to pH ∼7.5–8.0 with NaOH. Samples were then diluted 1:2–1:4 in the provided sample buffer and incubated for 2 h at RT with gentle shaking. The remainder of the assay was performed according to the ELISA kit protocol. Differential measurement of active and total TGF-β1 was accomplished by analyzing samples before and after the activating acidification, as this kit uses an antibody specific for the active form of TGF-β1. The amount of latent TGF-β1 was determined by subtracting the active fraction from the total fraction. Plates were read in a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm.
Statistical Analysis
Statistical significance was determined by one-way analysis of variance with Tukey's post hoc test for multiple comparisons, and a two-tailed unpaired t test for single comparisons using the Instat application (GraphPad Software, San Diego, CA). P values <0.05 were considered significant.
RESULTS
LM-332 Expression Is Transcriptionally Regulated in MDCK Cells as a Function of Confluence
Previous results from our laboratory demonstrated that MDCK cell cultures synthesized and deposited LM-332 only when subconfluent, with little or no synthesis occurring once confluence was achieved (Mak et al., 2006). To confirm and extend these findings, we examined MDCK cells cultured on Transwell permeable supports under conditions in which cultures are subconfluent 1 d after plating and reach full confluence after 3–4 d of growth.
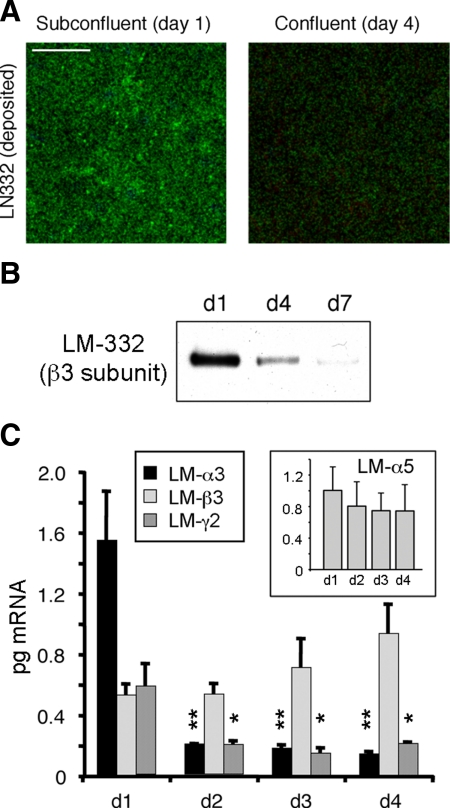
As shown in Figure 1, LM-332 was visible in a basal optical section by confocal immunofluorescence microscopy only in a subconfluent (day 1) culture but not in a confluent (day 4) culture (Figure 1A). Similarly, LM-332 (as detected by Western blotting with an antibody against the β3 subunit) was deposited onto the permeable support in significant amounts at day 1 of culture but was present in diminishing amounts at day 4 and, to an even lesser extent, at day 7, suggesting that not only was secretion and deposition of LM-332 reduced as confluence was achieved but also that any residual LM-332 was removed (Figure 1B).
Figure 1.
Laminin-332 (LM-332) expression is regulated as a function of confluence. (A) LM-332 deposition only occurs in subconfluent cells. Subconfluent (day 1) or confluent (day 4) MDCK cells cultured on 0.4-μm Transwell supports were immunostained for LM-332 with an anti-β3 subunit mAb (green). Confocal sections corresponding to the basal plane (deposited extracellular matrix [ECM]) are shown. Bar, 10 μm. (B) Significant amounts of LM-332 are deposited into the substratum only in subconfluent (day 1) cultures. Cells plated on Transwell supports were removed by treatment with 20 mM NH4OH at the indicated time points (days 1–7). Deposited ECM proteins were extracted, resolved by SDS-polyacrylamide gel electrophoresis and Western-blotted for LM-332 with an anti-β3 mAb. (C) Laminin α3 and γ2 subunit expression is transcriptionally regulated as a function of cell confluence. RNA from Transwell cultures was isolated at different time points (days 1–4) and analyzed by qRT-PCR using canine-specific primers. Inset, qRT-PCR for the α5 subunit of LM-511 (LM-α5). The histograms represent the average abundance of mRNAs from three independent experiments expressed in picograms ± SD. **p < 0.01 or *p < 0.05 relative to day 1 (d1) levels.
To determine whether decreased production of LM-332 was regulated at the transcriptional level, RNA extracted from cultures at different degrees of confluence was examined by qRT-PCR using primers specific for the canine α3, β3, and γ2 laminin subunit mRNAs. As shown in Figure 1C, mRNAs for all three subunits were expressed at day 1, with levels of α3 mRNA the highest. By day 2, however, amounts of both the α3 and γ2 mRNAs had significantly declined and remained low through day 4. Curiously, amounts of the β3 mRNA, as well as the α5 subunit mRNA of LM-511, continued to be produced at approximately the same level independently of confluence, These observations not only demonstrated that expression of LM-332 is transcriptionally regulated as a function of confluence but confirm previous protein biosynthesis data indicating that only the α3 and γ2 subunits are down-regulated as confluence is achieved, whereas the β3 subunit continues to be synthesized (Mak et al., 2006).
Exogenous TGF-β1 Stimulates LM-332 Expression in Confluent Cultures
Analysis of the promoter region of the human laminin α3A gene identified previously several transcriptional regulatory elements including three AP1 (Fra2/JunD) binding sites, a TGF-β response element, two KLF4 sites, and a noncanonical E-box that binds USF-1 (Virolle et al., 1998, 2002; Aberdam et al., 2000; Miller et al., 2001; Fitsialos et al., 2008). Of these, the TGF-β response element stood out because TGF-β is well known as a regulator of extracellular matrix gene expression and elevated levels of TGF-β have been observed in the kidney in response to ischemic injury, conditions where expression of LM-332 is also known to be increased (Basile et al., 1998; Ledbetter et al., 2000).
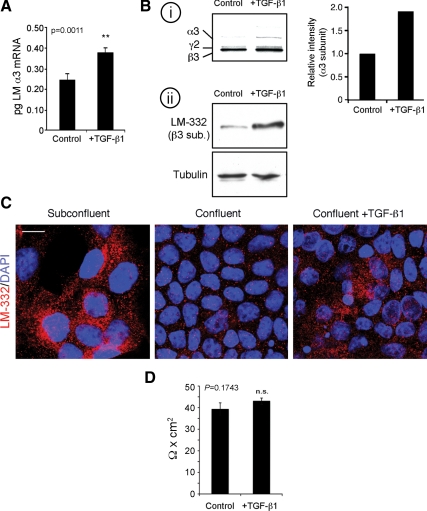
To test the involvement of TGF-β1 in regulation of LM-332 production in MDCK cells, exogenous activated TGF-β1 was added both apically and basolaterally to confluent (day 4) cultures of MDCK cells that normally do not express LM-332 (Figure 1). As illustrated in Figure 2A, addition of TGF-β1 for 6 h stimulated transcription of mRNA for the α3 subunit. In addition, increased synthesis of LM-332 was also evident in similarly treated cultures when analyzed either by metabolic-labeling and immunoprecipitation or Western blotting (Figure 2B). These findings were further supported by detection of LM-332 in treated cells by immunofluorescence (Figure 2C). Treatment with exogenous TGF-β1 did not compromise the epithelial barrier, as determined by measurement of transepithelial resistance (Figure 2D). Thus, under conditions in which MDCK cells do not normally express LM-332, TGF-β1 treatment induces LM-332 mRNA transcription and protein synthesis, suggesting a possible physiological role for TGF-β1 in the regulation of LM-332 expression.
Figure 2.
