Summary
Platelet activation and blood coagulation are essential for hemostasis and contribute to a variety of other biological processes such as inflammation, complement activation and tissue repair. Factor XII, originally called Hageman factor, plays an important role in the kallikrein-kinin system by activating prekallikrein. In the 1960s, a platelet activity that promoted factor XII activation was identified but its biochemical nature remained unknown. Inorganic polyphosphates (poly P) are polymers that consist of many phosphate residues linked by phosphoanhydride bonds. These polymers exist in all living organisms. In bacteria, poly P is important for growth and survival. Recently, poly P has been identified in human platelet dense granules. Studied have shown that upon platelet activation and secretion, poly P activates factor XII, indicating that it is most likely the elusive platelet factor XII activator. Poly P also regulates coagulation and fibrinolysis. In this review, we focus on early studies of factor XII and the identification of platelet factor XII activation activity, and discuss recent findings of poly P in factor XII activation and coagulation.
Keywords: factor XII, platelet, polyphosphate, coagulation
Introduction
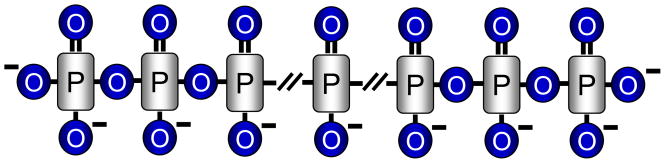
Phosphates are ubiquitous on earth. In addition to salt forms, inorganic phosphates also form linear polymers linked by phosphoanhydride bonds (Fig. 1). Such a polymer structure, called inorganic polyphosphate (poly P), can be made by dehydration of phosphates under extremely high temperature such as in volcanoes [1–4]. Like those in ATP, the phosphoanhydride bonds in poly P are energy-rich, which apparently has been exploited by microorganisms during evolution for survival and adaptation. Studies have shown that poly P is important in bacterial growth, motility, virulence and stress response [1–4].
Fig. 1.
A schematic presentation of inorganic Poly P.
In addition to bacteria and lower eukaryotes, poly P also exists in mammalian cells [5, 6]. Recently, human platelets have been found to contain significant amounts of poly P in their dense granules [7]. Studies to understand the functional significance of these seemingly primitive molecules have solved a long-standing puzzle in platelet-mediated factor XII activation.
Hageman Factor and the Waterfall/Cascade Hypothesis of Blood Coagulation
“Once in a rare while, a patient crosses the threshold and changes one’s life”, the late hematologist Oscar Ratnoff surmised 30 some years later [8]. Indeed, in July 1953 John Hageman, a 37-year-old brakeman of the Penn-Central Railroad in Ohio, changed not only Ratnoff’s life, but also the field of blood coagulation [8–10].
John Hageman had suffered duodenal ulcer and was to be operated at the Case Western Reserve University Hospitals in Cleveland. In a pre-operation exam, his blood unexpectedly failed to clot in test tubes, a finding troublesome enough for his procedure to be postponed. Oddly, Hageman had no history of bleeding problems even though he had tonsillectomy and dental extraction in the past [11, 12]. After being informed of this paradox [8], Ratnoff began to study Hageman’s blood, which led to the first report of Hageman trait, an inherited deficiency in blood coagulation [12]. The factor missing in individuals with Hageman trait was hence named Hageman factor.
Soon Ratnoff and others found that Hageman factor was activated upon encountering glass or other negatively charged surfaces [8, 13, 14], which explained why blood clotted faster in a glass tube than in a rubber tube, a phenomenon noticed by the English surgeon Joseph Lister a century ago [8]. It appeared, therefore, that Hageman factor acted at the initial phases of blood clotting. Starting in 1957, Ratnoff collaborated with Earl Davie to purify Hageman factor from human plasma [15]. This challenging work was accomplished in the early 1960s by using carboxymethylcellulose and DEAE chromatographies that just became available [15, 16].
