Summary of recent advances
Plants utilize circadian clocks to synchronize their physiological and developmental events with daily and yearly changes in the environment. Recent advances in Arabidopsis research have provided a better understanding of the molecular mechanisms of the circadian clock and photoperiodism. One of the most important questions is whether the mechanisms studied in Arabidopsis are conserved in other plants. Homologs of many Arabidopsis clock genes have been identified in various plants and some gene functions have been characterized. It seems that the circadian clocks in plants are similar. Recent success in molecular genetics has also revealed the mechanisms of photoperiodic flowering in cereals. The day-length sensing mechanisms appear to have diverged more between long-day plants and short-day plants than the circadian clock.
Introduction
Many organisms, including plants, possess an endogenous timekeeper known as a circadian clock that synchronizes biological events with daily environmental changes. Plants also use internal clocks to anticipate upcoming seasonal changes and adjust their physiology and development accordingly. There has been a tremendous amount of progress made recently in our understanding of the molecular mechanisms of circadian clocks, particularly in Arabidopsis thaliana. Recent studies of photoperiodic flowering regulation in Arabidopsis and other model plants have increased our knowledge of the mechanisms of photoperiodism in a wide variety of plant species at the molecular levels. Here we summarize recent advances in our studies of the clock and photoperiodism in various plants, and discuss to what degree the circadian clock mechanism and photoperiodism pathways are conserved among these plants.
The circadian clock in Arabidopsis
As we have the best understanding of the molecular regulation of the clock in Arabidopsis, our knowledge of Arabidopsis serves as a reference for other plant species. Here we introduce the major clock components and summarize the overall structure of the clock (see more details in recent reviews [1–3]).
The Arabidopsis circadian clock comprises multiple feedback regulations centered on two MYB transcription factors, CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) [1–3]. CCA1 and LHY, both of which are most abundant at dawn, directly activate the transcription of PSUEDO-RESPONSE REGULATOR9 (PRR9) and PRR7 in the morning [4]. CCA1 and LHY concomitantly suppress the expression of the other clock genes with afternoon to evening peaks, such as PRR5, TIMING OF CAB EXPRESSION1 (TOC1), CCA1 HIKING EXPEDITION (CHE), GIGANTEA (GI), LUX ARRHYTHMO (LUX) and EARLY FLOWERING4 (ELF4) [5–10]. PRR9, PRR7, and PRR5 proteins are expressed throughout the day. They physically associate with the CCA1 and LHY promoters and repress their transcription [11·]. As CCA1 and LHY protein levels decrease, CCA1/LHY-dependent repression fades away, facilitating accumulation of the evening clock gene transcripts. TOC1 (also known as PRR1) physically associates with the CCA1 and LHY promoters and participates in the transcriptional activation of both genes by partially interfering with the function of CCA1-specific transcriptional repressor CHE [10]. No PRR proteins possess DNA binding domains, yet they associate with the promoters of CCA1 and LHY and regulate their expression [10,11·]. It is of great interest to understand the precise molecular mechanisms by which PRR proteins induce CCA1 and LHY transcription. The GARP domain transcription factor LUX (also known as PHYTOCLOCK 1 (PCL1)) and the small nuclear protein ELF4 are also involved in CCA1 and LHY induction [6,7]. In addition to transcriptional feedback regulation, posttranslational regulation is built into the molecular circuits of the circadian clock. Three related photoreceptor F-box proteins, ZEITLUPE (ZTL), LOV KELCH PROTEIN2 (LKP2) and FLAVIN-BINDING KELCH REPEAT F-BOX 1 (FKF1), regulate the stability of both TOC1 and PRR5 to fine tune the pace and robustness of circadian clock oscillation [12]. GI protein also plays a role in this regulation by directly binding these F-box proteins [13,14]. Considering that Arabidopsis is a model organism for plants, an important question is how much the Arabidopsis clock system is conserved in other plants. In recent years, homologs of Arabidopsis clock genes have been identified in various plant species, enabling us to systematically compare the plant molecular clocks (Table 1).
Table 1.
Homologs of Arabidopsis clock genes in plants
| Gene names in A. thaliana Protein motifs and expression peak(ZT)a[65] | Homologs in other speciesb | Species | Characteristic remarks, gene expression peak (ZT)a | Refs |
|---|---|---|---|---|
|
CCA1 (ZTO) LHY (ZTO) Single MYB DNA binding domain |
OsCCA1/OsLHY | O. sativa | OsCCA1-OXc shows late flowering and long hypocotyl phenotypes (ZT0~4) | [26,52] |
|
| ||||
| PnLHY1, PnLHY2 | P. nigra | (ZTO~3) | [21] | |
|
| ||||
| CsLHY | C. sativa | (ZT4) | [23] | |
|
| ||||
| MYB1/LHY | P. sativum | (ZT-4~4) | [19,20··] | |
|
| ||||
| GmLCL1, GmLCL2 | G. max | (ZT1~3) | [15] | |
|
| ||||