Exogenous active TGF-β1 is sufficient to induce LM-332 expression in confluent cells. (A) TGF-β1 induces transcription of the laminin α3 subunit gene. Confluent MDCK cells untreated (control) or treated with 5 ng/ml active TGF-β1 for 6 h in serum-free medium (+TGF-β1) were collected and analyzed for laminin α3 subunit mRNA by q-RT-PCR. **p = 0.0011 (B) TGF-β1 induces LM-332 protein synthesis. i, untreated control (control) or TGF-β1-treated (+TGF-β1) confluent cultures were metabolically labeled with [35S]Met/Cys for 20 min and extracted with RIPA buffer. The extracts were immunoprecipitated with a polyclonal antibody against LM-332, and the immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis and detected by autoradiography. The graph represents the intensities of the α3 bands in this experiment. ii, equal amounts of total protein from RIPA lysates were analyzed by Western blot for LM-332 using a mAb against the β3 subunit with immunoblotting for tubulin as loading control. (C) Confocal immunofluorescence localization of LM-332 after TGF-β1 treatment. Subconfluent and confluent MDCK cell cultures without added exogenous TGF-β1, and TGF-β1-treated confluent MDCK cells were immunostained with a polyclonal antibody against LM-332 (red) and optical sections of a mid/basal plane visualized. Nuclei were stained with DAPI (blue). Bar, 10 μm. (D) Transepithelial resistance of MDCK cells is not affected by TGF-β1 treatment. Transepithelial electrical resistance of confluent MDCK cultures grown on Transwell supports in either the absence or presence of exogenous active TGF-β1 was measured with a Millicell-ERS device. The experiment was repeated four times, and the average is shown. n.s., not statistically significant, p = 0.1743.
Activin-like Kinase Activity of TβR-I Is Required for LM-332 Expression
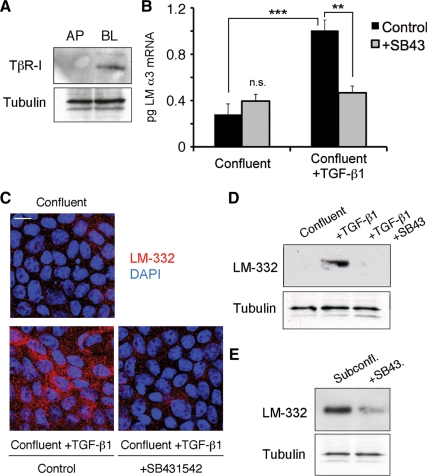
TGF-β normally signals through type I and II receptors that are believed to form a heterotetramer on the cell surface when ligated (Wrana et al., 2008). In MDCK cells, the TβR-I receptor was expressed on the basolateral surface of confluent cultures, as determined by domain-selective biotinylation and Western blotting (Figure 3A), confirming previous results (Murphy et al., 2004). To ascertain whether this receptor is used by MDCK cells in the expression of LM-332, cells were preincubated with SB431542, a selective inhibitor of activin receptor-like kinase (ALK) superfamily members, including TβR-I (Inman et al., 2002), for 30 min before addition of exogenous activated TGF-β1 for 6 h to both the apical and basolateral chambers of a confluent Transwell culture of MDCK cells. As shown in Figure 3B, transcription of the laminin α3 gene, as determined by qRT-PCR, and synthesis of LM-332, as determined by immunofluorescence and Western blotting, was nearly abolished in samples treated with exogenous TGF-β1 and the TβR-I receptor inhibitor relative to control cultures treated only with TGF-β1 and vehicle (Figure 3, B–D). Furthermore, when subconfluent cultures of MDCK cells, which synthesize LM-332 in the absence of exogenous TGF-β1 stimulation, were treated with the inhibitor under the same conditions, LM-332 synthesis was significantly diminished (Figure 3E). Overall, these results strongly suggest that the TGF-β1 receptor TβR-I is mediating signals leading to the expression of LM-332 in MDCK cells.
Figure 3.
Kinase activity of the TGF-β receptor type I (TβR-I) is required for LM-332 expression. (A) TβR-I is localized basolaterally in polarized MDCK cells. Confluent cultures of MDCK cells grown on permeable supports were biotinylated either apically (AP) or basolaterally (BL). Biotinylated proteins were captured on streptavidin-conjugated beads and immunoblotted with anti-TβR-I antibodies. The unbound protein fraction was used for immunoblotting with an mAb against tubulin as loading control. These experiments were repeated twice with similar results. (B) Inhibition of TβR-I signaling abolishes laminin α3 subunit mRNA expression. Confluent MDCK cells were pretreated with DMSO (control) or with 5 μM TβR-I kinase activity inhibitor SB431542 (+SB43) for 30 min. Then, cells were either incubated with or without exogenous TGF-β1 for 6 h and analyzed for laminin α3 subunit mRNA expression by qRT-PCR. n.s., not statistically significant; ***p < 0.001; **p < 0.01 (C) LM-332 protein expression is also diminished after blocking TβR-I signaling in TGF-β1–treated confluent MDCK cells. Confluent cultures of MDCK cells untreated or pretreated with SB431542 were then incubated with or without exogenous TGF-β1 for 6 h, and LM-332 expression was detected by immunofluorescence. Control/confluent, no TGF-β1 or SB431542; control/confluent + TGF-β1, treated with TGF-β1 but no SB431542; +SB431452/confluent+TGF-β1, treated with both TGF-β1 and SB431452. Bar, 10 μm. (D) Production of LM-332 was examined by Western blotting of MDCK cell extracts using an anti-β3 subunit monoclonal from cultures treated as described in C. SB43, treated with SB431452. (E) LM-332 expression is also dependent on TβR-I signaling in subconfluent cells. Subconfluent MDCK cell cultures without addition of exogenous TGF-β1 were incubated with or without SB431452 (SB43) for 18 h, and extracts were Western blotted for the β3 subunit of LM-332.
TGF-β1 Signaling through Smad2 and Smad 4 Is Responsible for Stimulating LM-332 Expression
The major signaling pathway downstream of TGF-β1 and its type I and II receptors involves Smad proteins (Schmierer and Hill, 2007; Schilling et al., 2008). However, TGF-β1 and its receptors are also known to signal through a variety of Rho-family small GTPases, several mitogen-activated protein kinase pathways, including those leading to extracellular signal-regulated kinase, p38, and c-Jun NH2-terminal kinase, and PP2A/p70S6K (Schilling et al., 2008). It was therefore important to determine whether Smads are the primary signal transducers involved in TGF-β1–mediated LM-332 expression. To accomplish this, Smad phosphorylation, complex formation, and nuclear localization were examined.
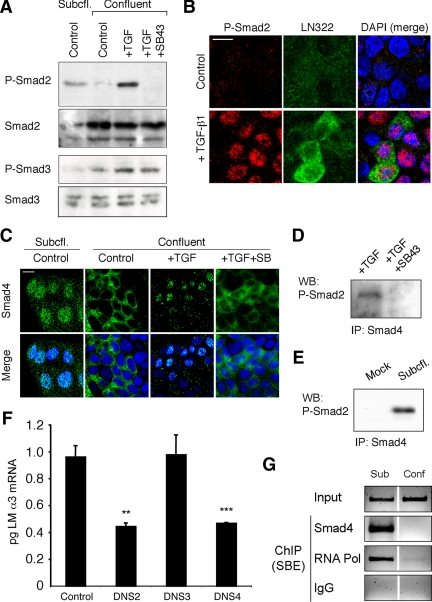
Ligated TGF-β1 type I and II receptors phosphorylate the so-called “receptor-activated” or R-Smads Smad 2 and -3. As shown in Figure 4A, phospho-Smad2 was detected in subconfluent MDCK cell cultures expressing LM-332 but not in confluent cultures. Stimulation of confluent cultures with exogenous activated TGF-β1–stimulated phosphorylation of Smad2, whereas inhibition of TβR-I with SB431542 blocked this phosphorylation. In contrast, Smad3 phosphorylation was low in subconfluent cultures, and baseline levels of Smad3 phosphorylation were only modestly affected by exogenous TGF-β1 and the inhibitor of TβR-I in confluent cultures (Figure 4A), suggesting that Smad2 but not Smad3 was involved in TGF-β1 signaling in MDCK cells under these conditions.
