Abstract
Introduction
We report a boy who received two allogeneic stem cell transplantations from umbilical cord donors to treat chronic granulomatous disease (CGD). The CGD was cured after the second transplantation, but 2½ years later, he was diagnosed with Duchenne muscular dystrophy (DMD).
Methods
Examinations of his DNA, muscle tissue, and myoblast cultures derived from muscle tissue were performed to determine whether any donor dystrophin was being expressed.
Results
The boy was found to have a large-scale deletion on the X chromosome that spanned the loci for CYBB and DMD. The absence of dystrophin led to muscle histology characteristic of DMD. Analysis of myofibers demonstrated no definite donor cell engraftment.
Discussion
This case suggests that umbilical cord-derived hematopoietic stem cell transplantation will not be efficacious in the therapy of DMD without additional interventions that induce engraftment of donor cells in skeletal muscle.
Keywords: Duchenne muscular dystrophy, chronic granulomatous disease, umbilical cord blood transplantation, stem cell therapy
Introduction
Individuals affected by chronic granulomatous disease (CGD)1,2 are vulnerable to recurrent, life-threatening infections3. The X-linked variant is caused by mutations in CYBB4. Duchenne muscular dystrophy (DMD) is a severe, progressive muscle disease caused by mutations in DMD, which encodes dystrophin5,6. CYBB and DMD are located near each other on Xp21.1, and large deletions involving both genes have previously been reported and studied7,8.
Hematopoietic stem cell transplantation has successfully treated hematologic malignancies, bone marrow failure, immunodeficiency syndromes, hemoglobinopathies, and inherited metabolic diseases9–12. Unrelated-donor umbilical cord blood transplantation (uUCBT) is curative in CGD13. A previous report described a boy who received a bone marrow transplantation to treat X-linked severe combined immune deficiency (SCID)14. He was later found to have a mutation indicating DMD, but he had a mild phenotype. A muscle biopsy performed after transplantation demonstrated that his skeletal muscle myofibers had a very small number of donor-derived nuclei, but numerous myofibers expressing endogenous dystrophin by exon skipping, not from donor nuclei.
We report findings on a boy with DMD and CGD due to a large deletion on Xp21.1. Unlike the previously reported case, he was treated with uUCBT rather than bone marrow transplantation prior to the diagnosis of DMD. Examination of his muscle tissue and cell cultures derived from this tissue demonstrated no definite evidence of donor-derived dystrophin protein and RNA expression, suggesting that improvements in either trafficking or engraftment of donor cells into myofibers are required before stem cell transplantation can be used to treat DMD.
Case Report
A full term infant boy was found to have lymphadenopathy, hepatosplenomegaly, elevated liver transaminases, and staphylococcal skin infections. He was diagnosed with CGD based on a nitroblue-tetrazolium test and mutation analysis of the CYBB gene. At 16 months, he underwent uUCBT after preparation with myeloablative chemotherapy using fludarabine, busulfan, cyclophosphamide, and anti-thymocyte globulin. Despite transplantation with a 5/6 HLA-matching, female, A+, cell dose of 8.53 × 107 nucleated cells/kg, he experienced graft rejection and autologous reconstitution. Approximately two months after the first transplant, he was given additional reduced intensity cytoreduction with alemtuzumab, fludarabine, and cyclophosphamide and transplanted with a second, 4/6 matching, male, A+ donor umbilical cord blood graft delivering a cell dose of 6.87 × 107 nucleated cells/kg. He gradually engrafted after the second transplant, and now maintains full (>98%) donor chimerism over three years post transplant. He did not have evidence of graft-versus-host disease, and his immune function normalized within one year of the second transplant. He has not had a serious infection since engraftment.
The patient pulled to a stand and cruised by one year but did not walk until 2 years. The delay was initially attributed to complications of his transplantations, but significantly elevated creatine kinase levels were noted at 3½ years. At 4 years, he had a shuffling gait with frequent tripping and falling, and he had difficulty rising from the floor.