| PvLHY | P. vulgaris | Induced by light. Putative Lhcb activator, (ZTO~6) | [16] | |
|
| ||||
| LgLHYH1, LgLHYH2 | L. gibba | LgLHYH1-OXd possesses low and advanced circadian rhythms. LgLHYH2-OXd shows arrhythmic phenotype. LgLHYH1 RNAid shows short period phenotype. LgLHYH1 (ZT-2~0), LgLHYH1 (ZT2~6) | [28,29] | |
|
| ||||
| LpLHYH1, LpLHYH2 | L. paucicostata | LpLHYH1 (ZT-4~2), LpLHYH2 (ZT4~4) | [28] | |
|
| ||||
| OtCCA1 | O. tauri | OtCCA1-OX shows arrhythmic phenotype, (ZT12~24) | [32··] | |
|
| ||||
| McCCA1/LHY | M. crystallinum | (ZTO) | [66] | |
|
| ||||
| PpCCA1a, PpCCA1b | P. patens | PpCCA1a (ZTO), PpCCA1b (ZT-4~0) | [30,31] | |
|
| ||||
|
TOC1/PRR1 (ZT10~12) Pseudo-receiver and CCT domains |
OsTOC1/OsPRR1 | O. sativa | OsPRR1-OX in Arabidopsis shows arrhythmic, small and short hypocotyl phenotypes, (ZT7~13) | [26,67,68] |
|
| ||||
| CsTOC1 | C. sativa | (ZT15~18) | [22·,23] | |
|
| ||||
| TOC1 | P. sativum | (ZT8~16) | [19,20··] | |
|
| ||||
| GmTOC1 | G. max | (ZT13~17) | [15] | |
|
| ||||
| BrPRR1 | B. rapa | (ZT9~18) | [69] | |
|
| ||||
| McTOC1 | M. crystallinum | (ZT7) | [66] | |
|
| ||||
| OtTOC1 | O. tauri | True response regulator, (ZT9~12) | [32··] | |
|
| ||||
|
PRRs Pseudo-receiver and CCT domains PRR9 (ZT2~4) PRR7(ZT6~8) PRR5 (ZT8~9) PRR3 (ZT10) |
OsPRR73, OsPRR37, OsPRR95, OsPRR59 | O. sativa | OsPRR37 partially rescue prr7-11 mutation. OsPRR73 (ZT4~10), OsPRR37 (ZT7~10), OsPRR95 (ZT10), OsPRR59 (ZT7~13) | [67,68,70] |
|
| ||||
| PPD(PRR) | H. vulgare | Flowering activator | [44] | |
|
| ||||
| PPD(PRR) | T. aestivum | Flowering activator | [45] | |
|
| ||||
| PPD(PRR) | T. durum | Flowering activator | [71] | |
|
| ||||
| CsPRR9, CsPRR7, CsPRR5 | C. sativa | CsPRR9 (ZT4~9), CsPRR7 (ZT7~12), CsPRR5 (ZT9~12) | [22] | |
|
| ||||
| PRR37, PRR59 | P. sativum | PRR37 (ZT8), PRR59 (ZT8) | [20··] | |
|
| ||||
| BrPRR9, BrPRR7, BrPRR5, BrPRR3 | B. rapa | BrPRR9 (ZT3~12), BrPRR7 (ZT12), BrPRR5 (ZT6~12), BrPRR3 (ZT12~15) | [69] | |
|
| ||||
| LgPRRH95 LgPRRH59 LgPRRH37 | L. gibba | LgPRRH95 (ZT6~12), LgPRRH59 (ZT8~16), LgPRRH37 (ZT6~12) | [28] | |
|
| ||||
| PpPRRa | P. patens | True response regulator, (ZT4~12) | [30·] | |
|
| ||||
|
LUX (ZTU) GARP DNA binding domain |
OsLUX | O. sativa | (ZT10~13) | [26] |
|
| ||||
|
ELF3 Unknown (ZT16) |
OsEF3 | O. sativa | Positive flowering regulator in both LD and SD. The loss of function mutant has short roots, but does not have clock phenotype. | [72] |
|
| ||||
| LgELF3H1 | L. gibba | LgELF3H1-OXd shows weak rhythmicity and long period. LgELF3H1 RNAid shows high expression with normal periodicity. LgELF3H1 (ZT16 in LD, ZT10 in SD) | [28,29] | |
|
| ||||
| McELF3 | M. crystallinum | (ZT9~21) | [66] | |
|
| ||||
|
ELF4 (ZT12) Unknown |
PsELF4/DNE | P. sativum | dne mutation affects clock gene expression and causes early flowering, (ZT12~16) | [19,20··] |
|
| ||||
| McELF4 | M. crystallinum | (ZT9~15) | [66] | |
|
| ||||
|
ZTL (constant) LKP2 (constant) FKF1 (ZT7~10) LOV, F-box and Kelch repeat domains [13] |
OsZTL1, OsZTL2, OsFKF1 | O. sativa | OsZTL1-OXc shows arrhythmic, late flowering, and long hypocotyl phenotypes. OsZTL1 (constant), OsZTL2 (constant), and OsFKF1 (ZT7~13) | [26] |
|
| ||||
| McZTL, McFKF1 | M. crystallinum | McZTL expression oscillates, (ZT9), McFKF1 (ZT9) | [66] | |
|
| ||||
|
GI (ZT8~10) Nuclear protein that interacts with ZTL family proteins |
OsGI | O. sativa | OsGI-OX plant is late flowering, (ZT8~12) | [51,73] |
|
| ||||
| HvGI | H. vulgare | (ZT15 in LD, ZT6 in SD) | [74] | |
|
| ||||
| TaGI | T. aestivum | TaGI-OXc shows early flowering and complements gi-2 mutantc, (ZT12 in LD, ZT8 in SD) | [75] | |
|
| ||||
| BdGI | B. distachyon | BdGI Interacts with AtZTL and AtCOP1 in yeast and rescues gi mutantc, (ZT12 in LD, ZT8 in SD) | [76] | |
|
| ||||
| LATE1 | P. sativum | late1 mutant shows short period and low amplitude rhythms and late flowering (ZT12 in LD and ZT8 in SD) | [19,20··] | |
|
| ||||
| MtGI | M. truncatula | (ZT8 in LD) | [18] | |
|
| ||||
| LgGIH1 | L. gibba |
LgGIH1-OXd shows very low amplitude, LgGIH1 RNAid shows arrhythmic, (ZT8 10) |
[28,29] | |
|
| ||||
| LpGIH1 | L. paucicostata | (ZT10~16) | [28] | |
|
| ||||
| McGI | M. crystallinum | (ZT9) | [66] | |
Gene name abbreviations: CCA1, CIRCADIAN CLOCK-ASSOCIATED 1; LHY, LATE ELONGATED HYPOCOTYL; TOC1, TIMING OF CAB EXPRESSION 1; PRRs, PSEUDO RESPONSE REGULATORs; LUX/PCL1, LUX ARRHYTHMO/ PHYTOCLOCK 1; ELF, EARLYFLOWERING; ZTL, ZEITLUPE; LKP2, LOV KELCH PROTEIN2; FKF1, FLAVIN-BINDING KELCH REPEAT F-BOX 1; GI, GIGANTEA; LCL, LHY AND CCA1 LIKE; PPD, PHOTOPERIOD; DNE, DIE NEUTRALIS; and LATE1, LATE BLOOMER1. Species abbreviations: A. thariana, Arabidopsis thariana; O. sativa, Oriza sativa; P. nigra, Populus nigra; C. sativa, Castanea sativa; P. sativum, Pisum sativum; G. max, Glycine max; P. vulgaris, Phaseolus vulgaris; L. gibba, Lemna gibba; L. paucicostata, Lemna paucicostata; O. tauri, Ostreococcus tauri; M. crystallinum, Mesembryanthemum crystallinum; P. patens, Physcomtirella patens; H. vulgare, Hordeum vulgare; T. aestivum, Triticum aestivum; B. rapa, Brassica rapa; and B. distachyon, Brachypodium distachyon.