Figure 4.
Phospho-Smad2 and Smad4 regulate LM α3 subunit gene transcription. (A) Differential phosphorylation of Smads. Subconfluent (Subcfl.) or confluent cultures either untreated (control) or treated with 5 ng/ml TGF-β1 for 6 h in the absence or presence of the TβR-I inhibitor SB431542 (+TGF-β1 or + TGF-β1+SB43, respectively) were analyzed by Western blotting for phospho-Smad2 (P-Smad2), total Smad2, P-Smad3, and total Smad3. (B) Phospho-Smad2 is localized to the nuclei after TGF-β1 treatment in confluent cells. Untreated (control) or TGF-β1–treated confluent cells (+TGF-β1) were stained with antibodies against P-Smad2 (red) and LM-332 (β3 subunit; green) and analyzed by confocal fluorescence microscopy. Nuclear staining with DAPI, blue. Bar, 10 μm. (C) Smad 4 is also localized to the nuclei in subconfluent and TGF-β1–treated confluent cells. Subconfluent MDCK cell cultures without added exogenous TGF-β1 and confluent cultures either untreated (control) or treated with of TGF-β1 in the presence or absence of SB431542 (SB) were stained with antibodies against Smad4 (green) and analyzed by confocal fluorescence microscopy. Nuclear staining with DAPI, blue. Bar, 10 μm. (D) Phosho-Smad2 and Smad4 form a complex dependent on TβR-I signaling. Confluent cultures treated with TGF-β1 to induce LM-332 expression in the absence or presence of the TβR-I inhibitor SB431542 (+TGF or +TGF+SB43, respectively) were extracted in RIPA buffer. Extracts were immunoprecipitated with an anti-Smad4 antibody (mouse) and Western blotted with anti-P-Smad2 antibody (rabbit). (E) P-Smad2 and Smad4 also form a complex in subconfluent cells. Extracts of subconfluent MDCK cell cultures without added exogenous TGF-β1 were immunoprecipitated with a control IgG (mock) or with an anti-Smad4 antibody (mouse), and Western blotted for P-Smad2 (rabbit). (F) DN Smad2 and Smad4, but not Smad3, impairs α3 subunit transcription in subconfluent cultures. Subconfluent MDCK cell cultures were transiently transfected with DN-Smad2, DN-Smad3, or DN-Smad4, and α3 subunit mRNA expression was analyzed by qRT-PCR after 24 h. **p < 0.05; ***p < 0.001 (G) Smad4 binds to an Smad binding element in the α3 subunit gene promoter. Subconfluent (6 h) or confluent (4 d) MDCK cell extracts were subjected to ChIP with anti-Smad4, RNA-polymerase II, or nonspecific antibodies (IgG). Enrichment of the SBE in the immunoprecipitated chromatin was determined by touchdown-PCR using primers specific for the α3 subunit promoter. Products were resolved by agarose gel electrophoresis (negative staining is shown) and compared with whole chromatin lysates (input).
Activation of transcription by Smads requires formation of a complex between a phosphorylated R-Smad, such as Smad2 and Smad3, and Smad4, followed by translocation of the complex into the nucleus where it interacts with the promoter region of target genes. As illustrated in Figure 4B, phospho-Smad2 was almost undetectable in confluent MDCK cell cultures by immunofluorescence but increased in intensity and concentrated in nuclei upon stimulation with exogenous TGF-β1. Smad4 was also localized in nuclei in subconfluent cultures of MDCK cells known to express LM-332 and concentrated in nuclei in confluent cultures that had been treated with exogenous TGF-β1 (Figure 4C). This localization was significantly reduced upon preincubation with the TβR-I inhibitor (Figure 4C).
To determine whether phospho-Smad2 and Smad4 interacted after TGF-β1 stimulation, cell extracts were immunoprecipitated with anti-Smad4 followed by Western blotting with anti-phospho-Smad2. As shown in Figure 4D, Smad-4 and phospho-Smad2 coimmunoprecipitated upon stimulation of a confluent culture with TGF-β1, and this association was abolished when the culture was pretreated with the TβR-I inhibitor. Similarly, coimmunoprecipitated phospho-Smad2 and Smad4 were also evident in subconfluent MDCK cells in the absence of exogenous TGF-β1 (Figure 4E), consistent with a role for Smad2/Smad4 signaling in LM-332 production in subconfluent cultures.
As a final proof that Smad signaling was responsible for TGF-β1–mediated LM-332 expression, dominant-negative constructs of Smad2, Smad3, and Smad4 were transiently expressed in MDCK cells. After culturing the transfected cells overnight under subconfluent conditions, RNA was extracted and the amount of laminin α3 transcripts estimated by qRT-PCR. As shown in Figure 4F, transfection with Smad2 and Smad4 dominant-negative constructs reduced the levels of laminin α3 mRNA relative to controls, whereas transfection with dominant-negative Smad3 had no effect.
Previous studies have demonstrated that TGF-β1 activation of laminin α3 expression in keratinocytes and carcinoma cell lines occurs via binding of Smad4 to a Smad binding element (SBE) in the α3 gene promoter. To confirm that this is also the case in MDCK cells, ChIP was carried out with an antibody against Smad4 and PCR primers demarcating a 246-nucleotide upstream region of the canine laminin α3 gene. As shown in Figure 4G, ChIP from chromatin extracted from subconfluent cells that produce LM-332 and immunoprecipitated with anti-Smad4 or anti-RNA-polymerase II yields an appropriately-sized PCR product, whereas chromatin from confluent cells not producing LM-332 and nonspecific IgG controls do not. Sequencing of the PCR product confirmed that the region containing the SBE was immunoprecipitated and amplified (data not shown). Together, the results of this experiment and the analysis of Smad phosphorylation, nuclear localization, complex formation, and dominant-negative Smad expression strongly support the central involvement of Smad2 and Smad4 in TGF-β1–stimulated expression of LM-332.
MDCK Cells Constitutively Secrete Latent TGF-β1 and Activate It Only under Subconfluent Conditions
In all previous experiments, MDCK cells were cultured in medium containing 5% FBS that presumably contains TGF-β1. Furthermore, this serum-containing medium was present in both the apical and basolateral chambers of Transwell cultures. Despite this, TGF-β1–stimulated expression of LM-332 only occurred under subconfluent conditions or when exogenous activated TGF-β1 was added to confluent cultures. TGF-β is an unusual growth factor in that it is secreted in an inactive or “latent” form and must be activated to enable its binding to cell surface receptors and elicitation of downstream effects. For this reason, it seemed as though TGF-β1 contributed from the serum would have to be differentially activated in MDCK cells as a function of confluence if it was indeed the physiological stimulus of LM-332 expression.
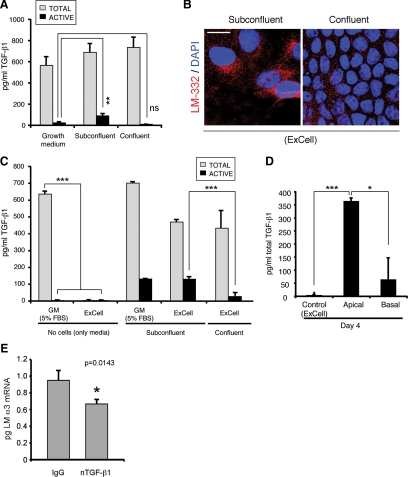
To test this, serum-containing growth medium and conditioned medium from subconfluent and confluent cultures were analyzed by an ELISA capable of distinguishing active from inactive TGF-β1. As shown in Figure 5, TGF-β1 is indeed present in serum-containing MDCK cell growth medium but is almost completely inactive (Figure 5A). In contrast, conditioned medium taken from subconfluent cultures had a significant fraction of activated TGF-β1, whereas the medium from confluent cultures only contained inactive TGF-β1 (Figure 5A).