With purified Hageman factor in hand, Ratnoff and Davie were able to show that it activated factor XI, which in turn activated factor IX [17]. These data, together with studies on additional blood clotting factors by other investigators at the time, suggested that coagulation was achieved by sequential activations that were progressively amplified, leading to thrombin generation and fibrin clot formation. In 1964, Davie and Ratnoff proposed their ‘Waterfall’ model of coagulation, marked by the ranking order of the reactions starting with Hageman factor (also called factor XII by then), factors XI, IX, VIII, X and V, prothrombin and fibrinogen [18]. Independently, Robert G. MacFarlane of Oxford proposed a remarkably similar model, which was called ‘Cascade’ [19]. Thus, a decade after John Hageman was introduced to Ratnoff, the basic principle of coagulation was established, setting the stage for many important future findings.
Shortly after Ratnoff reported the Hageman trait, we identified the first case of factor XII deficiency in France. This individual, named Pierrette Sartorio, was a healthy judo player. During an exam before her tonsillectomy, she had a prolonged partial thromboplastin time, suggesting a deficiency at early stages of coagulation. We contacted Ratnoff in Cleveland and obtained some of Mr. Hageman’s plasma samples. With Jean Bernard, we showed that Hageman’s plasma did not correct Sartorio’s partial thromboplastin time, thereby confirming that Sartorio had factor XII deficiency [20]. In recognition of Ratnoff’s research on blood coagulation, the International Society of Thrombosis and Haemostasis (ISTH) awarded him the Robert P. Grant Medal, the highest honor of the Society. This award was presented to him by Jacques Caen at the VIIIth ISTH Congress in Toronto in 1981.
Platelets and Factor XII Activation
In the early 1960s, we first reported the contact activation of coagulation in the presence of platelets, indicating a new role of platelets in initiating blood clotting [21]. By the mid 1960s, investigators in London and New York also showed that incubation of platelet-rich plasma with kaolin shortened the recalcification and Russell’s viper venom times in clotting assays [22–24]. The results suggested that exposure of platelets to a ‘foreign’ surface promoted coagulation and thus this platelet activity was called ‘platelet factor 3 availability’. It was noticed that this platelet activity was defective in thrombasthenic patients, possibly due to poor platelet responses to ADP.
We confirmed this finding in platelets from nine thrombasthenic patients. More importantly, we found that thrombasthenic platelets had a defect in factor XII activation, which accounted for the prolonged clotting times in platelet-rich plasma from thrombasthenic patients. We concluded, in a 1965 Nature paper, that in addition to the defects in platelet aggregation, thrombasthenic platelets had another defect in platelet factor 3 availability, which was important for factor XII activation [25]. At the time, the exact nature of this defect was unknown but might be associated with poor platelet secretion. In later years, platelet-mediated factor XII activation was studied extensively by other investigators [26–29], but the platelet factor XII activator missing in thrombasthenic patients remained elusive.
Biological Functions of Factor XII
From the very beginning, factor XII had an ‘identity crisis’ [8, 30], because individuals lacking it do not exhibit abnormal bleeding. In fact, John Hageman had gastrojejunostomy in 1954 without major complications. In 1968, he broke his left hemipelvis from a fall. After bedrest at St. Vincent Hospital in Toledo, he was on the way to recovery but died suddenly and unexplainably several days later. An autopsy showed that the death was caused by pulmonary thromboemboli [11]. It was incongruous that John Hageman, who had deficiency in a clotting factor, died of thrombosis. In a paper reporting Hageman’s demise, Ratnoff wrote: “As always, Mr. Hageman has left us with a plethora of questions”[11].
The question of factor XII function led investigators to believe that other factors initiate blood coagulation in vivo. It is now accepted that factor VII and tissue factor initiate thrombin generation and fibrin clot formation at sites of vessel injury [31–35]. Thrombin also activates factor XI, providing a positive feedback to enhance coagulation and prevent premature fibrinolysis [36–38]. Several other proteases such as hepsin and factor VII-activating protease were postulated to have a role in factor VII activation and hence initiation of coagulation [39, 40], but such a function in vivo has not yet been established [41–43].