Monocots are shown in bold.
Peak gene expression phases for RNA expression in the table are in Zeitgeber time (ZT). Light on is at ZT0 and light off is usually at ZT12. In LD conditions, light is turned off at ZT16, in SD conditions, light is turned off from ZT8.
The names of homologs are quoted from the original articles.
These experiments were conducted in Arabidopsis.
These results were obtained from transient assays.
Note: In Lotus japonicus, Carica papaya, and Vitis vinifera, many Arabidopsis clock homolog candidate genes are identified from DNA databases, however there is no other information; therefore these genes are excluded from this table.
Circadian clocks in other eudicots
Can a system resembling the Arabidopsis circadian clock be identified in other eudicots? As shown in Table 1, homologs of most Arabidopsis clock genes exist in legumes, soybeans (Glycine max), peas (Pisum sativum), common beans (Phaseolus vulgaris), and Medicago truncatula [15–18]. The expression patterns of many legume clock homologs resemble those of Arabidopsis clock genes, suggesting that the legume plant clock is similar to the Arabidopsis clock. Recently, the function of two pea clock genes was characterized. The LATE BLOOMER1 (LATE1) and the DIE NEUTRALIS (DNE) gene are the pea orthologs of Arabidopsis GI and ELF4. Pea plants flower earlier in long day (LD) conditions. Like Arabidopsis gi and elf4 mutants, late1 and dne mutants are both photoperiod insensitive and always flower late and early, respectively [19,20··]. In the late1 mutant, the expression of pea TOC1, ELF4 and MYB1 (LHY homolog) dampens and becomes arrhythmic under continuous weak light and dark conditions [19]. This clock phenotype is stronger than that of the Arabidopsis gi mutants, indicating that GI is essential for the rhythmic expression of the clock genes in peas, although we do not know how the molecular function of pea GI differs from Arabidopsis GI. Interestingly, circadian oscillation stops under continuous intense light in the wild type pea cultivar examined. The common spring-sown pea cultivars, including the one used in these studies, contain a mutation in the HIGH RESPONSE (HR) locus, which causes this conditional arrhythmicity [17,20··]. Therefore, the mutant phenotype observed could be a combinational effect with the hr mutation. Unlike the Arabidopsis elf4 mutant that shows the arrhythmic clock phenotype, the dne mutant exhibits a subtle effect on the rhythmic expression of the clock genes examined, although it shows a short period phenotype [20··]. Overexpression of DNE in the elf4 mutant complements flowering and hypocotyl growth phenotypes, indicating that DNE and ELF4 share a similar function at least for regulating these responses. The different contributions of LATE1 and DNE to the circadian clock could be pea specific. Further analysis deciphering the more precise role of LATE1 and DNE possibly with HR in the clock is awaited.
Homologs of Arabidopsis clock genes were also identified in perennial (woody) eudicots such as poplar (Populus trichocarpa and Populus nigra) [21] and chestnut (Castanea sativa) [22·,23](Table 1); however, the molecular function of these genes has not been characterized yet. In the chestnut, CsLHY,CsTOC1, CsPRR9, CsPRR7 and CsPRR5 mRNA levels oscillate similarly to Arabidopsis clock gene mRNAs during growth seasons; however, during winter dormancy their mRNA levels become constitutive. This is caused by exposure to low temperature (4 °C), but not by the dormant status of tissues, since endodormant tissues transferred to 22 °C regain daily oscillation without breaking dormancy [22·,23]. The Arabidopsis clock still sustains oscillation even at lower temperatures, although it is weakened [24]. Having similar expression patterns to many clock genes that peak at different times of the day indicates that similar feedback regulation among clock genes might exist in chestnuts. It is possible that some components in the network are more sensitive to lower temperatures, thus pausing their clock at some point. Stopping circadian-regulated physiology and development may help perennial trees cope with severe winter conditions. Although there is some species specific modification in the clock, overall expression patterns appear to be conserved within eudicots.
Circadian clocks in monocots
The divergence between eudicots and monocots is estimated to have occurred 200 million years ago [25]. We wondered whether monocots have evolved clock systems similar to eudicots. Recent studies have revealed the function of some clock genes in distant species of monocots, such as rice and duckweed, providing us with clues to help answer this question.
Homologs of most Arabidopsis clock genes exist in rice, although the rice genome only contains one CCA1/LHY homolog (Table 1). The circadian expression profiles of the putative rice clock genes highly overlap those of their Arabidopsis counterparts. In addition, ectopic overexpression of both OsPRR1 and OsZTL in Arabidopsis caused a similar arrhythmic clock phenotype to the TOC1 and ZTL overexpressors [26]. Overexpression of OsLHY in the rice callus also repressed rhythmic expression of the OsPRR1:LUC circadian reporter [27]. Moreover, OsPRR1 and OsPRR37 rescue the clock phenotypes of toc1 and prr7 mutants, respectively [26]. Therefore, these rice genes are orthologs of Arabidopsis clock genes. Even the posttranslational regulation of LHY is likely conserved in rice, as OsLHY protein also accumulates under light [27].
Duckweed is a free-floating aquatic monocot that provides an additional example of how circadian clocks in monocots are similar to Arabidopsis. Two species (Lemna gibba and L. paucicostata) have two LHY/CCA1 (LHYH1 and LHYH2), three PRRs (PRR95, PRR59 and PRR37), GI (GIH1) and ELF3 (ELF3H1) genes that show circadian expression patterns [28]. Transient transfection analysis with overexpression and RNA interference (RNAi) constructs of LHYH1, LHYH2, GIH1 and ELF3H1 revealed that these genes have similar functions to their Arabidopsis counterparts. (Table 1) [29]. Like the pea GI mutant, GIH1 RNAi induces arrhythmic expression of clock markers, suggesting that GIH1 plays an important role in the circadian clock in duckweed. Thus, the results obtained from several monocots suggest that both eudicots and monocots possess similar molecular circadian clocks.
