Abstract
Background
Smoking cessation is associated with weight gain, but the effects of smoking cessation on measures of body composition (BC) have not been adequately evaluated. The purpose of this study is to examine the effects of 16 months of cigarette abstinence on areas of BC measured by dual-energy x-ray absorptiometry (DXA).
Methods
One hundred fifty-two postmenopausal women participated in a smoking cessation study using the nicotine patch. Secondary analyses were conducted on data from 119 subjects (age 56 ± 7 years, range 41–78 years) who had had DXA scans at baseline and 16 months later. Participants were classified either as quitters (self-reported cigarette abstinence confirmed with exhaled carbon monoxide [co] ≤8 ppm at 3 and 16 months after quit date) or as continued smokers. BC was assessed using a General Electric Lunar DXA IQ machine. Four areas of BC (kg) were measured: whole body weight, fat mass, muscle mass, and functional skeletal muscle mass in arms and legs (ASM/ht2). Multivariate analysis of covariance (MANCOVA) assessed changes in BC in quitters vs. continued smokers between baseline and 16 months of follow-up. Increases in BC measures were evaluated as a function of increased calorie intake or change in physical activity, using linear regression.
Results
Quitters significantly increased body weight (p < 0.001), fat mass (p < 0.001), muscle mass (p = 0.04), and functional muscle mass (p = 0.004) over time, when baseline BC measures and other confounding factors were controlled. Regression analysis indicated change in BC could not be accounted for by calorie intake or physical activity.
Conclusions
Smoking cessation may be associated with increased fat and muscle mass in postmenopausal women. The novel finding of an increase in functional muscle mass suggests that smoking cessation could increase functional capacity. Further studies need to replicate these findings and examine mechanisms of these effects.
Introduction
Cigarette smoking cessation is associated with weight gain (i.e., 6–12 pounds/2.7–5.4 kg) in the first year of abstinence.1–6 The effect of smoking cessation on weight gain may be even greater in postmenopausal women, when weight gain naturally occurs.6 The mechanisms underlying weight gain with smoking cessation are likely multifaceted and may include increased calorie intake, decreased metabolism, and changes in physical activity.4,7–10 Weight gain from smoking cessation not only has become a barrier to quitting but also increases the risk of relapse among female smokers.11–15
Although the association between smoking cessation and weight gain is well established, it is not known if the effect is through increases in body fat, muscle, or both. The relationship between smoking and body fat is complex, with cross-sectional studies showing that smokers have higher waist circumference (a measure of central adiposity) than nonsmokers and also lower body mass index (BMI)16–21 and decreased overall body fat.22 In older populations, smoking is also associated with an increased risk of sarcopenia (age-related muscle loss).23–25 Consequently, it seems possible that smoking cessation could increase lean and fat tissue. If smoking cessation increases muscle mass, this may be an added inducement to persuade people to quit smoking.
The purpose of this report was to examine the effects of smoking cessation on body composition (BC) in postmenopausal women. We conducted a secondary analysis of a randomized, longitudinal trial evaluating nicotine vs. placebo patch treatment (3 month treatment and 1 month taper) on long-term smoking cessation rates in postmenopausal women.6,26 Dual-energy x-ray absorptiometry (DXA) scans were obtained before smoking cessation and again 16 months later.6 In addition to measuring bone mineral density (BMD), DXA technology provides a valid measurement of BC (lean and fat tissue) as well as functional muscle mass (appendicular skeletal mass/height2 [ASM/ht2]).27,28 We are not aware of any studies to date that have evaluated the effects of smoking cessation on changes in these BC measures.
Materials and Methods
Subjects
One hundred fifty-two postmenopausal women participated in a smoking cessation study examining the adjunctive use of the nicotine vs. placebo patch combined with group behavioral counseling for smoking cessation.6 This study was approved by the institutional review board at the University of Connecticut, and all subjects signed a written informed consent form.