Figure 5.
MDCK cells constitutively secrete endogenous TGF-β1 but only activate it under subconfluent conditions. (A) Latent TGF-β1 is activated only in subconfluent cells. Subconfluent and confluent cultures were grown in normal growth medium (containing 5% FBS) and the conditioned media were analyzed for total and active TGF-β1 by sandwich ELISA. **p < 0.01 (B) Cells grown in ExCell also express LM-332 only when subconfluent. MDCK cells were grown in ExCell, a defined serum-free growth media, on Transwell supports for 1 d (subconfluent) or 4 d (confluent); fixed; stained; and analyzed for LM-332 expression (red) by confocal immunofluorescence microscopy; nuclei were stained with DAPI (blue). Bar, 10 μm. (C) MDCK cells constitutively secrete latent TGF-β1. Conditioned media of cells grown in ExCell and normal serum-containing growth medium (GM) were analyzed for total and active TGF-β1 using sandwich ELISA. ***p < 0.001 (D) Confluent MDCK cells secrete latent TGF-β1 apically. MDCK cells were grown in ExCell serum-free medium on Transwell supports for 4 d to achieve full apicobasal polarization. Conditioned medium from either the apical or basal compartments was analyzed for total TGF-β1 by sandwich ELISA. *p < 0.05 (E) Neutralization of endogenous active TGF-β1 reduces LM α3 subunit expression. Subconfluent MDCK cell cultures grown in ExCell in the presence of 1 μg of neutralizing antibody against active TGF-β1 (nTGF-β1) or IgG as negative control were analyzed for α3 subunit mRNA levels by qRT-PCR. *p = 0.0143.
Although it was conceivable that MDCK cells depended upon exogenous TGF-β1 from serum to stimulate LM-332 expression, it was also possible that TGF-β1 was endogenously produced. To determine this, MDCK cells were grown in a commercial hormone-supplemented serum-free medium (ExCell), and production of LM-332 was analyzed. As shown in Figure 5B, subconfluent but not confluent MDCK cell cultures grown in ExCell in the absence of serum produce LM-332 detectable by immunofluorescence. When analyzed by ELISA, fresh ExCell medium completely lacked TGF-β1. However, conditioned ExCell medium from subconfluent and confluent cultures contained significant amounts of latent TGF-β1 presumably synthesized by MDCK cells (Figure 5C). Furthermore, because the medium analyzed was changed every day, and thus in contact with cells for not >1 d, these results suggested that synthesis of TGF-β1 by MDCK cells was not regulated as a function of confluence but was constitutive at all stages of culture growth. More importantly, ∼25% of endogenous TGF-β1 was activated in medium from subconfluent cultures, whereas much less was activated in conditioned medium from confluent cultures (Figure 5C). Because confluent cultures of MDCK cells are fully polarized, with apical and basal chambers of the Transwell supports isolated by tight junctions between the cells, it was of interest to determine whether endogenous TGF-β1 was secreted in a polarized manner. Indeed, ELISA analysis of total TGF-β1 in apical and basal media from confluent ExCell cultures indicated that most TGF-β1 produced by MDCK cells was secreted into the apical chamber, confirming previous observations from others (Figure 5D; Murphy et al., 2004). As a final confirmation that endogenous TGF-β1 was capable of stimulating LM-332 expression, subconfluent MDCK cells grown in ExCell were preincubated with a neutralizing TGF-β1 antibody (nTGF-β1) that specifically blocks the function of the active form, and laminin α3 mRNA expression was analyzed by qRT-PCR. As shown in Figure 5E, treatment with nTGF-β1, but not with a control IgG, significantly reduced α3 chain mRNA transcription, proving that endogenous active TGF-β1 is required for laminin α3 expression in MDCK cells.
Together, the results of the experiments described in this section demonstrate that MDCK cells constitutively synthesize and secrete TGF-β1 at all stages of confluence but only activate it in subconfluent cultures. Furthermore, this endogenous activated TGF-β1 is the physiological stimulator of laminin α3 expression.
MDCK Cells Use Integrin αVβ3 to Activate TGF-β1 in Subconfluent Cultures
The latency of TGF-β1 is due to its continued association with its propeptide (latency associated peptide [LAP]) after secretion. Activation has been postulated to occur through a variety of mechanisms including controlled proteolysis of LAP and possibly the latent TGF-β binding protein (LTBP) by plasmin or other proteases including matrix metalloendoproteases (MMPs), interaction with thrombospondin, integrin binding, or some combination of these mechanisms (Dabovic and Rifkin, 2008). In particular, the LAP of TGF-β1 contains an integrin RGD binding site, and integrins such as αVβ6 have been implicated in TGF-β activation (Dabovic and Rifkin, 2008). Because MDCK cells express integrin αVβ3, an RGD-binding integrin (Schoenenberger et al., 1994), and possibly other αV containing integrins (Teräväinen and Manninen, University of Oulu, Oulu, Finland; personal communication), it was conceivable that this receptor was playing a role in TGF-β1 activation.
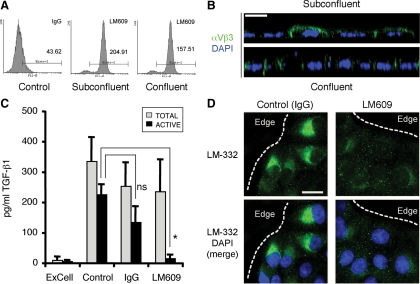
Initially, expression of αVβ3 was confirmed by flow cytometry and immunofluorescence using the LM609 monoclonal antibody (mAb) specific for the integrin. As shown in Figure 6A, flow cytometry detected αVβ3 on both subconfluent and confluent cells, although levels on confluent cells were slightly reduced (∼4.7-fold vs. ∼3.6-fold relative to controls). Expression was also evident in orthogonal (xz) sections of subconfluent and confluent cultures generated by confocal immunofluorescence microscopy. As expected, αVβ3 was localized to the basolateral plasma membrane in confluent cultures, as reported previously (Schoenenberger et al., 1994), but in subconfluent cultures is also present on the apical domain (Figure 6B).
Figure 6.
αVβ3 integrins regulate TGF-β1 activation and LM-332 expression. (A) MDCK cells express integrin αVβ3 on the cell surface. Subconfluent and confluent cells were incubated with monoclonal anti-αVβ3 (LM609) or mouse IgG (control), and analyzed by flow cytometry to determine cell surface expression levels. (B) Integrin αVβ3 is localized to the apical and lateral plasma membrane in subconfluent cells but is basolaterally polarized in confluent cultures of MDCK cells. Subconfluent and confluent (polarized) MDCK cell cultures grown in ExCell on Transwell supports were stained for αVβ3 integrins with the LM609 (green) and analyzed by confocal microscopy. XZ stacks (lateral views) are shown. Nuclei, blue. Bar, 10 μm. (C) Integrin αVβ3 participates in TGF-β1 activation. MDCK cells were pretreated with a nonspecific antibody (IgG) or anti-αVβ3 function blocking antibody (LM609) for 30 min and then grown for 18 h in ExCell (subconfluent cultures). The conditioned media was analyzed by sandwich ELISA to determine TGF-β1 activation. IgG, nonspecific immunoglobulin. n.s., not statistically significant; *p < 0.05 (D) Inhibition of αVβ3 integrin prevents LM-332 expression. Subconfluent MDCK cell cultures grown on Transwell supports in ExCell were pretreated with either anti-αVβ3 function blocking antibody (LM609) or a nonspecific antibody (IgG), and after 18 h LM-332 (green) was detected by confocal immunofluorescence. The dotted line indicates the edge of an “island” of cells. Nuclei, blue. Bar, 10 μm.