Circadian clocks in moss and alga
Currently there are some reports regarding the function of circadian clock proteins in plants other than angiosperms, such as a moss and a unicellular alga. The moss Physcomitrella patens possesses PpCCA1a, PpCCA1b and PpPRRa genes [30·]. The expression patterns of these genes show daily oscillation with morning peaks (PpCCA1a and PpCCA1b) and a mid-day peak (PpPRRa) under light-dark cycles and continuous dark conditions. Interestingly, their expression profiles become arrhythmic under continuous light conditions [30·]. Gene disruption is feasible in this moss by homologous recombination. The PpCCA1a PpCCA1b double disruptant presents shorter and dampened rhythms in clock-regulated gene expression, which resembles the Arabidopsis cca1 lhy double mutant phenotype [30·]. The double disruptant colony grows faster than the wild-type colony in LD [31], indicating the circadian clock regulates growth rates in this moss. Similar phenotypes of both the double mutants in Arabidopsis and Physcomitrella suggest that not only the functions of counterpart genes but also the structure of the clock gene networks are at least partly conserved between the two species. Since bryophytes are one of the earliest diverging groups of land plants, the basic structure of the current angiosperm clock formed long ago.
Even in the green unicellular alga Ostreococcus tauri, TOC1 and CCA1 appear to be conserved, although it does not have other Arabidopsis clock homologs [32··]. Ostreococcus TOC1 (and also moss PpPRRa) may function as a response regulator, because it conserves the aspartic acid residue required for phosphotransfer. OtTOC1 and OtCCA1 expression patterns are anti-phasic and OtCCA1 likely binds to the OtTOC1 promoter and regulates rhythmic expression. OtCCA1 and OtTOC1 overexpressors and the OtTOC1-antisense line all lead to arrhythmia of promoter LUC reporter activities [32··]. The Ostreococcus clock system seems to be similar to but relatively simpler than that of higher plants. Therefore, this type of the circadian clock may potentially be an ancestor of the land plant clocks.
Are photoperiodic pathways conserved in plants, like the circadian clocks?
In most plant species in which clock genes have been identified, the molecular characterization of clock proteins has just begun. However, it seems that the clock components and their function are likely conserved in land plants. In angiosperms, circadian clocks play important roles in seasonal flowering time regulation. Through circadian clock-regulated function, many plants measure changes in day length to precisely control flowering time to maximize reproductive success. Some plants respond to LD, and some respond to short days (SD), while others do not respond to any day length changes. Are the molecular mechanisms of day-length measurement conserved among plants that have different preferences? Among the plants that respond to the same day length, do they utilize similar mechanisms?
Photoperiodic flowering mechanism in Arabidopsis
The long-day photoperiod promotes flowering in Arabidopsis mainly through the function of CONSTANS (CO) and FLOWERING LOCUS T (FT). CO is a transcription activator of FT [33]. The circadian clock regulates the timing of CO gene expression and light and CO induces FT expression. Since daytime CO expression occurs only in LD, FT is induced under those conditions [33,34]. To accurately measure the difference in day length, clock-dependent timing regulation of CO is a crucial mechanism. During the day, CCA1 and LHY regulate the expression timing of PRR9, PRR7, PRR5, GI and FKF1, all of which regulate CO expression [4,8,35,36]. These three PRRs subsequently regulate the expression of the CO repressor, CYCLING DOF FACTOR1 (CDF1) [36]. CDF1 and other CDFs repress CO expression during the morning, and in the afternoon the FKF1-GI complex degrades CDFs to facilitate the expression of CO [14,37]. Once CO is expressed under light, CO protein is stabilized by light signals and induces FT expression [38]. FT protein is a florigen and it is synthesized in the leaf vasculature [39]. It is then translocated to the shoot apical meristem to initiate the expression of floral identity genes by binding to bZIP transcription factor FD [39,40]. FT expression is also regulated by many other transcription factors with redundant function [2]. Winter annual accessions require exposure to prolonged cold temperature (vernalization) to gain photoperiodic sensitivity. Without vernalization, FLC accumulates and directly represses the expression of FT, SUPPRESSOR OF OVER EXPRESSION OF CONSTANS 1 (SOC1) (another floral inducer) and FD [41]. Vernalization represses the expression of FLC by changing the chromatin status of the FLC locus, thus enabling plants to express FT in a day-length dependent manner [42].
Photoperiodic flowering mechanism in long-day plants
Temperate cereals such as barley and wheat are also long-day plants like Arabidopsis. Do these monocots have similar mechanisms to induce flowering? In barley and wheat, natural variations in photoperiod sensitivity are mainly controlled by Photoperiod (Ppd) loci, which regulate flowering time (Fig. 1) [43]. The Ppd-H1 gene has been determined to be a barley homolog of PRR7 [44]. The ppd-H1 mutation alters the circadian timing of barley CO (HvCO) expression and represses barley FT (HvFT1) expression, resulting in late flowering (Fig.1 and Fig 2) [44]. Conversely, some varieties of wheat carry a deletion in the promoter of the Ppd-D1 gene (wheat Ppd-H1), leading to high expression of Ppd genes. This causes up-regulation of wheat FT (TaFT) expression, leading to early flowering even in SD [45]. Arabidopsisprr7 prr5, prr9 prr7, and prr9 prr7 prr5 mutants also show an extreme late flowering phenotype (PRR9, PRR7 and PRR5 have overlapping function) and this phenotype is at least due to the alteration of CO expression [36]. Thus, the flowering function of PRRs in barley, wheat, and Arabidopsis may be similar.
Figure 1.
Photoperiodic flowering pathways in long-day and short-day plants. Long-day plants (Arabidopsis, barley and wheat) sense day-length increases and promote flowering in late spring and/or early summer. In these plants, photoperiodic flowering responses are regulated by the CO-FT modules. The circadian clocks regulate CO expression through the function of PRRs, FKF1, GI, and CDFs in Arabidopsis, and Ppd1 (a homolog of PRR7) in barley and wheat. Vernalization represses FLC expression in Arabidopsis, while it induces VRN1 expression in barley and wheat. VRN1 depresses the expression of VRN2, which represses FT expression. Therefore, vernalization releases the strong repression of FT and enables these plants to promote flowering in LD. In the short-day plant rice, the circadian clock regulates Hd1 (a homolog of CO) expression through OsGI function. Hd1 represses the expression of a rice FT (Hd3a) in LD, while it promotes the expression of Hd3a and RFT1 (another rice FT homolog) in SD. Phytochromes and SE5 signaling promotes Hd1-dependent repression of Hd3a in LD. The expression of a rice-specific floral inducer Ehd1 is regulated by activators (OsMADS50, OsMADS51, OsGI, and Ehd2/OsId1/RID) and repressors (Ghd7, SE5, and phys). To promote flowering Ehd1 induces RFT1 expression in LD and both Hd3a and RFT1 expression in SD. Cultivated rice plants grow in a wider range of latitudes with different changes in day-length conditions. Depending on where they grow, either the long-day or the short-day pathways control flowering. Grey dotted lines indicate potential interaction.