Women were eligible if they smoked at least 10 cigarettes per day and were postmenopausal (i.e., no menstruation for at least 1 year). Exclusion criteria included (1) taking oral corticosteroids, antiepileptics, or medication for prevention/treatment of osteoporosis other than hormone replacement therapy (HRT), (2) untreated parathyroid or thyroid disease, multiple myeloma, or insulin-dependent diabetes mellitus, (3) a recent fracture, (4) an ionized calcium concentration >1.36 mmol/L, (5) consuming more than two alcoholic drinks per day, (6) using nicotine replacement or tobacco products other than cigarettes, (7) an unstable psychiatric status (i.e., current major depressive disorder [MDD] as determined by a Structured Clinical Interview for DSM-IV (SCID) interview29 or psychosis); women who were currently taking antidepressants and who did not meet criteria for current MDD were not excluded from study participation, and (8) an unstable medical condition.
Women were randomly assigned with a 3:5 randomization scheme to nicotine patch (21 mg for 3 months, 14 mg for 2 weeks, 7 mg for 2 weeks) or placebo patch and received group behavioral counseling for smoking cessation. In addition, women were followed for approximately 12 months after patch discontinuation for cigarette abstinence and other measures. There was no overall advantage of the nicotine vs. the placebo patch on quit rates (35% quit rate in both groups) at the end of the follow-up period.6 We, therefore, combined the participants from both conditions for the analyses presented here. Complete data, including DXA scans at visit 2 (prior to treatment) and at visit 8 (16 months after quit date) were obtained for 119 women. At 16 months, 42 (35.3%) participants were considered abstinent from smoking (quitters), and 77 (64.7%) remained smokers.
Measures and instruments
Outcome variables
Four measures of BC were examined in this study: total body weight (kg), lean muscle (kg), fat tissue (kg), and functional muscle (ASM/ht2 in kg/m2). Tissue masses were determined using DXA with a DXA IQ scanner (GE Medical Systems Lunar, Madison, WI). All scans were obtained by the same certified technician. DXA is a valid and reliable means to collect total body fat and lean muscle.27 DXA measures BC by distinguishing between the soft tissue mass and the bone mineral content. The coefficients of variation (CV) for BC measurements (based on reproducibility scans) were 2.98% for fat and 1.85% for lean tissue. Fat tissue is separated from the soft tissue using the DXA machine software. Lean tissue is calculated from soft tissue by subtracting out the fat tissue. ASM was determined by combining the lean tissue mass of the arms and legs, excluding all other body regions from analysis.28 We adjusted ASM (kg) for height by dividing each by height2 (m2). Height was measured using a stadiometer, and total body weight was calculated by the DXA software as total body mass (kg). Previous studies define ASM/ht2 as functional muscle, as it includes the arm and leg skeletal muscles, which aid in our ability to move.28
Smoking cessation status
Subjects were categorized as quitters if they reported 7-day point prevalence abstinence confirmed with an exhaled carbon monoxide (CO) value of <8 ppm at visit 7 (3 months) and again at visit 8 (16 months).30 Subjects who did not report abstinence at visits 7 and 8 were considered smokers.
Potential mediators
Conceptually, mediators are factors through which interventions influence outcomes. Mediators lie on the causal path between the independent and dependent variables.31 It was hypothesized that smoking cessation could affect BC by altering food intake and exercise; both of which affect either calorie intake or calorie metabolism. Food intake was assessed using a 4-day food diary. Physical activity level was measured using the Physical Activity Scale for the Elderly (PASE). These were measured at baseline and at 3 months.
Food diary
One documented effect of smoking is to suppress appetite.8 Increase in appetite has been suggested as one reason that smoking cessation causes weight gain. To evaluate calorie intake, a 4-day example of each participants' daily eating habits was entered on dietary records. The dietary records were analyzed by using Food Processor II Nutrient Analysis Program software (ESHA Research, Inc., Salem, OR). Calorie intake was obtained from this food diary analysis.