Factor XII is believed to function primarily in the plasma kallikrein-kinin system, by converting prekallikrein to active kallikrein, which consequently converts high-molecular-weight kininogen to bradykinin [44–49]. The kallikrein-kinin system, also called the contact activation system, regulates vascular permeability, blood pressure, complement activation, bacterial infection and inflammatory response. Over the years, questions have been raised that factor XII deficiency might increase the risk of thrombosis. In several epidemiological studies, however, factor XII-deficient individuals did not appear to have higher overall incidences of thrombotic complications such as deep-vein thrombosis, myocardial infarction and stroke [50, 51]. Interestingly, in mouse models of thrombosis, lack of factor XII prevented the formation of platelet-rich occlusive thrombi [52], indicating that factor XII, while not essential for normal hemostasis, may play an important role in promoting thrombus growth under pathological conditions [44, 45, 53].
Role of Poly P in Hemostasis
Inorganic poly P was studied extensively by late Arthur Kornberg, starting his early days in the Cori lab at Washington University in St. Louis in the late 1940s [54]. Intrigued by the origin and function of poly P that was abundant in bacteria and yeast, Kornberg identified in E. coli an enzyme that assembled poly P from ATP [55]. However, unlike his work on DNA replication, for which he won a Nobel Prize in Physiology or Medicine in 1959, Kornberg’s early work on bacterial poly P was little appreciated and even failed to win him a faculty job at UC Berkeley [54]. Nevertheless, Kornberg’s passion for this “molecular fossil” never diminished for he, up until his death in 2007, continued to study the biosynthesis and function of poly P [56].
Poly P regulates growth and stress responses in microorganisms. In E. coli, for example, poly P levels may increase >100-fold in response to amino acid starvation. Poly P activates the ATP-dependent protease Lon to degrade ribosomal proteins, thereby providing necessary amino acids for bacterial survival [57]. In animals, poly P exists in most major organs including brain, heart, kidneys, liver and lungs [5]. Within cells, poly P can be detected in nuclei, plasma membrane, cytosol, mitochondria and microsomes. To date, however, the physiological function of poly P in most animal cells remains poorly understood [4].
Interestingly, human platelets contain significant amounts of poly P in their dense granules [7]. These platelet poly P polymers are of ~60–100 residues in length and released upon thrombin stimulation. Morrissey and co-workers were first to find that poly P activated the intrinsic pathway of blood clotting in human plasma-based assays [58], suggesting a role of poly P in coagulation. This seminal finding has been extended in a recent animal study. Renné and co-workers have reported that in mice purified platelet poly P activated factor XII and that the poly P-activated factor XII triggered the kallikrein-kinin system, producing high levels of bradykinin that increased vascular permeability and inflammatory response [59]. The results are significant and indicate that the negatively charged platelet poly P molecules serve as a ‘foreign’ surface, analogous to the glass surface, upon which factor XII is activated. The data also suggest that platelet poly P in the dense granules is most likely the elusive platelet factor XII activator we reported 45 years ago [25]. Impaired aggregation of thrombathenic platelets may prevent the release of poly P from the dense granules, thereby apparently ‘missing’ this factor.
In addition to factor XII activation, poly P modulates the activity of other proteins in blood coagulation and fibrinolysis. Morrissey and co-workers have shown that poly P enhanced thrombin-mediated factor V activation [58, 60]. The effect of poly P appeared to be mediated by its high affinity binding to thrombin exosite II [61], a positively charged site known for heparin binding [62–64]. Interestingly, poly P did not bind to thrombin exosite I, another positively charged site that interacts with many thrombin substrates, co-factors and inhibitors [62, 63, 65–68]. In the presence of poly P, fibrin clots were more resistant to fibrinolysis and this effect depended on thrombin-activatable fibrinolysis inhibitor (TAFI) but not factor XIII activity [58, 69]. Poly P was shown by electron microscopy to be incorporated directly into fibrin fibers, making fibrin strings thicker, stronger and thus more resistant to lysis [69].