His examination at 4 years was notable for hyperactivity, normal cranial nerves, mild hypotonia, calf pseudohypertrophy, heel cord contractures, and a he used the Gower maneuver to stand from the floor. These findings were unchanged at a follow-up examination at 5 years, and he did not have any motor regression in the interval. Serum chemistries demonstrated a creatine kinase level of 7,365 U/L [reference range 4–175], aldolase 89.9 U/L [3–12], alanine aminotransferase 648 U/L [3–30], aspartate aminotransferase 274 U/L [2–40], lactate dehydrogenase 997 U/L [110–295], and gamma glutamyl transpeptidase 17 U/L [12–55].
The proband had a hemizygous deletion of the entire CYBB gene. His mother was a heterozygous carrier, and his sister was not a carrier. High-resolution oligonucleotide array testing was performed on stored pre-transplant leukocyte-derived DNA, demonstrating a 6.1Mb deletion from Xp11.4-Xp21.1 (31,590,828 – 37,676,556), which includes the 5′ end of DMD, as well as FAM47A, TMEM47, FAM47B, MAGEB16, CXord22, CXorf59, CXorf30, FAM47C, PRRG1, LANCL3, XK, CYBB, and DYNLT3.
Materials and Methods
The proband, his mother, and his sister were enrolled in an institutionally-approved research protocol and contributed DNA samples. The proband underwent a clinical skeletal muscle biopsy, from which muscle tissue was obtained under the research protocol. This included frozen muscle sections, as well as fresh muscle tissue that was used to initiate myoblast cultures.
PCR-based mutation analysis was performed in both a clinical diagnostic laboratory and in a research laboratory, the latter using a set of DMD primers requiring uniform thermocycler conditions15.
Immunohistochemistry was performed using standard techniques14 on consecutive 10μm sections of snap-frozen muscle tissue using previously generated rabbit polyclonal antibodies: antibody 372 directed to dystrophin amino acids 762–20446 (located within the deleted region) at a dilution of 1:100; and antibody 373 targeted to amino acids 2060–31816 (a portion of dystrophin partially preserved in the proband) at a dilution of 1:2,000. A laminin antibody (Millipore) was used as a positive control at a dilution of 1:800.
Myoblasts were isolated and cultured from a sample of the patient’s fresh muscle tissue. Mononuclear cells were dissociated in collagenase D/dispase II16. Cells were expanded on gelatin-coated plates in growth medium consisting of high-glucose DMEM supplemented with 30% fetal bovine serum and 10 ng/ml basic fibroblast growth factor (bFGF). When cultures reached 70% confluence, cells were switched to differentiation medium consisting of low-glucose DMEM supplemented with 4% fetal bovine serum for 7 days.
Western blot analysis was performed on protein extracted from myoblast cultures using the above antibodies, as well as Dys1 (amino acids 1181–1388, Novocastra) and Dys2 (amino acids 3669–3685, the final 17 amino acids, Novocastra), diluted 1:1,000. A glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was used as a loading control.
Total RNA was extracted from differentiated cultures of the patient’s myotubes and control human myotubes using the QIAGEN QIAcube system, and reverse transcribed to cDNA. Reverse transcription polymerase chain reaction (RT-PCR) analysis was performed on 200ng and 500ng of cDNA using Applied Biosystems Taqman assays for dystrophin (DMD) exons 43–44 (Hs00240712_ml) and exons 68–69 (Hs00187805_ml); with ACTA1 (Hs00559403_ml), GAPDH (Hs99999905_ml), and MYH3 (Hs01074230_ml) as control assays.