PASE
BC may change with increased physical activity and, thus, mitigate other effects of quitting on weight gain. The PASE is a relatively brief, reliable, and valid instrument for the assessment of physical activity in older people. It has high test-retest reliability of 0.75 and has been correlated with measures of physical fitness, such as grip strength, leg strength, and balance.32
Statistical analyses
All continuous variables were checked for normality. Correlations were run to investigate any associations among predictors and outcomes. Baseline characteristics between treatment groups were compared using analysis of variance (ANOVA) or chi-square tests. Multivariate analyses of covariance (MANCOVA) with univariate posttests were used to determine if smoking cessation was associated with BC after 16 months. The four outcome variables assessed in the MANCOVA were total body weight (kg), lean mass (kg), fat mass (kg), and ASM/ht2 (kg/m2) at 16 months. Univariate posttests were performed to evaluate the effects of smoking cessation between smokers and quitters on each outcome independently. Covariates for the MANCOVA were the baseline values of all four BC measures.
A MANCOVA was also used to test the effects of smoking cessation on two potential mediators of weight gain (calorie intake and physical activity measured by PASE activity score), adjusting for baseline BC measures. Both mediator variables were measured at 3 months. Mediation analyses were conducted using four hierarchical linear regression analyses, one for each BC-dependent variable. For each analysis, the BC measures were evaluated as a function of smoking cessation (smokers vs. abstainers). In block 1, the following covariates were entered: baseline BC measure, age, nicotine treatment, number of cigarettes smoked per day at baseline, HRT use, antidepressant use, and years since menopause. In block 2, the mediator variables (calorie intake and PASE score) were entered as residualized change scores (i.e., calorie count or PASE score at 3 months after quit date with the baseline calories or PASE score value covaried out). All statistics were performed using SPSS version 16.0 software (SPSS, Chicago, IL).
Results
Baseline demographics and BC were not significantly different between the smokers and quitters (Table 1), with the exception of years postmenopause, number of cigarettes smoked per day, and antidepressant use. Smokers were 11 years postmenopausal, whereas quitters averaged 14 years (p < 0.05). Quitters smoked an average of 18 cigarettes a day at study entry, and continued smokers averaged 23 cigarettes per day (p < 0.05). Smokers were taking more antidepressants than quitters (p < 0.01). Physical activity (PASE score) was comparable between continued smokers and quitters (171 vs. 151, p = 0.18). The use of HRT was no different between smokers and nonsmokers; however, quitters were less likely to have a history of MDD, as previously reported.29
Table 1.
Baseline Characteristics
| Variable | Quitters (n = 42) | Smokers (n = 77) | p valuea |
|---|---|---|---|
| Age | 57.2 ± 7.4 | 55.0 ± 6.8 | 0.08 |
| Body mass index | 27.0 ± 5.6 | 26.9 ± 5.0 | 0.98 |
| Weight, kg | 68.9 ± 15.5 | 70.0 ± 14.4 | 0.70 |
| Whole body fat mass, kg | 27.1 ± 11.2 | 27.4 ± 10.4 | 0.84 |
| Whole body lean mass, kg | 39.4 ± 5.3 | 40.0 ± 5.1 | 0.51 |
| ASM/ht2, kg/m2 | 6.22 ± 0.77 | 6.21 ± 0.81 | 0.93 |
| Arm lean mass, kg | 4.1 ± 0.7 | 4.2 ± 0.7 | 0.54 |
| Leg lean, kg | 12.1 ± 1.9 | 12.3 ± 1.9 | 0.67 |
| Arm fat, % | 34.8 ± 9.4 | 35.5 ± 9.5 | 0.67 |
| Leg fat, % | 42.1 ± 8.7 | 40.9 ± 7.2 | 0.43 |
| Cigarettes smoked per day | 17.8 ± 4.9 | 22.9 ± 8.8 | 0.001 |
| Number of years smoked | 33.5 ± 11.3 | 34.8 ± 9.6 | 0.46 |
| Nicotine intervention patch, % | 38 | 40 | 0.82 |
| Years since menopause | 14.5 ± 10.1 | 10.6 ± 8.1 | 0.03 |
| Antidepressant medication, % | 15 | 43 | 0.01 |
| HRT use, % | 49 | 49 | 0.94 |
| Physical activity level or PASE score | 171 ± 86 | 151 ± 77 | 0.18 |
| Daily calorie intake | 1719 ± 584 | 1660 ± 431 | 0.54 |
| Caucasian, % | 86 | 90 | 0.22 |
| High school education or higher, % | 72 | 72 | 0.75 |
p values were based on independent t tests or chi-square tests.