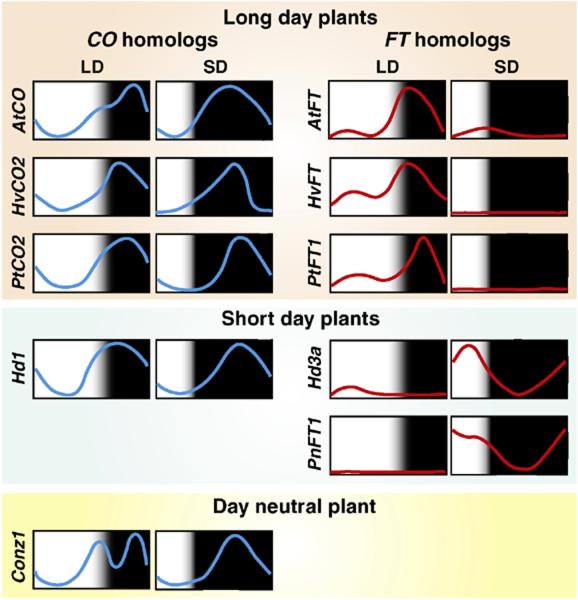
Figure 2.
The expression patterns of CO and FT in various plant species. CO expression profiles are similar in long-day and short-day plants under both long day (LD) and short day (SD) conditions. Even in the day neutral plant maize, conz1 (a homolog of rice Hd1) expression showed similar patterns [77]. These results suggest that the molecular mechanisms of CO transcriptional regulation may also be highly conserved among plants. In long-day plants, FT mRNA levels are high at the end of the day in LD and extremely low throughout the day in SD. In contrast, in short-day plants, FT mRNA levels are high in the morning in SD. White and black boxes designate day and night, respectively.
Winter varieties of barley, wheat, oat (Avena sativa) and rye (Secale cereale) require vernalization to induce flowering in LD [43]. The vernalization response in barley and wheat is mediated by a feedback regulatory loop formed by VERNALIZATION 1 (VRN1), VRN2, and VRN3 (these VRN genes are different from Arabidopsis VRN genes) [46–48]. None of the VRN genes encode FLC homologs. VRN1 is an APETALA1-like MADS-box transcription factor that is induced by vernalization [48]. VRN2 encodes a protein containing zinc-finger and CCT domains [47]. VRN2 inhibits FT induction and vernalization represses VRN2 expression. VRN3 is an FT gene [46]. Activation of VRN1 expression by vernalization decreases VRN2 expression to allow the long-day induction of FT (Fig 1). Thus, vernalization pathways in Arabidopsis and barley/wheat repress photoperiodic sensitivity by suppressing FT by utilizing different molecules.
In the poplar, a eudicot tree, LD induces flowering, while SD induces growth cessation and bud set. Poplar FT (PtFT1) regulates these two important developmental transitions [49]. As in Arabidopsis, the circadian clock regulates the timing of Poplar CO (PtCO2) and a high level of CO expression during the day seems to induce PtFT expression. (Fig. 2) Overexpression of PtFT1 induces precocious flowering and represses growth cessation and bud set. [49]. In the Norway spruce (Picea abies), a gymnosperm tree, the expression pattern of a FT homolog (PaFT4) correlates with the timing of bud set, indicating that FT plays an important role in developmental transition regulation in both angiosperms and gymnosperms [50].
The long-day plants examined use the CO-FT modules, and the expression patterns of both CO and FT in those plants are very similar (Fig. 2), suggesting that circadian clock-regulated CO expression mechanisms may also be conserved.
Photoperiodic flowering mechanism in short-day plants
Can the photoperiod measurement mechanism in long-day plants help us understand the mechanism in short-day plants? Success in QTL mapping on the heading date (flowering date) trait in rice enabled the identification of the genes involved in photoperiodic flowering regulation in rice. Some of the components identified are indeed orthologs of the Arabidopsis photoperiodic pathway components, but others are likely rice specific.
The rice ortholog of GI (OsGI) regulates diurnal expression of a rice CO gene, Heading date 1 (Hd1) [51]. In contrast to Arabidopsis CO, Hd1 possesses two opposite functions in the regulation of the rice FT gene, Heading date 3a (Hd3a). Hd1 inhibits Hd3a expression in LD but promotes its expression and subsequent flowering in SD (Fig. 1) [52,53]. The functional conversion of Hd1 on Hd3a repression and promotion in response to photoperiod changes is unclear, but phytochrome signaling may differently affect Hd1 activity in LD and SD [52,54].
Hd3a expression is also up-regulated by another strong rice-specific floral activator gene, Early Heading date 1 (Ehd1), which encodes a B-type response regulator [55]. Although Ehd1 expression is low in LD, Ehd1 antagonizes Hd1 function to enable rice to flower under these conditions (Fig 1). The expression of RICE FLOWERING LOCUS T1 (RFT1), the closest Hd3a homolog, induces flowering in LD, although RFT1 also promotes flowering in the absence of Hd3a in SD [56··]. Ehd1 expression is positively regulated by OsMADS50 (a rice homolog of SOC1) in LD, and by OsMADS51 in SD [57,58]. A rice ortholog of the maize Indeterminate1 (ld1) gene OsId1 (also known as Ehd2 and RID1) also induces Ehd1 expression in both LD and SD (Fig. 1) [59–61]. As a negative regulator of flowering, Ghd7, which contains a CCT domain related to VRN2 in barley and wheat, inhibits flowering through the repression of the Ehd1-Hd3a pathway [62··]. Phytochrome signaling through PhyB also negatively regulates Ehd1-RFT1 expression and down-regulates Hd3a expression through Hd1 activity in LD [56··]. In addition, the mutation in PHOTOPERIOD SENSITIVITY 5 (SE5), which encodes a heme oxygenase implicated in phytochrome chromophore biosynthesis, has a similar effect on rice FT expression regulation [54]. Cultivated rice plants grow throughout a wide area, spanning large latitudinal changes with diverse day-length changes [63]. Having two discrete floral regulators (Hd1 and Edh1) independently regulated by day-length conditions enables rice plants to adjust to environments with different day-length changes throughout the year.