Results
Mutation analysis
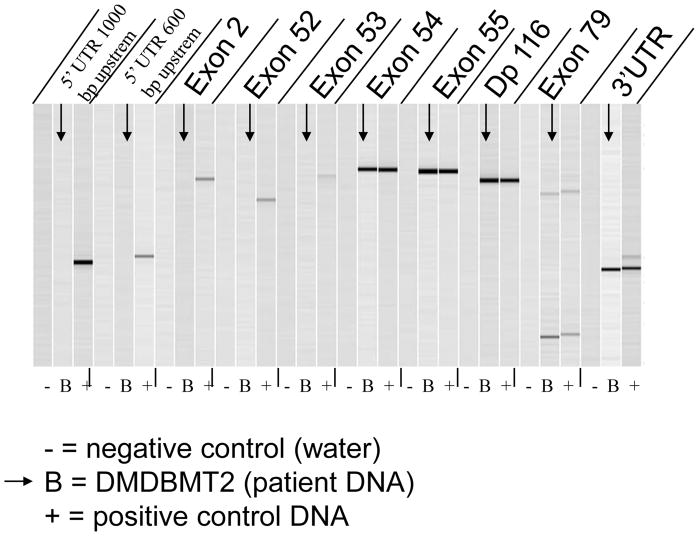
PCR demonstrated a hemizygous deletion in exons 1–53 of DMD in DNA obtained from the proband’s skin fibroblasts prior to transplantation (Fig. 1) and after transplantation. There was no deletion in DNA obtained from the proband’s leukocytes after transplantation. The deletion was predicted to affect the actin-binding N-terminal domain and the rod domain but not the dystroglycan-binding C-terminal domain (Fig. 2a). Promoters for the Dp116 isoform (in intron 55) and the Dp71 isoform (in intron 62) were expected to be preserved. The proband’s mother was found to be a carrier, but the sister was not.
Figure 1.
Capillary electrophoresis image of PCR amplification products using dystrophin primers. No PCR products are observed between exons 1 and 53 of dystrophin in DNA obtained from the patient’s skin fibroblasts prior to transplantation, while normal exon products are observed from exon 54 through exon 79. N, negative control; B, patient; P, positive control.
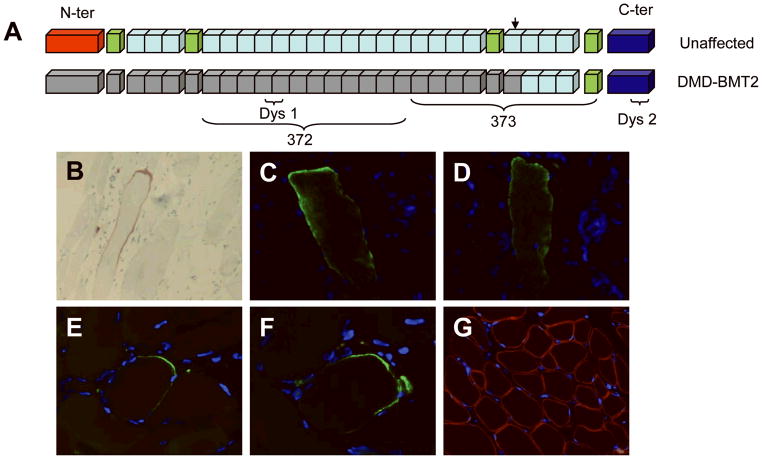
Figure 2.
(A) Diagram of dystrophin protein with major regions and antibody epitopes illustrated. The epitopes of antibodies 372 and Dys1 are entirely in the region of the patient’s deletion, while antibody 373’s epitope straddles the boundary of the deletion, and Dys 2 binds to the C-terminal domain. (B – G) Immunohistochemistry of sections of snap-frozen quadriceps tissue obtained from the patient at 4 years of age. (B) A single muscle fiber stained positive with Dys1 antibody, visualized using horseradish peroxidase. (C – D) A myofiber that stained positive with the 372 antibody in 2 independent sections. (E – F) The 373 antibody detected dystrophin expression in another myofiber in multiple sections. (G) Laminin-2 control antibody stains the sarcolemma diffusely in a representative section.
Histology
Hematoxylin and eosin (H&E)-stained paraffin sections of skeletal muscle tissue demonstrated rounded fibers with excessive variation in fiber diameter, basophilic regenerating fibers, scattered fibers undergoing myophagocytosis and focal inflammatory infiltrates. H&E-stained frozen sections additionally showed increased internalized nuclei, atrophic and hypertrophic fibers, and increased endomysial connective tissue, typical of DMD.