ASM/ht2, appendicular skeletal mass/height2; HRT, hormone replacement therapy; PASE, physical activity scale for the elderly.
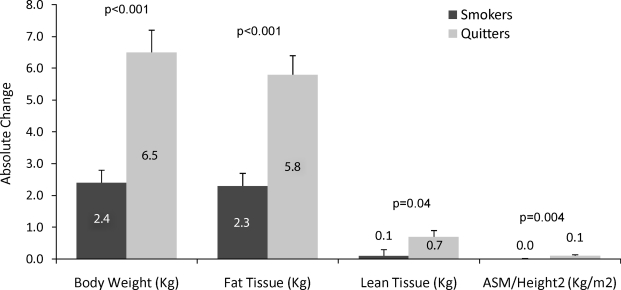
Overall, our analysis of BC showed a significant effect from smoking cessation, such that those who quit smoking gained significantly more weight. Women who had quit smoking significantly increased in body weight, fat mass, lean mass, and ASM/ht2 (multivariate tests, Wilks' λ = 0.76, p < 0.001). Quitters gained 14 pounds (6.5 kg); most of the weight was fat (13 pounds/5.8 kg), 1.5 pounds (0.7 kg) was muscle, and <0.5 pound (0.1 kg/m2) was functional muscle. Figure 1 displays unadjusted absolute changes in each type of BC measure.
FIG. 1.
Unadjusted body composition changes in smokers vs. quitters after 16 months. p values based on univariate posttests from the MANCOVA analysis. Bars represent the mean absolute change from baseline to 16 months in each body composition measures. Error bars represent the variability within each smoking status grouping. MANCOVA, multivariate analysis of covariance.
The MANCOVA results revealed smoking cessation as a significant predictor of all BC measures. Univariate posttests comparing quitters and smokers confirmed that body weight, fat, lean muscle, and functional muscle significantly increased in quitters (Fig. 1). MANCOVA results for the mediators showed that smoking cessation was not significantly associated with changes in physical activity levels or calorie intake. Univariate posttests confirmed no differences between quitters and continued smokers. Although the difference was not significant, the average calorie intake in quitters at 3 months was marginally higher (1712 ± 602) than in continued smokers (1547 ± 538) (p = 0.12). The average PASE score was lower in quitters (169 ± 75) than in smokers (175 ± 91) (p = 0.15). Quitters reported eating more calories daily and engaging in less physical activity than smokers, but these differences were not statistically significant.
Each of the linear regression models confirmed that smoking cessation, adjusting for all of the covariates, maintained a significant effect on each of the four different types of BC (Table 2). The percent of explained variance for these models was very high (≥89%). Smoking cessation remained a significant predictor of BC even when mediators (calorie intake and PASE change scores) were added in block 2 of each of the models. Given the failure of calorie intake change or PASE score change to account for any of the variance attributable to smoking cessation, the results indicate that smoking cessation did not act to increase BC by increasing calorie intake or by decreasing physical activity.
Table 2.
