1. Introduction
Schizotypal personality disorder (SPD) represents a diagnostic phenotype in the schizophrenia spectrum between schizophrenia and healthy controls (HC), with genetic, biological and behavioral characteristics shared with chronic schizophrenia (Siever and Davis, 2004). The symptoms of SPD, while less severe, mirror those of schizophrenia, including psychotic-like symptoms, social deficits, and cognitive impairment. Since SPD patients share these underlying spectrum characteristics with typical schizophrenia, insights into the pathophysiology of SPD may improve our understanding not only of this disorder, but of schizophrenia itself (Siever and Davis, 2004). Another particularly important question is what biological characteristics protect the SPD patients from the emergence of severe psychosis. This phenotypic difference has been hypothesized to be related to a better regulated (less responsive) striatal dopaminergic system in SPD compared to schizophrenia (Kirrane and Siever, 2000; Siever and Davis, 2004).
Hyperdopaminergic activity in the striatum is thought to be related to positive symptoms and hypodopaminergic activity in the prefrontal cortex to be related to negative ones (Davis, 1991; Siever et al., 1994). Dopamine activity can be measured both in imaging and pharmacological intervention studies. Imaging studies have shown that patients with schizotypal personality disorder have higher striatal dopamine release in response to amphetamine than healthy patients, but lower than schizophrenia patients (Abi-Dargham et al., 2004). In one study (Mitropoulou et al., 2004) SPDs evidenced a blunted cortisol and normal dopaminergic responses to 2-deoxyglucose (DG), in contrast to increased cortisol and dopamine responses to 2DG in schizophrenia patients.
Visual contrast detection reflects dopaminergic functioning in the early visual system (Harris et al., 1990; Masson et al., 1993). Dopamine enhances visual contrast detection by inhibiting the surrounding areas of the retinal neural unit’s receptive field via the D2 receptors (Djamgoz et al., 1997). Studies in Parkinson’s Disease patients (Bodis-Wollner, 1990), patients with phenylketonuria (PKU) (Diamond and Herzberg, 1996), and animals and humans with dopaminergic intervention (Corbe et al., 1992; Boumghar et al., 1997) suggest these retinal dopamine activity may in some cases parallel dopamine activity in the brain. Although the cellular mechanisms of striatal dopamine release are incompletely understood, SPD patients demonstrate less subcortical hyperdopaminergia than patients with schizophrenia, consistent with the hypothesis that SPD patients have less subcortical dopaminergic hyper-responsiveness compared to patients with schizophrenia (Abi-Dargham et al., 2004; Siever and Davis, 2004). Furthermore, SPD patients are hypothesized to have reduced dopamine activity in the prefrontal cortex (Siever and Davis, 2004) and their abnormal working memory and cognitive performance (Mitropoulou et al, 2005) are inversely correlated with dopamine and memory Siever et al., 1993 and can be partially normalized by dopaminergic agents (Siegel et al., 1996; Kirrane et al., 2003; McClure et al., 2009). If retinal D2 receptor activity mirrors that of the brain, reduced to normal visual contrast sensitivity might be expected in SPD, in contrast to the increased contrast sensitivity in schizophrenia (Kéri et al., 1998; Chen et al., 2003).
Disparate results have been reported for visual contrast detection in schizophrenia patients: Some studies show decreased contrast sensitivity (Slaghuis, 1998; Butler et al., 2001, Kéri et al., 2002; Chen et al., 2004), others describe excessive sensitivity (Kéri et al., 1998; Chen et al., 2003), and another reports no difference (Chen et al., 1999). One factor that may account for these disparate findings is the different antipsychotic drugs administered to these patients. One study hypothesized that the differences were due to the relative potency and the respective binding strength of the different classes of antipsychotic drugs (Chen et al., 2003). The typical antipsychotics, potent dopamine-antagonists, were associated with the lowest visual contrast sensitivity. Atypical antipsychotics, on the other hand, are less potent dopamine antagonists and thus do not lower contrast sensitivity as dramatically. Thus, patients with schizophrenia on typical antipsychotic agents had lower contrast sensitivity than HC, while those on atypical agents had the same sensitivity as HC, and those who were not medicated had higher sensitivity (Chen et al., 2003). Indeed in the two studies that have tested unmedicated patients thus far, superior visual contrast detection was demonstrated in schizophrenia, even compared to HC.