Immunohistochemistry
Staining for dystrophin with Dys1 (rod domain), Dys2 (C terminus), and Dys 3 (N terminus) demonstrated one fiber that was positive for all three antibodies (Fig. 2b), but dystrophin expression was otherwise absent in all sections examined. Stains for α-, β-,γ-, and δ-sarcoglycan were variably reduced, and stains for β-dystroglycan, merosin, spectrin, caveolin, and dysferlin were normal (data not shown). Twenty-six 10μm serial sections of the patient’s frozen muscle tissue were immunostained by alternating antibodies 372 (specific for donor-derived epitopes) and 373, either alone or in conjunction with the anti-laminin antibody. One fiber positive for the 372 antibody was detected in 2 serial sections (Fig. 2c, 2d), suggesting rare expression of donor-derived dystrophin.
Positive staining with the 373 antibody was seen in another myofiber in multiple sections (Fig. 2e, 2f), however this myofiber was negative with the 372 antibody, suggesting that it was likely expressing endogenous dystrophin. Laminin-2 control stains demonstrated intact muscle sarcolemma (Fig. 2g).
Myoblast culture
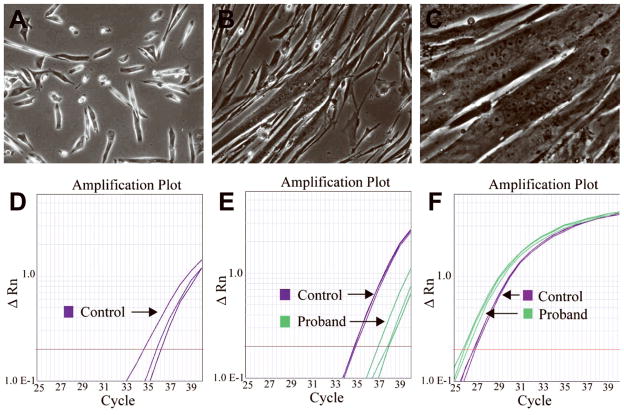
To address whether satellite cells of donor origin that could express full-length dystrophin were present, primary myoblast cultures were expanded and allowed to differentiate, leading to the formation of myotubes (Fig. 3a–c).
Figure 3.
(A – C) Photomicrographs of the patient’s myoblast culture illustrating (A) the myoblasts in proliferation medium, with formation of myotubes at (B) 2 days and (C) 7 days following addition of differentiation medium. (D – F) Taqman RT-PCR analysis of cDNA reverse-transcribed from RNA extracted from the patient and control subject’s myoblast culture after differentiation. Standard Applied Biosystems protocols were used. (D) Expression of dystrophin exons 43–44 is low in control tissue and absent in the patient’s tissue (purple, control). (E) Low expression of dystrophin exons 68–69 is observed in both patient and control tissue, with higher expression in control (purple, control; green, patient). (F) Control assay for GAPDH (purple, control; green, patient) showed equivalent expression levels in both patient and control tissue. Control assays for ACTA1 and MYH3 demonstrated similar results (data not shown).
Immunostaining with antibodies 372 and 373 revealed complete absence of dystrophin-expressing myotubes, suggesting that no satellite cells of donor origin or truncated dystrophin protein of host origin were present (data not shown). Protein extracts from these cultures were analyzed by western blot using 372, 373, Dys1, and Dys2 dystrophin antibodies. There was no evidence of dystrophin in protein extracted from the patient’s cultured myoblasts, while cultures from an unaffected control yielded a band at the expected molecular weight (data not shown).
RT-PCR
Quantitative PCR demonstrated that cultured differentiated myotubes from a control subject expressed dystrophin exons 43–44 (Fig. 3d) and 68–69 (Fig. 3e), while the patient’s myotubes expressed only exons 68–69 (Fig. 3d–e). The expression of exons 68–69 was lower in the patient compared to control, likely due to the presence of dystrophin isoforms that are not primarily expressed in skeletal muscle. The control genes ACTA1, GAPDH, and MYH3 were expressed at equal or greater levels in the patient compared to control (Fig. 3f).