Effects of Smoking Cessation on Body Composition: 2 Blocks of Linear Regression Models
| |
|
β |
95% CI |
t |
p value |
R2 |
β |
95% CI |
t |
p value |
R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable at 16 months | Variables entered | Block 1 | Block 2 | ||||||||
| Body weight | Age | −0.07 | −0.23-0.09 | −0.85 | 0.22 | 0.94 | −0.04 | −0.22-0.13 | −0.47 | 0.64 | 0.93 |
| Body weight (kg) at baseline | 0.99 | 0.91-1.03 | 32.67 | <0.001 | 0.97 | 0.91-1.04 | 30.47 | <0.001 | |||
| Treatment group | 0.47 | −1.49-2.43 | 0.48 | 0.63 | 1.12 | −1.05-3.28 | 1.03 | 0.31 | |||
| Number of cigarettes/day at baseline | 0.11 | −0.00-0.21 | 2.06 | 0.04 | 0.13 | 0.02-0.25 | 2.37 | 0.02 | |||
| HRT use | 1.59 | −0.46-3.64 | 1.55 | 0.13 | 1.06 | −1.32-3.45 | 0.89 | 0.37 | |||
| Antidepressant medications | 0.64 | −1.42-2.70 | 0.62 | 0.54 | 0.24 | −2.11-2.58 | 0.20 | 0.84 | |||
| Years since menopause | 0.08 | −0.04-0.20 | 1.37 | 0.17 | 0.02 | −0.08-0.20 | 0.36 | 0.72 | |||
| Smoking cessation | 5.09 | 3.02-7.15 | 4.91 | <0.001 | 5.58 | 3.43-7.72 | 5.19 | <0.001 | |||
| Calorie intake residualized change | 0.003 | 0.001-0.005 | 2.55 | 0.01 | |||||||
| PASE residualized change | −0.005 | −0.02-0.01 | −0.76 | 0.45 | |||||||
| Fat mass (kg) | Age | −0.02 | −0.15-0.12 | −0.29 | 0.77 | 0.90 | 0.002 | −0.15-0.16 | 0.03 | 0.98 | 0.89 |
| Fat mass (kg) at baseline | 0.92 | 0.85-0.99 | 25.64 | <0.001 | 0.91 | 0.84-0.99 | 23.15 | <0.001 | |||
| Treatment group | 0.50 | −1.18-2.18 | 0.59 | 0.55 | 0.99 | −0.92-2.90 | 1.04 | 0.30 | |||
| Number of cigarettes/day at baseline | 0.08 | −0.00-0.17 | 1.88 | 0.06 | 0.10 | 0.005-0.20 | 2.10 | 0.04 | |||
| HRT use | 1.88 | 0.13-3.64 | 2.14 | 0.04 | 1.40 | −0.69-3.50 | 1.34 | 0.19 | |||
| Antidepressant medications | 1.12 | −0.64-2.89 | 1.27 | 0.21 | 0.62 | −1.45-2.69 | 0.60 | 0.55 | |||
| Years since menopause | 0.07 | −0.03-0.17 | 1.30 | 0.20 | 0.03 | −0.09-0.15 | 0.52 | 0.60 | |||
| Smoking cessation | 4.48 | 2.71-6.25 | 5.05 | <0.001 | 4.85 | 2.96-6.73 | 5.13 | <0.001 | |||
| Calorie intake residualized change | 0.002 | 0.00-0.004 | 1.69 | 0.10 | |||||||
| PASE residualized change | −0.004 | −0.015-0.008 | −0.64 | 0.53 | |||||||
| Lean mass (kg) | Age | −0.04 | −0.10-0.02 | −1.21 | 0.23 | 0.93 | −0.03 | −0.09-0.04 | −0.88 | 0.38 | 0.94 |
| Lean mass (kg) at baseline | 1.03 | 0.96-1.09 | 31.57 | <0.001 | 1.03 | 0.97-1.10 | 31.41 | <0.001 | |||
| Treatment group | −0.15 | −0.89-0.60 | −0.39 | 0.70 | −0.006 | −0.79-0.78 | −0.016 | 0.99 | |||
| Number of cigarettes/day at baseline | 0.02 | −0.02-0.06 | 1.12 | 0.27 | 0.03 | −0.01-0.07 | 1.28 | 0.21 | |||
| HRT use | −0.25 | −1.02-0.53 | −0.63 | 0.53 | −0.21 | −1.06-0.65 | −0.48 | 0.63 | |||
| Antidepressant medications | −0.38 | −1.15-0.40 | −0.97 | 0.34 | −0.19 | −1.03-0.65 | −0.45 | 0.65 | |||
| Years since menopause | 0.01 | −0.03-0.06 | 0.65 | 0.52 | −0.01 | −0.06-0.04 | −0.44 | 0.66 | |||
| Smoking cessation | 0.68 | −0.10-1.46 | 1.73 | 0.09 | 0.81 | 0.04-1.58 | 2.10 | 0.04 | |||
| Calorie intake residualized change | 0.001 | 0.00-0.002 | 2.83 | 0.006 | |||||||
| PASE residualized change | −0.001 | −0.006-0.003 | −0.61 | 0.54 | |||||||
| ASM/ht2 (kg/m2) | Age | −0.01 | −0.02-−0.002 | −2.29 | 0.02 | 0.91 | −0.01 | −0.03-−0.003 | −2.48 | 0.02 | 0.90 |
| ASM/ht2 at baseline | 0.98 | 0.90-1.05 | 25.97 | <0.001 | 1.01 | 0.93-1.09 | 24.24 | <0.001 | |||
| Treatment group | 0.03 | −0.10-0.16 | 0.52 | 0.61 | 0.06 | −0.08-0.21 | 0.88 | 0.38 | |||