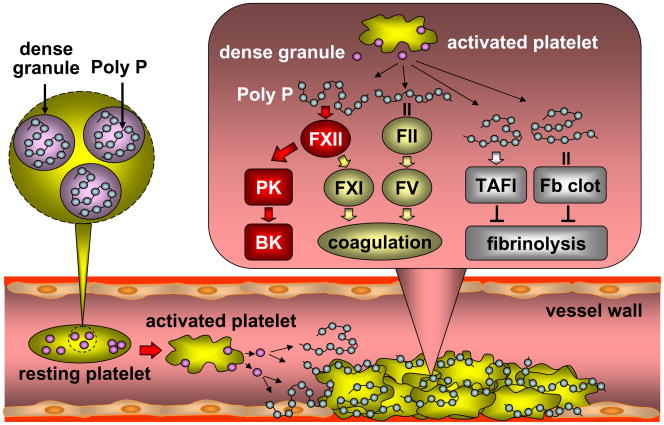
Together these recent data demonstrate that platelet poly P plays an important role in regulating the contact activation system, blood coagulation, and fibrinolysis. Upon platelet aggregation, poly P is secreted from the dense granules to activate factor XII and thus the kallikrein-kinin system. Poly P also promotes coagulation by indirectly activating factor XI and by strengthening fibrin fibers and inhibiting clot lysis (Fig. 2). In mice, infusion of purified platelet poly P caused lethal thrombosis [59]. Consistently, in a mouse model of Hermansky-Pudlak syndrome with defects in platelet dense granules, impaired platelet dense granule secretion significantly reduced arterial thrombosis and inflammation after vascular injury [70].
Fig. 2.
Poly P activates the kallikrein-kinin system and coagulation and inhibits fibrinolysis. Upon platelet activation, poly P is released from the dense granules to activate factor XII (FXII), which in turn activates prekallikrein (PK), consequently increasing bradykinin (BK) levels. Activated FXII also activates factor XI (FXI). By binding to thrombin (FII), poly P enhances thrombin-mediated factor V (FV) activation, promoting coagulation. In addition, Poly inhibits fibrinolysis by increasing TAFI activity and binding to fibrin (Fb) clots, making them more resistant to lysis.
Perspective
It is amazing that studies in totally separated fields have converged half a century later, leading to the identification of poly P as the long-sought platelet factor XII activator. Jacques Monod, who together with François Jacob, won a Nobel Prize in Physiology or Medicine in 1965 for their work on gene regulation, once said: “Je cherche à comprendre (I search to understand).” It is a great pleasure, indeed, for us to finally understand a long-standing question in blood coagulation and platelets.
The profound effect of poly P, an ancient molecule abundant in bacteria, on blood coagulation is intriguing. During evolution, the primary function of coagulation is as important for host defense as is for hemostasis. In the horseshoe crab, a primitive invertebrate that lacks adaptive immunity, hemolymph coagulation serves as a major mechanism in their innate immunity. Upon encountering bacterial lipopolysaccharides, serine proteases from hemocytes are activated sequentially to form coagulin clots to trap and kill invading microbes [71, 72]. The hemolymph coagulation cascade in the horseshoe crab is strikingly similar to the blood coagulation cascade in mammals. In bacteria and low eukaryotes, poly P is stored in acidocalcisomes, an acidic and calcium-rich compartment that is similar to platelet dense granules [4, 7]. It is possible, therefore, that platelet poly P-mediated factor XII activation has evolved from an ancient host defense system to battle inflammation and vascular injury.
As we recognize the role of platelet poly P in coagulation, we are stimulated to ask more questions. As for now, for example, we know little about how poly P is made in platelets. If platelet poly P promotes coagulation, can we use phosphatases, which degrade poly P, as an anti-thrombotic and anti-inflammatory agent? Recent studies of a phosphatase in mouse thrombosis models have produced promising results [59]. Along this line, then, is there any correlation between plasma phosphatase levels and risks of inflammation or thrombosis? If platelet poly P-mediated factor XII activation is important for a firm fibrin clot, is it possible that clots made in individuals lacking factor XII are weaker and prone to detach? Could this be an explanation for the pulmonary thromboembolism that ended John Hageman’s life? Science is a never-ending endeavor. Chercher à comprendre, as we all will continue to do.
Acknowledgments
The authors wish to dedicate this article to Oscar Ratnoff who passed away on May 20, 2008 in Cleveland. We thank J. Evan Sadler for helpful suggestions, and Anne-Sophie Caen and Shannon Wu for proofreading. This work was supported in part by grants from the Ralph Wilson Medical Research Foundation and the NIH (R01HL089298, R01HL089298-S1 to Q.W.).