The LM609 antibody is capable of blocking cell adhesion to vitronectin and fibronectin. To determine whether it also had an effect on TGF-β1 activation in MDCK cells, subconfluent ExCell cultures were pretreated with LM609 or a nonspecific IgG for 30 min, and TGF-β1 activation was determined by ELISA after 18 h. Under these conditions, LM609 dramatically inhibited TGF-β1 activation but not secretion (Figure 6C). To find out whether LM609 also blocked expression of LM-332, MDCK cells were cultured in ExCell in the presence of LM609 or control IgG. As shown in Figure 6D, treatment of subconfluent cultures with LM609 almost completely abrogated LM-332 expression.
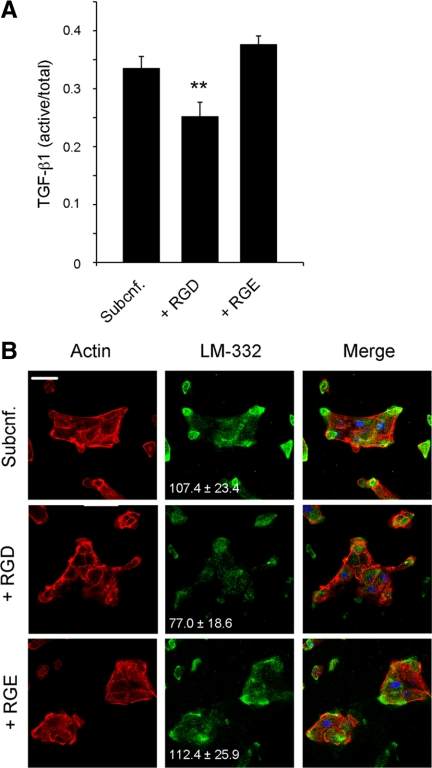
Because αVβ3 integrin can bind LAP, which is an RGD-containing protein (Ludbrook et al., 2003), we next treated subconfluent MDCK cell cultures with either soluble RGD or RGE peptides and measured TGF-β1 activation and LM-332 production. As shown in Figure 7A, the presence of soluble RGD, but not RGE, significantly reduced TGF-β1 activation. In a similar way, LM-332 expression was greatly diminished as shown by immunofluorescence (Figure 7B).
Figure 7.
TGF-β1 activation and LM-332 expression in subconfluent MDCK cells is regulated by RGD. (A) The RGD peptide inhibits TGF-β1 activation. Subconfluent MDCK cells were grown in ExCell for 18 h in the absence (Subcnf.) or presence of either RGD or RGE peptides (1 mM). The conditioned media were collected and analyzed for TGF-β1 activation by ELISA. Histograms represent the ratio of active versus total TGF-β1. **p < 0.01 (B) RGD inhibits LM-332 expression. Cell cultures corresponding to A were immunostained for LM-332 with a polyclonal antibody (green). Actin, red; nuclei, blue. The numbers indicate relative units of fluorescence (±SD) for the green channel (LM-332). Bar, 10 μm.
Overall, these experiments demonstrate that the RGD-binding integrin αVβ3 is essential for the activation of TGF-β1 in MDCK cells and confirm that this activation is required for expression of LM-332 in subconfluent cultures.
Mechanical Wounding of Confluent MDCK Cell Cultures Is Sufficient to Activate TGF-β1 and Stimulate LM-332 Expression
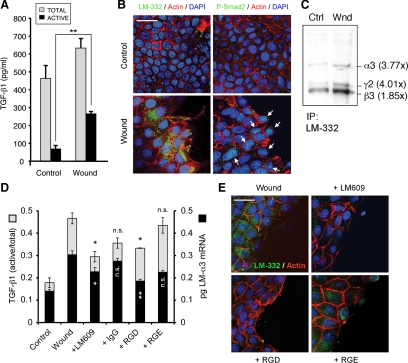
Previous results demonstrated that TGF-β1 activation and consequent LM-332 expression occurs exclusively in subconfluent cultures of MDCK cells. Subconfluence is an in vitro phenomenon created by plating cells at low density. Epithelia in vivo are rendered “subconfluent” by wounding or injury that opens gaps in the continuous epithelium. To determine whether wounding was equivalent to subconfluence with regard to TGF-β1 activation and LM-332 expression, confluent cultures of MDCK cells were mechanically wounded by scraping multiple times with a pipette tip and analyzed. As shown in Figure 8, wounding of confluent cultures produced both an apparent increase in the total amount of TGF-β1 in the medium as well as significantly more activation (Figure 8A). Furthermore, increased LM-332 synthesis and phospho-Smad2 were evident by immunofluorescence at the wound edge (Figure 7B) and by immunoprecipitation of LM-332 after metabolic-labeling of the wounded culture (Figure 7C), suggesting that expression of LM-332 is regulated in this situation in a manner identical to that established for subconfluent cultures.
Figure 8.
Wounded confluent MDCK monolayers express LM-332. (A) Wounded epithelial monolayers activate TGF-β1. Conditioned media from a confluent MDCK cell culture (control) and a culture wounded by scraping with a pipette tip were analyzed by sandwich ELISA for total and active TGF-β1. **p < 0.01 (B) LM-332 expression is localized to the cells close to the wound edge. A confluent MDCK cell monolayer (control) and a culture injured by scraping (Wound) were analyzed after 6 h for LM-332 expression (green; left) or P-Smad2 (green/arrows; right). F-actin, red; nuclei, blue. Bar, 25 μm. (C) LM-332 synthesis is up-regulated in wounded cultures. Confluent MDCK cell monolayers (Ctrl) or wounded cultures (Wnd) were metabolically labeled with [35S]Met/Cys for 20 min 6 h after wounding, and synthesized LM-332 was analyzed by immunoprecipitation. Autoradiography revealed increased levels of α3, β3, and γ2 subunit synthesis in wounded monolayers compared with the unwounded control. The numbers between parentheses indicate fold-increase in band intensity compared with the control. Note that α3 and γ2 subunits show a similar fold-increase, consistent with coregulation of the α3 and γ2 mRNAs. (D) Blocking αVβ3 integrin with LM609 or RGD prevents TGF-β1 activation and LM-α3 mRNA expression. Confluent MDCK cell monolayers were treated with either anti-αVβ3 function blocking antibody (LM609), a nonspecific antibody (IgG), RGD or RGE peptides in ExCell. After 6 h, the conditioned media were analyzed for TGF-β1 activation (gray bars), and the cells were analyzed for LM-α3 mRNA expression by qRT-PCR (black bars). n.s., not statistically significant compared with “wound”; *p < 0.05; **p < 0.01 (E) Wounded MDCK monolayers treated as described in D were analyzed by immunofluorescence for LM-332 with a polyclonal antibody (green). F-actin, red; nuclei, blue. Bar, 25 μm.
To determine whether the LM609 antibody or RGD peptides were also able to impair TGF-β1 activation and LM-332 expression as in subconfluent cultures, wounded monolayers were treated with either LM609, IgG, RGD, or RGE. As shown in Figure 8D, TGF-β1 activation was significantly diminished by the treatment with LM609 or RGD (compared with their respective controls, IgG and RGE). This reduction in TGF-β1 activation was accompanied with a reduction in α3 subunit mRNA expression (Figure 8D) and LM-332 expression shown by immunofluorescence (Figure 8E).
In summary, these experiments clearly show that subconfluent cultures and wounded monolayers similarly activate endogenous TGF-β1, which signal via Smads and stimulate LM-332 production in a mechanism dependent on LM609/RGD.