Recent studies reported that SPD patients’ performance was normal in visual contrast detection (O’Donnell et al., 2006) and abnormal in other visual tasks such as those requiring temporal integration or working memory (Cadenhead et al., 1999; Farmer et al., 2000; Siever et al., 2004). This pattern of results, while interesting, poses a question as to whether visual processing in SPD is still normal when basic temporal integration is involved. Temporal integration and storage is a fundamental aspect of visual functioning (Supèr et al., 2001) and the temporal dynamics of contrast detection is modulated by dopamine (Masson et al., 1993). In this study, we evaluated contrast detection of SPD patients under the conditions for which visual information is temporally integrated.
Moreover, in order to demonstrate whether the visual contrast detection in question is specifically related to a schizophrenia spectrum disorder or mainly due to personality disorders unrelated to schizophrenia, it is important to examine SPD patients in comparison to patients who have other personality disorders (OPDs), and thus are not hypothesized to have a dopamine abnormality affecting contrast detection. This study proposed to determine whether SPD patients differ from patients with other personality disorders and HCs in their ability to detect visual contrast.
2. Experimental/Materials and methods
2.1 Subjects
Twenty-one subjects with DSM-IV diagnosed schizotypal personality disorder, eighteen healthy controls, and twelve subjects with a personality disorder unrelated to schizophrenia (with less than 2 schizotypal traits) were recruited for this study by advertisements and word of mouth. All participants were between the ages of 18 and 65 and were studied as outpatients. The subject groups had no significant differences in age, gender, or years of education (Table 1).
Table 1.
Demographic information
| Characteristic | Patients with Schizotypal Personality Disorder (N=21*) |
Healthy Volunteers (N=18) |
Patients with Personality Disorders Unrelated to Schizophrenia (N=12*) |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Gender | ||||||
| Male | 12 | 57.1% | 7 | 38.9% | 7 | 58.3% |
| Female | 9 | 42.8% | 11 | 61.1% | 5 | 41.7% |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | ||||||
| Both genders | 40.8 | 11.5 | 35 | 9.8 | 38.2 | 15 |
| Years of Education | ||||||
| Both genders | 15.2 | 3.0 | 17.4 | 2.9 | 16.9 | 1.7 |
Data from one SPD patient and one OPD patient were discarded due to being extreme outliers
After informed consent was obtained, participants completed a medical evaluation and any participants with a history of systemic medical illnesses, serious eye disorders, a history of serious head trauma, or positive toxicology screens were excluded. The patients were evaluated for medical illnesses by a standard battery of blood tests, including an SMA-18. A medical history was also taken, where patients were asked about current or past eye problems. Patients were also excluded if they met criteria for a psychotic disorder or bipolar I, met lifetime criteria for substance dependence, had substance abuse in the last 6 months, or were currently taking psychiatric medication.
Diagnostic evaluations were conducted by doctoral-level clinical psychologists using the Structured Clinical Interview for DSM-IV for Axis I disorders and the Structured Interview for DSM-IV Personality for Axis II disorders (First et al., 1997). Diagnoses were given in a consensus meeting with an expert diagnostician in which the clinical interviewer presented all available information on each participant. Healthy control participants had no history of Axis I or Axis II disorders, and no first degree relatives with Axis I disorders. Those participants who had personality disorders unrelated to schizophrenia primarily consisted of patients with avoidant personality disorder, and all had less than 2 schizotypal traits. Patients with Schizotypal Personality Disorder met DSM-IV criteria according to the SIDP, all possessing at least 5 schizotypal traits.
2.2 Experimental procedure
The stimulus for contrast detection was a sinusoidal grating at varying contrast levels. Subjects sat in a dark room at a distance of 57 cm away from the computer screen. They were given 5 minutes to accommodate to the low light conditions before beginning the experiment. The task presented to the subjects was to detect the presence of the stimulus at varying temporal frequencies. In each trial the subject was exposed to 2 temporal intervals, each paired with a distinct auditory tone. The auditory tone demarcates one interval from another. Each interval lasted 300 msec. The gratings were presented in one of the intervals, either the first or the second, and in the other interval, a blank screen of same luminance level was displayed. Between the two intervals, there was a 500 msec break.