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
References
- 1.Harold FM. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966;30:772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulaev I, Kulakovskaya T. Polyphosphate and phosphate pump. Annu Rev Microbiol. 2000;54:709–734. doi: 10.1146/annurev.micro.54.1.709. [DOI] [PubMed] [Google Scholar]
- 4.Rao NN, Gomez-Garcia MR, Kornberg A. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 5.Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 6.Pisoni RL, Lindley ER. Incorporation of [32P]orthophosphate into long chains of inorganic polyphosphate within lysosomes of human fibroblasts. J Biol Chem. 1992;267:3626–3631. [PubMed] [Google Scholar]
- 7.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 8.Ratnoff OD. The George M. Kober lecture. The legacy of John Hageman: new dividends. Trans Assoc Am Physicians. 1985;98:cli–clxi. [PubMed] [Google Scholar]
- 9.Ratnoff OD. A quarter century with Mr. Hageman Thromb Haemost. 1980;43:95–98. [PubMed] [Google Scholar]
- 10.Roberts HR. Oscar Ratnoff: his contributions to the golden era of coagulation research. Br J Haematol. 2003;122:180–192. doi: 10.1046/j.1365-2141.2003.04459.x. [DOI] [PubMed] [Google Scholar]
- 11.Ratnoff OD, Busse RJ, Sheon RP. The demise of John Hageman. N Engl J Med. 1968;279:760–761. [Google Scholar]
- 12.Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955;34:602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biggs R, Sharp AA, Margolis J, Hardisty RM, Stewart J, Davidson WM. Defects in the early stages of blood coagulation: a report of four cases. Br J Haematol. 1958;4:177–191. doi: 10.1111/j.1365-2141.1958.tb03848.x. [DOI] [PubMed] [Google Scholar]
- 14.Ratnoff OD, Rosenblum JM. Role of Hageman factor in the initiation of clotting by glass; evidence that glass frees Hageman factor from inhibition. Am J Med. 1958;25:160–168. doi: 10.1016/0002-9343(58)90023-8. [DOI] [PubMed] [Google Scholar]
- 15.Davie EW. A brief historical review of the waterfall/cascade of blood coagulation. J Biol Chem. 2003;278:50819–50832. doi: 10.1074/jbc.X300009200. [DOI] [PubMed] [Google Scholar]
- 16.Ratnoff OD, Davie EW. The purification of activated Hageman factor (activated factor XII) Biochemistry. 1962;1:967–975. doi: 10.1021/bi00912a005. [DOI] [PubMed] [Google Scholar]
- 17.Ratnoff OD, Davie EW, Mallett DL. Studies on the action of Hageman factor: evidence that activated Hageman factor in turn activates plasma thromboplastin antecedent. J Clin Invest. 1961;40:803–819. doi: 10.1172/JCI104314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davie EW, Ratnoff OD. Waterfall Sequence for Intrinsic Blood Clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane RG. An Enzyme Cascade in the Blood Clotting Mechanism, and Its Function as a Biochemical Amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 20.Caen J, Bernard J. Deficit en facteur Hageman. Rev Hematol. 1958;13:154–162. [PubMed] [Google Scholar]
- 21.Caen J, Haas F. Interaction des plaquettes et du plasma dans la phase initiale de la coagulation. Technique d’étude chez le sujet normal du rôle des plaquettes. Effet du contact. Rev Fr Et Clin Biol. 1960;5:146–152. [PubMed] [Google Scholar]
- 22.Hardisty RM, Dormandy KM, Hutton RA. Thrombasthenia. Studies on Three Cases. Br J Haematol. 1964;10:371–387. doi: 10.1111/j.1365-2141.1964.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 23.Hardisty RM, Hutton RA. The Kaolin Clotting Time of Platelet-Rich Plasma: a Test of Platelet Factor-3 Availability. Br J Haematol. 1965;11:258–268. doi: 10.1111/j.1365-2141.1965.tb06586.x. [DOI] [PubMed] [Google Scholar]
- 24.Spaet TH, Cintron J. Studies on Platelet Factor-3 Availability. Br J Haematol. 1965;11:269–275. doi: 10.1111/j.1365-2141.1965.tb06587.x. [DOI] [PubMed] [Google Scholar]