A different photoperiodic response than those in Arabidopsis and in rice is found in the short-day eudicot Pharbitis (Ipomoea nil). Clock-regulated Pharbitis FT (PnFT) expression (Fig. 2) promotes flowering specifically in SD. In addition, the pattern of PnFT mRNA abundance is not highly correlated with the expression of PnCO, indicating that the photoperiod response in Pharbitis is controlled by a different mechanism for day-length sensing than that described for Arabidopsis and rice [64].
Although regulating FT expression seems to be the common final outcome of photoperiodic regulation in short-day plants, both rice and Pharbitis evolved their own regulatory mechanisms in addition to or separate from the CO-FT module, which is likely conserved in long-day plants.
Concluding remarks
Arabidopsis plays an important role as the model plant for increasing our understanding of the circadian clock and photoperiodism at molecular levels. The knowledge gained from Arabidopsis research has helped us to identify components and analyze the function of the circadian clock and photoperiodism in other plant species. Based on our current understanding, the circadian clock seems to be conserved in many plant species. However, our knowledge regarding the function of clock genes and the molecular architecture of clocks in plants other than Arabidopsis is still quite limited. More time is needed to truly understand how conserved the molecular clock systems are in plants.
In contrast, we have rapidly accumulated knowledge regarding the molecular mechanisms of photoperiodic flowering pathways in several plants, such as Arabidopsis, rice, and barley/wheat. The molecular mechanisms of photoperiod measurement are more diverse between long-day plants and short-day plants than between eudicots and monocots. Even in the short-day plant rice, the CO-FT module still exists and thus it could be a prototype of day-length sensing mechanisms. It would be interesting to find out whether other long-day plants use the CO-FT module and also to find out how other short-day plants measure changes in day length for flowering regulation.
Acknowledgements
I apologize to colleagues whose work was not cited due to space constrains. Preparation of this review was supported by the National Institute of Health (R01GM079712).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
· of special interest
·· of outstanding interest
- 1.Harmer SL. The circadian system in higher plants. Annu Rev Plant Biol. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 2.Imaizumi T. Arabidopsis circadian clock and photoperiodism: time to think about location. Curr Opin Plant Biol. 2010;13:83–89. doi: 10.1016/j.pbi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Más P, Yanovsky MJ. Time for circadian rhythms: plants get synchronized. Curr Opin Plant Biol. 2009;12:574–579. doi: 10.1016/j.pbi.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol. 2005;15:47–54. doi: 10.1016/j.cub.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 5.Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293:880–883. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- 6.Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma-Bognar L, Nagy F, Millar AJ, Amasino RM. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature. 2002;419:74–77. doi: 10.1038/nature00954. [DOI] [PubMed] [Google Scholar]
- 7.Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci U S A. 2005;102:10387–10392. doi: 10.1073/pnas.0503029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, et al. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell. 2005;17:2255–2270. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T. PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol. 2005;46:686–698. doi: 10.1093/pcp/pci086. [DOI] [PubMed] [Google Scholar]
- 10.Pruneda-Paz JL, Breton G, Para A, Kay SA. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science. 2009;323:1481–1485. doi: 10.1126/science.1167206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ·11.Nakamichi N, Kiba T, Henriques R, Mizuno T, Chua NH, Sakakibara H. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell. 2010 doi: 10.1105/tpc.109.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that PRR9, PRR7 and PRR5 physically associate with the promoters of CCA1 and LHY and repress their expression. Together with [10], we now know that most PRR proteins exist on the CCA1 and LHY promoters throughout the day.
- 12.Baudry A, Ito S, Song YH, Strait AA, Kiba T, Lu S, Henriques R, Pruneda-Paz JL, Chua NH, Tobin EM, et al. F-Box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell. 2010 doi: 10.1105/tpc.109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han L, David K, Putterill J, Nam HG, Somers DE. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature. 2007;449:356–360. doi: 10.1038/nature06132. [DOI] [PubMed] [Google Scholar]
- 14.Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Wang H, Gao P, Xu J, Xu T, Wang J, Wang B, Lin C, Fu YF. Analysis of clock gene homologs using unifoliolates as target organs in soybean (Glycine max) J Plant Physiol. 2009;166:278–289. doi: 10.1016/j.jplph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Kaldis AD, Kousidis P, Kesanopoulos K, Prombona A. Light and circadian regulation in the expression of LHY and Lhcb genes in Phaseolus vulgaris. Plant Mol Biol. 2003;52:981–997. doi: 10.1023/a:1025433529082. [DOI] [PubMed] [Google Scholar]
- 17.Weller JL, Hecht V, Liew LC, Sussmilch FC, Wenden B, Knowles CL, Vander Schoor JK. Update on the genetic control of flowering in garden pea. J Exp Bot. 2009;60:2493–2499. doi: 10.1093/jxb/erp120. [DOI] [PubMed] [Google Scholar]
- 18.Paltiel J, Amin R, Gover A, Ori N, Samach A. Novel roles for GIGANTEA revealed under environmental conditions that modify its expression in Arabidopsis and Medicago truncatula. Planta. 2006;224:1255–1268. doi: 10.1007/s00425-006-0305-1. [DOI] [PubMed] [Google Scholar]
- 19.Hecht V, Knowles CL, Vander Schoor JK, Liew LC, Jones SE, Lambert MJ, Weller JL. Pea LATE BLOOMER1 is a GIGANTEA ortholog with roles in photoperiodic flowering, deetiolation, and transcriptional regulation of circadian clock gene homologs. Plant Physiol. 2007;144:648–661. doi: 10.1104/pp.107.096818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ··20.Liew LC, Hecht V, Laurie RE, Knowles CL, Vander Schoor JK, Macknight RC, Weller JL. DIE NEUTRALIS and LATE BLOOMER 1 contribute to regulation of the pea circadian clock. Plant Cell. 2009;21:3198–3211. doi: 10.1105/tpc.109.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]; The author demonstrates that late1 and dne mutations affect not only the flowering phenotype but also the rhythmic expression of clock genes in the mutants. In addition, this paper shows that DNE is the ortholog of the Arabidopsis EARLY FLOWERING4 gene.