Discussion
Cell-based therapeutic approaches to muscular dystrophy have encountered numerous obstacles, due largely to inefficient engraftment of donor cells. Factors contributing to this inefficiency included low survival of the donor cells, inadequate migration, and poor fusion17.
Among progenitor cells, bone marrow-derived cells have been shown to be capable of fusing into myofibers. In mice, these progenitors are also able to transition into a phase in vivo where they express markers characteristic of muscle satellite stem cells, suggesting a possible re-programming prior to fusion18. However, the frequency of these events is extremely low. Our previous report also suggests that the fusion of donor cells to mature muscle fibers is inefficient14.
Umbilical cord blood cells (UCBC) are another source of stem cells, and are curative in CGD13 and other blood–related diseases. Studies in mice suggest that UCBCs contain progenitor(s) capable of contributing to skeletal muscle, but it is not known whether UCBCs can contribute to skeletal muscle myofibers in humans19. Our data indicate that by itself, uUCBT does not lead to significant engraftment of donor cells into skeletal muscle, and did not improve the phenotype of DMD.
It is not clear why blood-derived stem cells do not spontaneously engraft in skeletal muscle tissue. Without further processing and selection, hematopoietic stem cells derived directly from donors are heterogeneous, and a number of subpopulations have been identified, some of which appear to have better myogenic potential than others. In mouse and dog models of muscular dystrophy, some of these subpopulations have recently shown potential for engraftment into skeletal muscles, including side population (SP) cells20–22, mesangioblasts23, subpopulations of satellite cells24,25, and myoblasts deficient in myoD26. It would be interesting to determine whether subpopulations of umbilical cord cells have greater myogenic potential than the population as a whole, and whether transplanting such subpopulations may lead to novel candidates for cell-based therapy of muscular dystrophy. However, those studies are beyond the scope of this work.
It is disappointing that stem cell transplantation did not serendipitously lead to a therapy for DMD in our patient. However, it is tantalizing that donor-derived cells expressing normal dystrophin are present in this patient’s circulation. Refinements in the selection of cell subpopulations and interventions to facilitate engraftment of donor cells into skeletal muscle tissue may, in the future, lead to the development of stem cell transplantation as a treatment for DMD.
Acknowledgments
The authors wish to thank the patient and his family for participating in these research efforts and for sharing their thoughts about therapeutic interventions. The DNA Diagnostic Laboratory at Children’s Hospital Boston assisted with mutation analysis. Funding for this project was provided by: K08 NS048180 (PBK), MDA114353 (BTD, PBK), NIH/NINDS 5P50NS040828 (EG, LMK), the Howard Hughes Medical Institute (LMK), the Manton Center for Orphan Disease Research (LMK), and the Bernard and Alva B. Gimbel Foundation (LMK).