| Number of cigarettes/day at baseline | 0.005 | −0.002-0.012 | 1.29 | 0.20 | 0.005 | −0.003-0.012 | 1.32 | 0.19 | |||
| HRT use | −0.12 | −0.26-0.017 | −1.75 | 0.08 | −0.17 | −0.33-−0.013 | −2.16 | 0.03 | |||
| Antidepressant medications | −0.05 | −0.19-0.09 | −0.71 | 0.48 | −0.07 | −0.23-0.08 | −0.92 | 0.36 | |||
| Years since menopause | 0.002 | −0.006-0.01 | 0.51 | 0.61 | 0.002 | −0.007-0.01 | 0.47 | 0.64 | |||
| Smoking cessation | 0.15 | 0.015-0.29 | 2.20 | 0.03 | 0.15 | 0.01-0.30 | 2.14 | 0.04 | |||
| Calorie intake residualized change | 0.00 | 0.00-0.00 | 1.89 | 0.06 | |||||||
| PASE residualized change | 0.00 | 0.00-0.001 | −0.12 | 0.91 | |||||||
CI, confidence interval.
Discussion
The results of this study indicated that all measures of BC were influenced by smoking cessation. Sustained abstinence from smoking was associated with increases in total body weight, fat mass, and lean and functional muscle mass. Most of the weight gain in those who quit smoking was due to an increase in fat mass, although there was also a statistically significant increase in lean and functional muscle mass. We consider this a novel and important finding on the benefits of quitting smoking. Functional muscle changes were not large in this project, but they may be clinically significant.
The results of this study are consistent with those of other studies indicating that smoking cessation is associated with an increased body weight. Further, it extends the literature by showing that the majority of weight gain was due to an increase in body fat. Given that caloric changes and physical activity did not significantly change with quitting, it seems probable that most of the changes in body weight resulted from changes in metabolism, as noted in some previous studies.7–10
An interesting finding was that quitting was also associated with an increase in lean and functional muscle. This finding is consistent with a few cross-sectional studies that show that smoking is associated with an increased risk of sarcopenia.23–25 As far as we are aware, this is the first study to examine the effects of smoking cessation on muscle mass. Although the mechanisms by which smoking causes sarcopenia are unknown, two recent studies found that smoking impairs protein muscle synthesis and increases expression of genes associated with inhibition of muscle growth and catabolism.33,34 There may be a metabolic effect by which smoking impairs muscle synthesis, whereas there may be an epigenetic mechanism where smoking increases expression of genes associated with impaired muscle functioning. Future studies could further elucidate mechanisms, examine potential gene by environment interactions, and evaluate whether changes in functional muscle mass with smoking cessation translate into improved functional capacity. Future directions may also include determining if adding an exercise program to smoking treatment increases cessation rates and has further beneficial effects on BC measures (i.e., increases functional muscle mass and decreases in fat mass) in postmenopausal women.
It is noteworthy that, if replicated, the results of this study may be useful clinically. Weight-concerned smokers have poorer smoking treatment outcomes compared with smokers with lower levels of weight concerns.8 Informing women that some of the weight gain may be due to an increase in muscle mass could help women better accept weight gain with smoking cessation and thereby improve smoking treatment outcomes.