- 21.Takata N, Saito S, Tanaka Saito C, Nanjo T, Shinohara K, Uemura M. Molecular phylogeny and expression of poplar circadian clock genes, LHY1 and LHY2. New Phytol. 2008 doi: 10.1111/j.1469-8137.2008.02714.x. [DOI] [PubMed] [Google Scholar]
- ·22.Ibañez C, Ramos A, Acebo P, Contreras A, Casado R, Allona I, Aragoncillo C. Overall alteration of circadian clock gene expression in the chestnut cold response. PLoS One. 2008;3:e3567. doi: 10.1371/journal.pone.0003567. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that expression of the clock-associated genes in chestnut oscillates at normal growth temperatures but becomes constant at low temperatures. This conditional arrhythmicity is not to linked to dormancy, as it was shown that even endodormant plants can maintain the oscillation of clock genes when these plants are transferred to normal temperature conditions.
- 23.Ramos A, Pérez-Solís E, Ibáñez C, Casado R, Collada C, Gómez L, Aragoncillo C, Allona I. Winter disruption of the circadian clock in chestnut. Proc Natl Acad Sci U S A. 2005;102:7037–7042. doi: 10.1073/pnas.0408549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreps JA, Simon AE. Environmental and genetic effects on circadian clock-regulated gene expression in Arabidopsis. Plant Cell. 1997;9:297–304. doi: 10.1105/tpc.9.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe KH, Gouy M, Yang YW, Sharp PM, Li WH. Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc Natl Acad Sci U S A. 1989;86:6201–6205. doi: 10.1073/pnas.86.16.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami M, Tago Y, Yamashino T, Mizuno T. Comparative overviews of clock-associated genes of Arabidopsis thaliana and Oryza sativa. Plant Cell Physiol. 2007;48:110–121. doi: 10.1093/pcp/pcl043. [DOI] [PubMed] [Google Scholar]
- 27.Ogiso E, Takahashi Y, Sasaki T, Yano M, Izawa T. The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol. 2010;152:808–820. doi: 10.1104/pp.109.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miwa K, Serikawa M, Suzuki S, Kondo T, Oyama T. Conserved expression profiles of circadian clock-related genes in two Lemna species showing long-day and short-day photoperiodic flowering responses. Plant Cell Physiol. 2006;47:601–612. doi: 10.1093/pcp/pcj027. [DOI] [PubMed] [Google Scholar]
- 29.Serikawa M, Miwa K, Kondo T, Oyama T. Functional conservation of clock-related genes in flowering plants: overexpression and RNA interference analyses of the circadian rhythm in the monocotyledon Lemna gibba. Plant Physiol. 2008;146:1952–1963. doi: 10.1104/pp.107.114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ·30.Okada R, Kondo S, Satbhai SB, Yamaguchi N, Tsukuda M, Aoki S. Functional characterization of CCA1/LHY homolog genes, PpCCA1a and PpCCA1b, in the moss Physcomitrella patens. Plant J. 2009;60:551–563. doi: 10.1111/j.1365-313X.2009.03979.x. [DOI] [PubMed] [Google Scholar]; Together with [31], the authors demonstrated that PpCCA1a and PpCCA1b genes are involved in regulation of the circadian clock and growth rate under LD conditions. This is the first demonstration of physiological function of the circadian clock in non-angiosperm land plants.
- 31.Okada R, Satbhai SB, Aoki S. Photoperiod-dependent regulation of cell growth by PpCCA1a and PpCCA1b genes encoding single-myb clock proteins in the moss Physcomitrella patens. Genes Genet Syst. 2009;84:379–384. doi: 10.1266/ggs.84.379. [DOI] [PubMed] [Google Scholar]
- ··32.Corellou F, Schwartz C, Motta JP, Djouani-Tahri el B, Sanchez F, Bouget FY. Clocks in the green lineage: comparative functional analysis of the circadian architecture of the picoeukaryote ostreococcus. Plant Cell. 2009;21:3436–3449. doi: 10.1105/tpc.109.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides evidence that the green unicellular alga Ostreococcus tauri conserves only CCA1 and TOC1 homologs, and also provides their overexpresser and knockdown phenotype. The author suggests the clockwork of Ostreococcus is likely to be more complex than a simple TOC1 and CCA1 single loop mechanism
- 33.Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 34.Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- 35.Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature. 2003;426:302–306. doi: 10.1038/nature02090. [DOI] [PubMed] [Google Scholar]
- 36.Nakamichi N, Kita M, Niimura K, Ito S, Yamashino T, Mizoguchi T, Mizuno T. Arabidopsis clock-associated pseudo-response regulators PRR9, PRR7 and PRR5 coordinately and positively regulate flowering time through the canonical CONSTANS-dependent photoperiodic pathway. Plant Cell Physiol. 2007;48:822–832. doi: 10.1093/pcp/pcm056. [DOI] [PubMed] [Google Scholar]
- 37.Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell. 2009;17:75–86. doi: 10.1016/j.devcel.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 39.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 40.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 41.Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 2006;20:898–912. doi: 10.1101/gad.373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung S, Amasino RM. Remembering winter: toward a molecular understanding of vernalization. Annu Rev Plant Biol. 2005;56:491–508. doi: 10.1146/annurev.arplant.56.032604.144307. [DOI] [PubMed] [Google Scholar]
- 43.Greenup A, Peacock WJ, Dennis ES, Trevaskis B. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot. 2009;103:1165–1172. doi: 10.1093/aob/mcp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner A, Beales J, Faure S, Dunford RP, Laurie DA. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science. 2005;310:1031–1034. doi: 10.1126/science.1117619. [DOI] [PubMed] [Google Scholar]
- 45.Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.) Theor Appl Genet. 2007;115:721–733. doi: 10.1007/s00122-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci U S A. 2006;103:19581–19586. doi: 10.1073/pnas.0607142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science. 2004;303:1640–1644. doi: 10.1126/science.1094305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci U S A. 2003;100:6263–6268. doi: 10.1073/pnas.0937399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science. 2006;312:1040–1043. doi: 10.1126/science.1126038. [DOI] [PubMed] [Google Scholar]
- 50.Gyllenstrand N, Clapham D, Källman T, Lagercrantz U. A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiol. 2007;144:248–257. doi: 10.1104/pp.107.095802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature. 2003;422:719–722. doi: 10.1038/nature01549. [DOI] [PubMed] [Google Scholar]
- 52.Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 2002;16:2006–2020. doi: 10.1101/gad.999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson SD. Plant responses to photoperiod. New Phytol. 2009;181:517–531. doi: 10.1111/j.1469-8137.2008.02681.x. [DOI] [PubMed] [Google Scholar]
- 54.Andrés F, Galbraith DW, Talón M, Domingo C. Analysis of PHOTOPERIOD SENSITIVITY5 sheds light on the role of phytochromes in photoperiodic flowering in rice. Plant Physiol. 2009;151:681–690. doi: 10.1104/pp.109.139097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 2004;18:926–936. doi: 10.1101/gad.1189604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ··56.Komiya R, Yokoi S, Shimamoto K. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development. 2009;136:3443–3450. doi: 10.1242/dev.040170. [DOI] [PubMed] [Google Scholar]; This study reports the role of the RFT1 gene (the closest homolog to Hd3a) as a key activator of long-day flowering and showed that the RFT1 protein acts as a mobile florigenic signal, like FT and Hd3a. The authors suggested a new regulatory mechanism by which RFT1 expression is positively regulated by OsMADS50 and Ehd1.