DISCUSSION
Previous results from our laboratory demonstrated that MDCK cells synthesize LM-332 only at subconfluent density (Mak et al., 2006). In this study we have explored these observations further to determine the mechanism responsible for regulated synthesis. We report that expression of the α3 and γ2 chains is transcriptionally controlled as a function of confluence and that expression of at least the α3 subunit is stimulated by TGF-β1 acting through Smad2/Smad4 binding to an SBE found in the α3 promoter. Furthermore, we find that MDCK cells constitutively secrete inactive TGF-β1 independently of state of confluence but only activate it when subconfluent through a mechanism involving integrin αVβ3.
Transcriptional regulation of LM-332 expression is known to be complex. Early work showed that TGF-β stimulated LM-332 expression in keratinocytes (Korang et al., 1995), and the laminin α3A promoter was found to have three AP-1 sites (linked to stimulation by TGF-β), and an SRE (Virolle et al., 1998). Later work also implicated other factors (Miller et al., 2000, 2001; Virolle et al., 2002; Sathyanarayana et al., 2003a,b; Fitsialos et al., 2008). More recently, Smad4 was shown to regulate the synthesis of all three LM-332 chains (Olsen et al., 2000; Zboralski et al., 2008).
Our results are consistent with a central role for TGF-β1 and Smad signaling in the regulation of LM-332 expression. In confluent cultures of MDCK cells that do not express LM-332, expression is stimulated by the addition of active TGF-β1. This artificially activated expression and normal expression in subconfluent cells is blocked by an inhibitor of the TβR-I receptor that also blocks Smad2 phosphorylation, formation of Smad2/Smad4 complexes, and translocation of Smad4 to the nucleus. Transient expression of Smad2 and Smad4 (but not Smad3) dominant-negative constructs suppresses laminin α3 transcription, and Smad4, presumably in a complex with phospho-Smad2, binds to an SBE in the laminin α3 promoter when LM-332 expression is activated.
We find that confluent MDCK cells secrete inactive TGF-β1 apically, in agreement with Murphy et al. (2004). Our work extends these observations by describing an activation mechanism that is dependent on the integrin αVβ3, based on that the specificity of the function blocking antibody LM609 (Cheresh and Spiro, 1987; Cheresh et al., 1989; Takagi et al., 1997). Under polarized conditions, inactive TGF-β1 presumably is unable to interact with either the activation machinery dependent on αVβ3 or with its receptors, all of which are on the basolateral surface below the tight junction. Our findings are also potentially consistent with a recent study by Geng et al. (2009) who examined TGF-β signaling in the mouse proximal tubule cell line BUMPT-306, and concluded that response to exogenous activated TGF-β1, as measured by Smad phosphorylation, declined with confluence (Geng et al., 2009). Given that the cells in this study were grown on solid substrata instead of permeable supports, it is possible that the observed reduction in signaling was due, in part, to the inability of TGF-β in the medium above the cells to access its receptors sequestered below the tight junctions.
Several αV-containing integrins have been implicated in TGF-β activation. Particularly, αVβ3 has been shown to bind through an RGD motif the LAP that sequesters TGF-β (Ludbrook et al., 2003; Dabovic and Rifkin, 2008; Goodwin and Jenkins, 2009). Our results showing that soluble RGD peptides can block TGF-β1 activation (and thus LM-332 expression) support these findings. Other details about the mechanism of TGF-β1 activation in MDCK cells are unknown at this point. Some studies suggest that integrin-dependent activation of TGF-β also requires mechanical stretching of the extracellular matrix-associated LAP, metalloendoproteases, or a combination to release the active TGF-β1 (Hyytiainen et al., 2004; Dabovic and Rifkin, 2008; Wells and Discher, 2008). Indeed, some matrix metalloendoproteases are only active in subconfluent MDCK cells (Moyano, Zuk, and Matlin, unpublished), but it is not clear whether they participate in the activation mechanism. In subconfluent MDCK cells, αVβ3 is present on the free surface where it could bind the inactive complex and relocate it to the substratum for activation via mechanical tension caused by cell spreading. Further studies are necessary to discriminate between these possibilities.
Our results indicate that expression of LM-332 occurs primarily through activation of Smad2 and not Smad3. Consistent with this, transient expression of dominant-negative Smad2 but not Smad3 constructs in subconfluent MDCK cells suppresses expression of the laminin α3 mRNA. In their study of TGF-β and its receptors in MDCK cells, Murphy et al. (2004) observed activation of both Smad2 and Smad3 when active TGF-β2 was added to the basolateral side of polarized cells. The basis for the apparent discrepancy between our results and those of Murphy et al. (2004) is unclear.
It is well known that differential activation of Smad2 and Smad3 can occur, but the exact molecular basis for this and downstream consequences are not known (Brown et al., 2007; Fu et al., 2009; Petersen et al., 2010). After binding of TGF-β1 to its receptors, phosphorylation of Smads by TβR-I is regulated by accessory proteins such as Smad anchor for receptor activation (Wrana et al., 2008). In MDCK cells, it is possible that such proteins are responsible for activation of Smad2 over Smad3 under subconfluent conditions. In MDCK and other renal cells, it is conceivable that activation of Smad2 is more important in injury repair responses, whereas Smad3 is involved in more dramatic alterations in cells leading to epithelial–mesenchymal transformation and fibrosis. In support of this, Phanish et al., 2006 found that Smad2 was responsible for increases in MMP-2 expression in a transformed proximal tubule cell line, whereas changes related to epithelial–mesenchymal transdifferentiation such as loss of E-cadherin were Smad3 dependent.
Our laboratory reported previously that LM-332 expression in the rat kidney is significantly up-regulated during epithelial regeneration after ischemic injury (Zuk and Matlin, 2002). If the in vitro results reported here are applied to regenerative events in the rat kidney, then it would be anticipated that TGF-β is involved in the up-regulation of LM-332. In fact, Basile et al. (1998) reported increased levels of TGF-β1 mRNA and its receptors in the rat kidney after ischemic injury, and suggested that TGF-β1 was responsible for increased expression of a variety of extracellular matrix-related genes. Expression of laminin isoforms was not examined. Basile's group also injected neutralizing TGF-β1 antibodies into the circulation of postischemic rats and observed that the rates of renal tubular epithelial repair and improvement in physiological measures of injury were not impacted by the antibody (Spurgeon et al., 2005). Given our findings and others (Murphy et al., 2004) demonstrating that TGF-β1 is secreted apically in MDCK cells, it is reasonable to ask whether the circulating anti-TGF-β1 antibodies in the Basile experiments, which could only access the tubular epithelium by passing through the interstitial space, were effective in neutralizing TGF-β1 at the level of the epithelium. The well-recognized presence of TGF-β in the urine of normal individuals suggests that the polarity of the TGF-β response described here is not just an in vitro phenomenon (Twardzik et al., 1982; Twardzik and Sherwin, 1985; Goumenos et al., 2002).
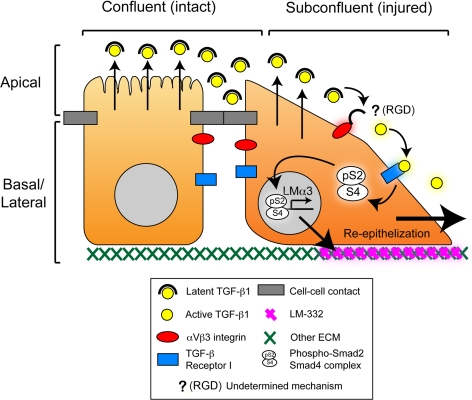
In summary, our results describe a novel mechanism for regulation of LM-332 expression in epithelial cells (Figure 9). In it, renal epithelial cells, and possibly other epithelial cells, constitutively produce all of the components necessary to initiate LM-332 expression, including latent TGF-β, its receptors, and TGF-β activation machinery. When the cells are confluent and polarized, TGF-β can neither be activated nor signal because it is separated from the activation machinery and its receptors by the tight junctional barrier. When that barrier is breached, then TGF-β signaling ensues and LM-332 expression commences without delay. To our knowledge, this is only the second example of a signaling mechanism whose regulation is dependent in part on the integrity of an epithelial barrier (Vermeer et al., 2003), and the first such instance involving a component of the extracellular matrix. An implication of this proposed mechanism is that production of LM-332 is perhaps the most, proximal event after breakdown of the epithelial barrier. As such, it is conceivable that LM-332, acting through its receptors, helps to reprogram injured epithelial cells for restoration of epithelial continuity.