Abbreviations
- bFGF
basic fibroblast growth factor
- CGD
chronic granulomatous disease
- DMD
Duchenne muscular dystrophy
- DMD
dystrophin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H&E
hematoxylin and eosin
- RT-PCR
reverse transcription polymerase chain reaction
- SCID
severe combined immune deficiency
- UCBC
umbilical cord blood cell
- uUCBT
unrelated umbilical cord blood transplantation
References
- 1.Berendes H, Bridges RA, Good RA. A fatal granulomatosus of childhood: the clinical study of a new syndrome. Minn Med. 1957;40(5):309–312. [PubMed] [Google Scholar]
- 2.Bridges RA, Berendes H, Good RA. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child. 1959;97(4):387–408. [PubMed] [Google Scholar]
- 3.Di Matteo G, Giordani L, Finocchi A, Ventura A, Chiriaco M, Blancato J, Sinibaldi C, Plebani A, Soresina A, Pignata C, Dellepiane RM, Trizzino A, Cossu F, Rondelli R, Rossi P, De Mattia D, Martire B. Molecular characterization of a large cohort of patients with Chronic Granulomatous Disease and identification of novel CYBB mutations: an Italian multicenter study. Mol Immunol. 2009;46(10):1935–1941. doi: 10.1016/j.molimm.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, Orkin SH. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 5.Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 7.Francke U, Ochs HD, de Martinville B, Giacalone J, Lindgren V, Disteche C, Pagon RA, Hofker MH, van Ommen GJ, Pearson PL, et al. Minor Xp21 chromosome deletion in a male associated with expression of Duchenne muscular dystrophy, chronic granulomatous disease, retinitis pigmentosa, and McLeod syndrome. Am J Hum Genet. 1985;37(2):250–267. [PMC free article] [PubMed] [Google Scholar]
- 8.Kunkel LM, Monaco AP, Middlesworth W, Ochs HD, Latt SA. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321(17):1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 10.Ballen KK, Haley NR, Kurtzberg J, Lane TA, Lindgren BR, Miller JP, Newman B, McCullough J. Outcomes of 122 diverse adult and pediatric cord blood transplant recipients from a large cord blood bank. Transfusion. 2006;46(12):2063–2070. doi: 10.1111/j.1537-2995.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzberg J. Update on umbilical cord blood transplantation. Curr Opin Pediatr. 2009;21(1):22–29. doi: 10.1097/mop.0b013e32832130bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, Ciocci G, Carrier C, Stevens CE, Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335(3):157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 13.Parikh SH, Szabolcs P, Prasad VK, Lakshminarayanan S, Martin PL, Driscoll TA, Kurtzberg J. Correction of chronic granulomatous disease after second unrelated-donor umbilical cord blood transplantation. Pediatr Blood Cancer. 2007;49(7):982–984. doi: 10.1002/pbc.21365. [DOI] [PubMed] [Google Scholar]
- 14.Gussoni E, Bennett RR, Muskiewicz KR, Meyerrose T, Nolta JA, Gilgoff I, Stein J, Chan YM, Lidov HG, Bonnemann CG, Von Moers A, Morris GE, Den Dunnen JT, Chamberlain JS, Kunkel LM, Weinberg K. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110(6):807–814. doi: 10.1172/JCI16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett RR, Schneider HE, Estrella E, Burgess S, Cheng AS, Barrett C, Lip V, Lai PS, Shen Y, Wu BL, Darras BT, Beggs AH, Kunkel LM. Automated DNA mutation detection using universal conditions direct sequencing: application to ten muscular dystrophy genes. BMC Genet. 2009;10(1):66. doi: 10.1186/1471-2156-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlath GK, Gussoni E. Human myoblasts and muscle-derived SP cells. Methods Mol Med. 2005;107:97–110. doi: 10.1385/1-59259-861-7:097. [DOI] [PubMed] [Google Scholar]
- 17.Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 18.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111(4):589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 19.Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., Jr Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem Cells. 2004;22(6):981–993. doi: 10.1634/stemcells.22-6-981. [DOI] [PubMed] [Google Scholar]
- 20.Bachrach E, Perez AL, Choi YH, Illigens BM, Jun SJ, del Nido P, McGowan FX, Li S, Flint A, Chamberlain J, Kunkel LM. Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve. 2006;34(1):44–52. doi: 10.1002/mus.20560. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4(3):217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luth ES, Jun SJ, Wessen MK, Liadaki K, Gussoni E, Kunkel LM. Bone marrow side population cells are enriched for progenitors capable of myogenic differentiation. J Cell Sci. 2008;121(Pt 9):1426–1434. doi: 10.1242/jcs.021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 24.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25(4):885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 25.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134(1):37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asakura A, Hirai H, Kablar B, Morita S, Ishibashi J, Piras BA, Christ AJ, Verma M, Vineretsky KA, Rudnicki MA. Increased survival of muscle stem cells lacking the MyoD gene after transplantation into regenerating skeletal muscle. Proc Natl Acad Sci U S A. 2007;104(42):16552–16557. doi: 10.1073/pnas.0708145104. [DOI] [PMC free article] [PubMed] [Google Scholar]