The strengths of this study are a well-defined subject population, biochemical measures of cigarette abstinence, and good retention rates, given the length of follow-up. Limitations of the study include some missing mediator values at 3 months (particularly in the smokers) that could influence regression outcomes. There was no measure to account for metabolism changes over the 16 months that may have influenced BC. Measurements of muscle strength (e.g., hand grip) that were used in other studies examining sarcopenia23,25 could have been useful in examining whether even small changes in functional muscle were clinically significant. Waist/hip ratio measurements could have been useful to determine if distribution of body fat may have been altered with smoking cessation.
In conclusion, smoking cessation is associated with an increase in fat and muscle mass in postmenopausal women. Further studies are needed to replicate these findings and to examine mechanisms of these effects.
Acknowledgments
This study was funded by The Patrick and Catherine Weldon Donaghue Medical Research Foundation and the General Clinical Research Center and the University of Connecticut M01RR6192 and by grant R01 DA024872. We thank the Claude Pepper Older American Independence Center for originally supporting this project.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Flegal KM. Troiano RP. Pamuk ER. Kuczmarski RJ. Campbell SM. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;333:1165–1170. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA. Segal MR. Myers AH. Stampfer MJ. Willett W. Speizer FE. Weight change in relation to smoking cessation in women. J Smoking Rel Dis. 1992;3:145–153. [Google Scholar]
- 3.Ferrara CM. Kumar M. Nicklas B, et al. Weight gain and adipose tissue metabolism after smoking cessation in women. Int J Obes Rel Metab Disord. 2001;25:1322–1326. doi: 10.1038/sj.ijo.0801716. [DOI] [PubMed] [Google Scholar]
- 4.Filozof C. Fernandez Pinilla MC. Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 5.Williamson DF. Madans J. Anda RF. Kleinman JC. Giovino GA. Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 6.Oncken C. Prestwood K. Kleppinger A. Wang Y. Cooney J. Raisz L. Impact of smoking cessation on bone mineral density in postmenopausal women. J Womens Health. 2006;15:1141–1150. doi: 10.1089/jwh.2006.15.1141. [DOI] [PubMed] [Google Scholar]
- 7.Chiolero A. Faeh D. Paccaud F. Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 8.Perkins KA. Marcus MD. Levine MD, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consul Clin Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- 9.Berlin I. Endocrine and metabolic effects of smoking cessation. Curr Med Res Opin. 2009;25:527–534. doi: 10.1185/03007990802707626. [DOI] [PubMed] [Google Scholar]
- 10.French SA. Hennrikus DJ. Jeffery RW. Smoking cessation, dietary intake, and physical activity in a sample of working adults. Health Psychol. 1996;15:448–454. doi: 10.1037//0278-6133.15.6.448. [DOI] [PubMed] [Google Scholar]
- 11.Nides MA. Rakos RF. Gonzales D, et al. Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. J Consul Clin Psychol. 1995;63:60–69. doi: 10.1037//0022-006x.63.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen G. Pechacek TF. Attitudes toward smoking cessation among men and women. J Behav Med. 1987;10:129–137. doi: 10.1007/BF00846421. [DOI] [PubMed] [Google Scholar]
- 13.French SA. Jeffery RW. Klesges LM. Forster JL. Weight concerns and change in smoking behavior over two years in a working population. Am J Public Health. 1995;85:720–722. doi: 10.2105/ajph.85.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French SA. Jeffery RW. Pirie PL. McBride CM. Do weight concerns hinder smoking cessation efforts? Addict Behav. 1992;17:219–226. doi: 10.1016/0306-4603(92)90027-s. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services. Women and smoking: A report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2001. [Google Scholar]