Figure 9.
Proposed model for the regulation of LM-332 expression by TGF-β1 and the epithelial barrier. Confluent (polarized) epithelial cells form an intact epithelium with differentiated apical and basolateral domains. Latent TGF-β1 is secreted apically but is separated from the activation machinery (composed at least by integrin αVβ3) and the TGF-β Receptor I (TβR-I) by intact epithelial junctional complexes. When the epithelium is wounded (cell–cell contacts disrupted), latent TGF-β1 is able to interact with integrin αVβ3 which activates it by an unidentified mechanism dependent on RGD [indicated by “? (RGD)”; see Discussion]. Activated TGF-β1 then binds to TβR-I, initiating transcription and expression of LM-332 and facilitating the restoration of a continuous epithelium (reepithelialization).
ACKNOWLEDGMENTS
We thank Drs. Aki Manninen, Jonathan Jones, and Joel Collier for critically reading the manuscript and Drs. Tong-Chuan He, Kameswara Badri, and Lucia Schuger for helpful suggestions. This research was supported by National Institutes of Health [NIH] grant R01 DK-068568 and the Digestive Diseases Research Core Center of the University of Chicago grant NIH P30 DK-42086.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0523) on September 15, 2010.
REFERENCES
- Aberdam D., Virolle T., Simon-Assmann P. Transcriptional regulation of laminin gene expression. Microsc. Res. Tech. 2000;51:228–237. doi: 10.1002/1097-0029(20001101)51:3<228::AID-JEMT3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Aumailley M., et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Aumailley M., El Khal A., Knoss N., Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003;22:49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- Basile D. P., Martin D. R., Hammerman M. R. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-β in repair. Am. J. Physiol. 1998;275:F894–F903. doi: 10.1152/ajprenal.1998.275.6.F894. [DOI] [PubMed] [Google Scholar]
- Brown K. A., Pietenpol J. A., Moses H. L. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-β signaling. J. Cell. Biochem. 2007;101:9–33. doi: 10.1002/jcb.21255. [DOI] [PubMed] [Google Scholar]
- Bruckner P. Suprastructures of extracellular matrices: paradigms of functions controlled by aggregates rather than molecules. Cell Tissue Res. 2010;339:7–18. doi: 10.1007/s00441-009-0864-0. [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Mostov K. E. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Champliaud M. F., Burgeson R. E., Marinkovich M. P., Yurchenco P. D. Self-assembly of laminin isoforms. J. Biol. Chem. 1997;272:31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin αVβx) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989;57:59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- Dabovic B., Rifkin D. B. TGF-β bioavailability: latency, targeting, and activation. In: Derynck R., Miyazono K., editors. The TGF-β Family. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 179–202. [Google Scholar]
- Eaton S., Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Fitsialos G., et al. HIF1 transcription factor regulates laminin-332 expression and keratinocyte migration. J. Cell Sci. 2008;121:2992–3001. doi: 10.1242/jcs.029256. [DOI] [PubMed] [Google Scholar]
- Frank D. E., Carter W. G. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J. Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Fu Y., Chang A., Chang L., Niessen K., Eapen S., Setiadi A., Karsan A. Differential regulation of transforming growth factor β signaling pathways by Notch in human endothelial cells. J. Biol. Chem. 2009;284:19452–19462. doi: 10.1074/jbc.M109.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H., et al. Inhibition of autoregulated TGF-β signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am. J. Pathol. 2009;174:1291–1308. doi: 10.2353/ajpath.2009.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Stack M. S. Proteolytic modification of laminins: functional consequences. Microsc. Res. Tech. 2000;51:238–246. doi: 10.1002/1097-0029(20001101)51:3<238::AID-JEMT4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Giannelli G., Antonaci S. Biological and clinical relevance of Laminin-5 in cancer. Clin. Exp. Metastasis. 2000;18:439–443. doi: 10.1023/a:1011879900554. [DOI] [PubMed] [Google Scholar]
- Goldfinger L. E., Jiang L., Hopkinson S. B., Stack M. S., Jones J. C. Spatial regulation and activity modulation of plasmin by high affinity binding to the G domain of the α3 subunit of laminin-5. J. Biol. Chem. 2000;275:34887–34893. doi: 10.1074/jbc.M006652200. [DOI] [PubMed] [Google Scholar]
- Goodwin A., Jenkins G. Role of integrin-mediated TGF-β activation in the pathogenesis of pulmonary fibrosis. Biochem. Soc. Trans. 2009;37:849–854. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- Gottardi C. J., Dunbar L. A., Caplan M. J. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am. J. Physiol. 1995;268:F285–F295. doi: 10.1152/ajprenal.1995.268.2.F285. [DOI] [PubMed] [Google Scholar]
- Goumenos D. S., Tsakas S., El Nahas A. M., Alexandri S., Oldroyd S., Kalliakmani P., Vlachojannis J. G. Transforming growth factor-β1 in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol. Dial. Transplant. 2002;17:2145–2152. doi: 10.1093/ndt/17.12.2145. [DOI] [PubMed] [Google Scholar]
- Guess C. M., Quaranta V. Defining the role of laminin-332 in carcinoma. Matrix Biol. 2009;28:445–455. doi: 10.1016/j.matbio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelers I. H., Olivo C., Mertens A. E., Pegtel D. M., van der Kammen R. A., Sonnenberg A., Collard J. G. The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J. Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill K. J., McLean W. H. The α3 polypeptide chain of laminin 5, insight into wound healing responses from the study of genodermatoses. Clin. Exp. Dermatol. 2005;30:398–404. doi: 10.1111/j.1365-2230.2005.01842.x. [DOI] [PubMed] [Google Scholar]
- Hyytiainen M., Penttinen C., Keski-Oja J. Latent TGF-β binding proteins: extracellular matrix association and roles in TGF-β activation. Crit. Rev. Clin. Lab. Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- Inman G. J., Nicolás F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Jamora C., Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Dehart G. W., Gonzales M., Goldfinger L. E. Laminins: an overview. Microsc. Res. Tech. 2000;51:211–213. doi: 10.1002/1097-0029(20001101)51:3<211::AID-JEMT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Hopkinson S. B., Goldfinger L. E. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Korang K., Christiano A. M., Uitto J., Mauviel A. Differential cytokine modulation of the genes LAMA3, LAMB3, and LAMC2, encoding the constitutive polypeptides, α3, β3, and γ2, of human laminin 5 in epidermal keratinocytes. FEBS Lett. 1995;368:556–558. doi: 10.1016/0014-5793(95)00740-z. [DOI] [PubMed] [Google Scholar]
- Korbie D. J., Mattick J. S. Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat. Protoc. 2008;3:1452–1456. doi: 10.1038/nprot.2008.133. [DOI] [PubMed] [Google Scholar]
- Ledbetter S., Kurtzberg L., Doyle S., Pratt B. M. Renal fibrosis in mice treated with human recombinant transforming growth factor-β2. Kidney Int. 2000;58:2367–2376. doi: 10.1046/j.1523-1755.2000.00420.x. [DOI] [PubMed] [Google Scholar]