- 25.Castaldi PA, Larrieu MJ, Caen J. Availability of platelet Factor 3 and activation of factor XII in thrombasthenia. Nature. 1965;207:422–424. doi: 10.1038/207422a0. [DOI] [PubMed] [Google Scholar]
- 26.Majerus PW, Miletich JP. Relationships between platelets and coagulation factors in hemostasis. Annu Rev Med. 1978;29:41–49. doi: 10.1146/annurev.me.29.020178.000353. [DOI] [PubMed] [Google Scholar]
- 27.Walsh PN. Platelet coagulant activities and hemostasis: a hypothesis. Blood. 1974;43:597–605. [PubMed] [Google Scholar]
- 28.Walsh PN. Platelet-mediated trigger mechanisms in the contact phase of blood coagulation. Semin Thromb Hemost. 1987;13:86–94. doi: 10.1055/s-2007-1003478. [DOI] [PubMed] [Google Scholar]
- 29.Walsh PN, Griffin JH. Contributions of human platelets to the proteolytic activation of blood coagulation factors XII and XI. Blood. 1981;57:106–118. [PubMed] [Google Scholar]
- 30.Wright IS. Concerning the functions and nomenclature of blood clotting factors, with a preliminary report of the profile of blood clotting factors in young males. Ann Intern Med. 1959;51:841–850. doi: 10.7326/0003-4819-51-5-841. [DOI] [PubMed] [Google Scholar]
- 31.Broze GJ., Jr Tissue factor pathway inhibitor and the revised theory of coagulation. Annu Rev Med. 1995;46:103–112. doi: 10.1146/annurev.med.46.1.103. [DOI] [PubMed] [Google Scholar]
- 32.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 33.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 35.Morrissey JH. Tissue factor: a key molecule in hemostatic and nonhemostatic systems. Int J Hematol. 2004;79:103–108. doi: 10.1532/ijh97.03167. [DOI] [PubMed] [Google Scholar]
- 36.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 37.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266:7353–7358. [PubMed] [Google Scholar]
- 38.von dem Borne PA, Meijers JC, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86:3035–3042. [PubMed] [Google Scholar]
- 39.Kazama Y, Hamamoto T, Foster DC, Kisiel W. Hepsin, a putative membrane-associated serine protease, activates human factor VII and initiates a pathway of blood coagulation on the cell surface leading to thrombin formation. J Biol Chem. 1995;270:66–72. doi: 10.1074/jbc.270.1.66. [DOI] [PubMed] [Google Scholar]
- 40.Romisch J, Feussner A, Vermohlen S, Stohr HA. A protease isolated from human plasma activating factor VII independent of tissue factor. Blood Coagul Fibrinolysis. 1999;10:471–479. [PubMed] [Google Scholar]
- 41.Kanse SM, Parahuleva M, Muhl L, Kemkes-Matthes B, Sedding D, Preissner KT. Factor VII-activating protease (FSAP): vascular functions and role in atherosclerosis. Thromb Haemost. 2008;99:286–289. doi: 10.1160/TH07-10-0640. [DOI] [PubMed] [Google Scholar]
- 42.Wu Q. Gene targeting in hemostasis. Hepsin Front Biosci. 2001;6:D192–200. doi: 10.2741/a604. [DOI] [PubMed] [Google Scholar]
- 43.Wu Q, Yu D, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest. 1998;101:321–326. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 45.Gailani D, Renne T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 46.Maas C, Govers-Riemslag JW, Bouma B, Schiks B, Hazenberg BP, Lokhorst HM, Hammarstrom P, ten Cate H, de Groot PG, Bouma BN, Gebbink MF. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb Haemost. 2007;98:77–83. [PubMed] [Google Scholar]
- 48.Schmaier AH. The elusive physiologic role of Factor XII. J Clin Invest. 2008;118:3006–3009. doi: 10.1172/JCI36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5:2323–2329. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- 50.Koster T, Rosendaal FR, Briet E, Vandenbroucke JP. John Hageman’s factor and deep-vein thrombosis. Leiden thrombophilia Study. Br J Haematol. 1994;87:422–424. doi: 10.1111/j.1365-2141.1994.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 51.Zeerleder S, Schloesser M, Redondo M, Wuillemin WA, Engel W, Furlan M, Lammle B. Reevaluation of the incidence of thromboembolic complications in congenital factor XII deficiency--a study on 73 subjects from 14 Swiss families. Thromb Haemost. 1999;82:1240–1246. [PubMed] [Google Scholar]