- 57.Kim SL, Lee S, Kim HJ, Nam HG, An G. OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol. 2007;145:1484–1494. doi: 10.1104/pp.107.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryu CH, Lee S, Cho LH, Kim SL, Lee YS, Choi SC, Jeong HJ, Yi J, Park SJ, Han CD, et al. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ. 2009;32:1412–1427. doi: 10.1111/j.1365-3040.2009.02008.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu C, You C, Li C, Long T, Chen G, Byrne ME, Zhang Q. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci U S A. 2008;105:12915–12920. doi: 10.1073/pnas.0806019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol. 2008;148:1425–1435. doi: 10.1104/pp.108.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, Kim CM, Ryu CH, Park SH, Xuan YH, et al. Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J. 2008;56:1018–1029. doi: 10.1111/j.1365-313X.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- ··62.Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008;40:761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]; The authors identified rice Ghd7, a gene encoding a CCT domain, and showed that the gene has a key role in the repression of photoperiodic flowering by negatively regulating Ehd1 and Hd3a expression. This paper also proposed that natural variation in Ghd7 is an important regulator in the adaptation of rice cultivars to local areas at different latitudes.
- 63.Izawa T. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot. 2007;58:3091–3097. doi: 10.1093/jxb/erm159. [DOI] [PubMed] [Google Scholar]
- 64.Hayama R, Agashe B, Luley E, King R, Coupland G. A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis. Plant Cell. 2007;19:2988–3000. doi: 10.1105/tpc.107.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamilton EE, Kay SA. SnapShot: circadian clock proteins. Cell. 2008;135:368–368. e361. doi: 10.1016/j.cell.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 66.Boxall SF, Foster JM, Bohnert HJ, Cushman JC, Nimmo HG, Hartwell J. Conservation and divergence of circadian clock operation in a stress-inducible Crassulacean acid metabolism species reveals clock compensation against stress. Plant Physiol. 2005;137:969–982. doi: 10.1104/pp.104.054577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T. The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol. 2003;44:1229–1236. doi: 10.1093/pcp/pcg135. [DOI] [PubMed] [Google Scholar]
- 68.Murakami M, Tago Y, Yamashino T, Mizuno T. Characterization of the rice circadian clock-associated pseudo-response regulators in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2007;71:1107–1110. doi: 10.1271/bbb.70048. [DOI] [PubMed] [Google Scholar]
- 69.Kim JA, Yang TJ, Kim JS, Park JY, Kwon SJ, Lim MH, Jin M, Lee SC, Lee SI, Choi BS, et al. Isolation of circadian-associated genes in Brassica rapa by comparative genomics with Arabidopsis thaliana. Mol Cells. 2007;23:145–153. [PubMed] [Google Scholar]
- 70.Murakami M, Matsushika A, Ashikari M, Yamashino T, Mizuno T. Circadian-associated rice pseudo response regulators (OsPRRs): insight into the control of flowering time. Biosci Biotechnol Biochem. 2005;69:410–414. doi: 10.1271/bbb.69.410. [DOI] [PubMed] [Google Scholar]
- 71.Wilhelm EP, Turner AS, Laurie DA. Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.) Theor Appl Genet. 2009;118:285–294. doi: 10.1007/s00122-008-0898-9. [DOI] [PubMed] [Google Scholar]
- 72.Fu C, Yang XO, Chen X, Chen W, Ma Y, Hu J, Li S. OsEF3, a homologous gene of Arabidopsis ELF3, has pleiotropic effects in rice. Plant Biol (Stuttg) 2009;11:751–757. doi: 10.1111/j.1438-8677.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 73.Hayama R, Izawa T, Shimamoto K. Isolation of rice genes possibly involved in the photoperiodic control of flowering by a fluorescent differential display method. Plant Cell Physiol. 2002;43:494–504. doi: 10.1093/pcp/pcf059. [DOI] [PubMed] [Google Scholar]
- 74.Dunford RP, Griffiths S, Christodoulou V, Laurie DA. Characterisation of a barley (Hordeum vulgare L.) homologue of the Arabidopsis flowering time regulator GIGANTEA. Theor Appl Genet. 2005;110:925–931. doi: 10.1007/s00122-004-1912-5. [DOI] [PubMed] [Google Scholar]
- 75.Zhao XY, Liu MS, Li JR, Guan CM, Zhang XS. The wheat TaGI1, involved in photoperiodic flowering, encodes an Arabidopsis GI ortholog. Plant Mol Biol. 2005;58:53–64. doi: 10.1007/s11103-005-4162-2. [DOI] [PubMed] [Google Scholar]
- 76.Hong SY, Lee S, Seo PJ, Yang MS, Park CM. Identification and molecular characterization of a Brachypodium distachyon GIGANTEA gene: functional conservation in monocot and dicot plants. Plant Mol Biol. 2010;72:485–497. doi: 10.1007/s11103-009-9586-7. [DOI] [PubMed] [Google Scholar]
- 77.Miller TA, Muslin EH, Dorweiler JE. A maize CONSTANS-like gene, conz1, exhibits distinct diurnal expression patterns in varied photoperiods. Planta. 2008;227:1377–1388. doi: 10.1007/s00425-008-0709-1. [DOI] [PubMed] [Google Scholar]