- Li S., Edgar D., Fassler R., Wadsworth W., Yurchenco P. D. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Ludbrook S. B., Barry S. T., Delves C. J., Horgan C. M. The integrin αVβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem. J. 2003;369:311–318. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak G. Z., Kavanaugh G. M., Buschmann M. M., Stickley S. M., Koch M., Goss K. H., Waechter H., Zuk A., Matlin K. S. Regulated synthesis and functions of laminin 5 in polarized Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2006;17:3664–3677. doi: 10.1091/mbc.E05-11-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C., Raymond K., Kreft M., Sachs N., Janssen H., Sonnenberg A. Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J. Cell Sci. 2009;122:278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- Marinkovich M. P. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- Masunaga T. Epidermal basement membrane: its molecular organization and blistering disorders. Connect Tissue Res. 2006;47:55–66. doi: 10.1080/03008200600584157. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. A., Chung J., Lo D., Jones J. C., Thimmapaya B., Weitzman S. A. Inhibition of laminin-5 production in breast epithelial cells by overexpression of p300. J. Biol. Chem. 2000;275:8176–8182. doi: 10.1074/jbc.275.11.8176. [DOI] [PubMed] [Google Scholar]
- Miller K. A., Eklund E. A., Peddinghaus M. L., Cao Z., Fernandes N., Turk P. W., Thimmapaya B., Weitzman S. A. Kruppel-like factor 4 regulates laminin α3A expression in mammary epithelial cells. J. Biol. Chem. 2001;276:42863–42868. doi: 10.1074/jbc.M108130200. [DOI] [PubMed] [Google Scholar]
- Miner J. H., Yurchenco P. D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Murphy S. J., Dore J. J., Edens M., Coffey R. J., Barnard J. A., Mitchell H., Wilkes M., Leof E. B. Differential trafficking of transforming growth factor-β receptors and ligand in polarized epithelial cells. Mol. Biol. Cell. 2004;15:2853–2862. doi: 10.1091/mbc.E04-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B. P., Ryan M. C., Gil S. G., Carter W. G. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr. Opin. Cell Biol. 2000;12:554–562. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Jou T. S., Pollack A. L., Zhang Q., Hansen S. H., Yurchenco P., Mostov K. E. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Odenthal U., Haehn S., Tunggal P., Merkl B., Schomburg D., Frie C., Paulsson M., Smyth N. Molecular analysis of laminin N-terminal domains mediating self-interactions. J. Biol. Chem. 2004;279:44504–44512. doi: 10.1074/jbc.M402455200. [DOI] [PubMed] [Google Scholar]
- Olsen J., Lefebvre O., Fritsch C., Troelsen J. T., Orian-Rousseau V., Kedinger M., Simon-Assmann P. Involvement of activator protein 1 complexes in the epithelium-specific activation of the laminin gamma2-chain gene promoter by hepatocyte growth factor (scatter factor) Biochem. J. 2000;347:407–417. doi: 10.1042/0264-6021:3470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M., Pardali E., van der Horst G., Cheung H., van den Hoogen C., van der Pluijm G., Ten Dijke P. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene. 2010;29:1351–1361. doi: 10.1038/onc.2009.426. [DOI] [PubMed] [Google Scholar]
- Phanish M. K., Wahab N. A., Colville-Nash P., Hendry B. M., Dockrell M. E. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGF-β1 responses in human proximal-tubule epithelial cells. Biochem J. 2006;393:601–607. doi: 10.1042/BJ20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana U. G., Padar A., Huang C. X., Suzuki M., Shigematsu H., Bekele B. N., Gazdar A. F. Aberrant promoter methylation and silencing of laminin-5-encoding genes in breast carcinoma. Clin. Cancer Res. 2003a;9:6389–6394. [PubMed] [Google Scholar]
- Sathyanarayana U. G., Toyooka S., Padar A., Takahashi T., Brambilla E., Minna J. D., Gazdar A. F. Epigenetic inactivation of laminin-5-encoding genes in lung cancers. Clin Cancer Res. 2003b;9:2665–2672. [PubMed] [Google Scholar]
- Schilling S. H., Hjelmeland A. B., Rich J. N., Wang X.-F. TGF-β: a multipotential cytokine. In: Derynck R., Miyazono K., editors. The TGF-β Family. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 45–77. [Google Scholar]
- Schmierer B., Hill C. S. TGF-β-SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Schock F., Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 2002;18:463–493. doi: 10.1146/annurev.cellbio.18.022602.131838. [DOI] [PubMed] [Google Scholar]
- Schoenenberger C. A., Zuk A., Zinkl G. M., Kendall D., Matlin K. S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J. Cell Sci. 1994;107:527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Sehgal B. U., DeBiase P. J., Matzno S., Chew T. L., Claiborne J. N., Hopkinson S. B., Russell A., Marinkovich M. P., Jones J. C. Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J. Biol. Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon K. R., Donohoe D. L., Basile D. P. Transforming growth factor-β in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am. J. Physiol. Renal Physiol. 2005;288:F568–F577. doi: 10.1152/ajprenal.00330.2004. [DOI] [PubMed] [Google Scholar]
- Takagi J., Kamata T., Meredith J., Puzon-McLaughlin W., Takada Y. Changing ligand specificities of αVβ1 and αVβ3 integrins by swapping a short diverse sequence of the β subunit. J. Biol. Chem. 1997;272:19794–19800. doi: 10.1074/jbc.272.32.19794. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Sherwin S. A. Transforming growth factor (TGF) activity in human urine: synergism between TGF-β and urogastrone. J. Cell. Biochem. 1985;28:289–297. doi: 10.1002/jcb.240280407. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Sherwin S. A., Ranchalis J., Todaro G. J. Transforming growth factors in the urine of normal, pregnant, and tumor-bearing humans. J. Natl. Cancer Inst. 1982;69:793–798. [PubMed] [Google Scholar]
- Vermeer P. D., Einwalter L. A., Moninger T. O., Rokhlina T., Kern J. A., Zabner J., Welsh M. J. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- Virolle T., Coraux C., Ferrigno O., Cailleteau L., Ortonne J. P., Pognonec P., Aberdam D. Binding of USF to a non-canonical E-box following stress results in a cell-specific derepression of the lama3 gene. Nucleic Acids Res. 2002;30:1789–1798. doi: 10.1093/nar/30.8.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virolle T., Monthouel M. N., Djabari Z., Ortonne J. P., Meneguzzi G., Aberdam D. Three activator protein-1-binding sites bound by the Fra-2.JunD complex cooperate for the regulation of murine laminin α3A (lama3A) promoter activity by transforming growth factor-β. J. Biol. Chem. 1998;273:17318–17325. doi: 10.1074/jbc.273.28.17318. [DOI] [PubMed] [Google Scholar]
- Wells R. G., Discher D. E. Matrix elasticity, cytoskeletal tension, and TGF-β: the insoluble and soluble meet. Sci. Signal. 2008;1:pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Ozdamar B., Le Roy C., Benchabane H. Signaling receptors of the TGF-β family. In: Derynck R., Miyazono K., editors. The TGF-β Family. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 151–177. [Google Scholar]
- Yu W., Datta A., Leroy P., O'Brien L. E., Mak G., Jou T. S., Matlin K. S., Mostov K. E., Zegers M. M. β1-Integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Patton B. L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Wadsworth W. G. Assembly and tissue functions of early embryonic laminins and netrins. Curr. Opin. Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Zboralski D., Bockmann M., Zapatka M., Hoppe S., Schoneck A., Hahn S. A., Schmiegel W., Schwarte-Waldhoff I. Divergent mechanisms underlie Smad4-mediated positive regulation of the three genes encoding the basement membrane component laminin-332 (laminin-5) BMC Cancer. 2008;8:215. doi: 10.1186/1471-2407-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Feng X., We R., Derynck R. Receptor-associated Smad homologues synergize as effectors of the TGF-β response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- Zuk A., Matlin K. S. Induction of a laminin isoform and α3β1 integrin in renal ischemic injury and repair in vivo. Am. J. Physiol. Renal Physiol. 2002;283:F971–F984. doi: 10.1152/ajprenal.00176.2002. [DOI] [PubMed] [Google Scholar]