- 52.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renne T, Gailani D. Role of Factor XII in hemostasis and thrombosis: clinical implications. Expert Rev Cardiovasc Ther. 2007;5:733–741. doi: 10.1586/14779072.5.4.733. [DOI] [PubMed] [Google Scholar]
- 54.Kornberg A. ATP and inorganic pyro- and polyphosphate. Protein Sci. 1993;2:131–132. doi: 10.1002/pro.5560020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornberg A, Kornberg SR, Simms ES. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim Biophys Acta. 1956;20:215–227. doi: 10.1016/0006-3002(56)90280-3. [DOI] [PubMed] [Google Scholar]
- 56.Fuller RS. A tribute to Arthur Kornberg 1918–2007. Nat Struct Mol Biol. 2008;15:2–17. doi: 10.1038/nsmb0108-2. [DOI] [PubMed] [Google Scholar]
- 57.Kuroda A, Nomura K, Ohtomo R, Kato J, Ikeda T, Takiguchi N, Ohtake H, Kornberg A. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli Science. 2001;293:705–708. doi: 10.1126/science.1061315. [DOI] [PubMed] [Google Scholar]
- 58.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutch NJ, Myles T, Leung LL, Morrissey JH. Polyphosphate binds with high affinity to exosite II of thrombin. J Thromb Haemost. 2009;11:548–555. doi: 10.1111/j.1538-7836.2009.03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bode W. The structure of thrombin: a janus-headed proteinase. Semin Thromb Hemost. 2006;32 (Suppl 1):16–31. doi: 10.1055/s-2006-939551. [DOI] [PubMed] [Google Scholar]
- 63.Leung LL, Gibbs CS. Modulation of thrombin’s procoagulant and anticoagulant properties. Thromb Haemost. 1997;78:577–580. [PubMed] [Google Scholar]
- 64.Sheehan JP, Sadler JE. Molecular mapping of the heparin-binding exosite of thrombin. Proc Natl Acad Sci U S A. 1994;91:5518–5522. doi: 10.1073/pnas.91.12.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Q, Picard V, Aiach M, Sadler JE. Activation-induced exposure of the thrombin anion-binding exosite. Interactions of recombinant mutant prothrombins with thrombomodulin and a thrombin exosite-specific antibody. J Biol Chem. 1994;269:3725–3730. [PubMed] [Google Scholar]
- 66.Wu Q, Tsiang M, Sadler JE. Localization of the single-stranded DNA binding site in the thrombin anion-binding exosite. J Biol Chem. 1992;267:24408–24412. [PubMed] [Google Scholar]
- 67.Wu Q, Sheehan JP, Tsiang M, Lentz SR, Birktoft JJ, Sadler JE. Single amino acid substitutions dissociate fibrinogen-clotting and thrombomodulin-binding activities of human thrombin. Proc Natl Acad Sci U S A. 1991;88:6775–6779. doi: 10.1073/pnas.88.15.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye J, Rezaie AR, Esmon CT. Glycosaminoglycan contributions to both protein C activation and thrombin inhibition involve a common arginine-rich site in thrombin that includes residues arginine 93, 97, and 101. J Biol Chem. 1994;269:17965–17970. [PubMed] [Google Scholar]
- 69.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112:2810–2816. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.King SM, McNamee RA, Houng AK, Patel R, Brands M, Reed GL. Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation. 2009;120:785–791. doi: 10.1161/CIRCULATIONAHA.108.845461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwanaga S. The molecular basis of innate immunity in the horseshoe crab. Curr Opin Immunol. 2002;14:87–95. doi: 10.1016/s0952-7915(01)00302-8. [DOI] [PubMed] [Google Scholar]
- 72.Muta T, Iwanaga S. The role of hemolymph coagulation in innate immunity. Curr Opin Immunol. 1996;8:41–47. doi: 10.1016/s0952-7915(96)80103-8. [DOI] [PubMed] [Google Scholar]