- 16.Bamia C. Trichopoulou A. Lenas D. Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Rel Metab Disord. 2004;28:1091–1096. doi: 10.1038/sj.ijo.0802697. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor E. Khaw KT. Cigarette smoking and increased central adiposity. Ann Intern Med. 1989;111:783–787. doi: 10.7326/0003-4819-111-10-783. [DOI] [PubMed] [Google Scholar]
- 18.Canoy D. Wareham N. Luben R, et al. Cigarette smoking and fat distribution in 21,828 British men and women: A population-based study. Obes Res. 2005;13:1466–1475. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 19.Pisinger C. Jorgensen T. Waist circumference and weight following smoking cessation in a general population: The Inter99 study. Prev Med. 2007;44:290–295. doi: 10.1016/j.ypmed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Jee SH. Lee SY. Nam CM. Kim SY. Kim MT. Effect of smoking on the paradox of high waist-to-hip ratio and low body mass index. Obes Res. 2002;10:891–895. doi: 10.1038/oby.2002.122. [DOI] [PubMed] [Google Scholar]
- 21.Sarni SE. Silventoinen K. Rissanen A. Sarlio-Lahteenkorva S. Kaprio J. Recurrent dieting and smoking among Finnish men and women. Obesity. 2007;15:1851–1859. doi: 10.1038/oby.2007.219. [DOI] [PubMed] [Google Scholar]
- 22.Stavropoulos-Kalinoglou A. Metsios GS. Panoulas VF, et al. Cigarette smoking associates with body weight and muscle mass of patients with rheumatoid arthritis: A cross-sectional, observational study. Arthritis Res Ther. 2008;10(R59) doi: 10.1186/ar2429. . Epub May 20, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JS. Auyeung TW. Kwok T. Lau EM. Leung PC. Woo J. Associated factors and health impact of sarcopenia in older Chinese men and women: A cross-sectional study. Gerontology. 2007;53:404–410. doi: 10.1159/000107355. . Epub August 16, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner RN. Koehler KM. Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. Erratum in Am J Epidemiol 1999;149:1161. [DOI] [PubMed] [Google Scholar]
- 25.Castillo EM. Goodman-Gruen D. Kritz-Silverstein D. Morton DJ. Wingard DL. Barrett-Connor E. Sarcopenia in elderly men and women: The Rancho Bernardo study. Am J Prev Med. 2003;25:226–231. doi: 10.1016/s0749-3797(03)00197-1. Erratum in Am J Prev Med 2004;27:265. [DOI] [PubMed] [Google Scholar]
- 26.Oncken C. Prestwood K. Cooney JL, et al. Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tobacco Res. 2002;4:451–458. doi: 10.1080/1462220021000018399. [DOI] [PubMed] [Google Scholar]
- 27.Lukaski HC. Soft tissue composition and bone mineral status: Evaluation by dual-energy x-ray absorptiometry. J Nutrition. 1993;123:438–443. [PubMed] [Google Scholar]
- 28.Wang ZM. Visser M. Ma R, et al. Skeletal muscle mass: Evaluation of neutron activation and dual-energy x-ray absorptiometry methods. J Appl Physiol. 1996;80:824–831. doi: 10.1152/jappl.1996.80.3.824. [DOI] [PubMed] [Google Scholar]
- 29.Oncken C. Cooney J. Feinn R. Lando H. Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addict Behav. 2007;32:296–309. doi: 10.1016/j.addbeh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Stookey GK. Katz BP. Olson BL. Drook CA. Cohen SJ. Evaluation of biochemical validation measures in determination of smoking cessation. J Dent Res. 1987;66:1597. doi: 10.1177/00220345870660101801. [DOI] [PubMed] [Google Scholar]
- 31.Kenny DA. Calsyn RJ. Morse GA. Klinkenberg WD. Winter JP. Trusty ML. Evaluation of treatment programs for persons with severe mental illness: Moderator and mediator. Eval Rev. 2004;28:294. doi: 10.1177/0193841X04264701. [DOI] [PubMed] [Google Scholar]
- 32.Washburn RA. Smith KW. Jette AM. Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 33.Petersen AM. Magkos F. Atherton P, et al. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab. 2007;293:E843–848. doi: 10.1152/ajpendo.00301.2007. . Epub July 3, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Vanni HM. Kazeros A. Wang R, et al. Cigarette smoking induces overexpression of fat depleting gene AZGP1 in the human. Chest. 2009;135:1197–1208. doi: 10.1378/chest.08-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]