Subjects were asked to indicate whether the stimulus was present in the first or the second interval by pressing a designated key. The spatial frequency of the stimulus was 0.5 cycles/degree. The temporal frequencies was 0 (static), 1, 5, 10 or 15 Hz. The low spatial frequency grating (0.5 cycles/degree) was selected for this study because using this stimulus in previous studies yielded significant group differences between schizophrenia patients and healthy controls (e.g. Chen et al., 2004). It was found that visual processing in schizophrenia patients were more impaired in the presence of temporally modulated, than static, stimuli (Kéri et al., 2002). This study thus includes a series of temporal frequency conditions (0, 1, 5, 10, 15 Hz) and the spatial frequency was kept constant.
A psychophysical method was employed to measure contrast detection threshold (inverse of contrast sensitivity). The adaptive staircase method determined a perceptual threshold for each stimulus condition, which is defined as the minimum contrast level, or difference in luminosity between the bars of the sinusoidal grating, needed for a subject to perform the task at equivalent 79% accuracy level. Throughout a testing session, the contrast level in a trial was increased if an incorrect response was produced in a previous trial, and decreased if three correct responses were produced consecutively. The subjects underwent evaluation under the 5 temporal frequency conditions and 2 times each, which resulted in a total of 10 testing sessions.
The entire procedure took approximately 40 minutes to complete.
2.3 Neuropsychological tests
The Paced Auditory Serial Addition Test (PASAT) is a test of verbal working memory and attention. It requires subjects to listen to a list of 50 randomly selected numbers, with a 2 second pause after each number is spoken. Subjects are asked to add each adjacent pair of numbers and to report each answer verbally. The dependent measure for this test is defined as the number of times a subject responds correctly (Gronwall, 1977). The PASAT is difficult for patients with SPD due to the combination of memory, attention, and mental manipulation required (Mitropoulou et al., 2005). This specific deficit has also been correlated with interpersonal factors in SPD patients (Mitropoulou et al., 2002).
In the N-Back test, subjects watch sequences of letters appear on a computer screen. Each sequence represents one of three conditions. Subjects are instructed to respond each time they see a specific letter (0-Back condition), when they see a letter that is the same as the previous letter (1-Back condition), or when they see a letter that is the same as the one that they saw two letters back (2-Back condition). Subjects complete each condition three times in random order. The dependent measure for this test is calculated separately for each condition, and is defined as the proportion of each subject’s responses that are correct (Gevins and Cutillo, 1993). The N-Back test in particular is more difficult for patients with SPD (McClure et al., 2008) due to the temporal integration and context processing required. This test is of particular interest for contrast detection because Abi-Dargham (2003) has demonstrated a relationship between N-Back scores and dopaminergic activity in SPD patients.
The Trail-Making Test requires subjects to connect a series of numbers in sequential order. The dependent measure used for this assessment is the number of seconds that the subject requires in order to complete the task (Reitan et al., 1958). The trail-making test is used to assess verbal/spatial perception and psychomotor speed. SPD patients have demonstrated poor performance in this task thus it is a potentially useful comparison when studying visual perceptual deficits (Mitropoulou et al., 2005).
The Wechsler Adult Intelligence Scale – Revised (WAIS-R) is a widely used and well-validated intelligence test. This study used the vocabulary and block design portions of the WAIS-R to assess subjects’ verbal intelligence and visuospatial ability. For the purpose of this study, the dependent measure for this test is defined as a subject’s age-adjusted scaled scores (Wechsler, 1981).
3. Results
3.1 Demographics and clinical variables
The subject groups did not have significant differences in age, gender, or years of education (Table 1). No significant correlation was found between the SPD patients’ mean contrast detection threshold, and the severity of their illness. Illness severity was defined as the number of DSM-IV SPD symptoms met by each patient.
3.2 Visual contrast detection
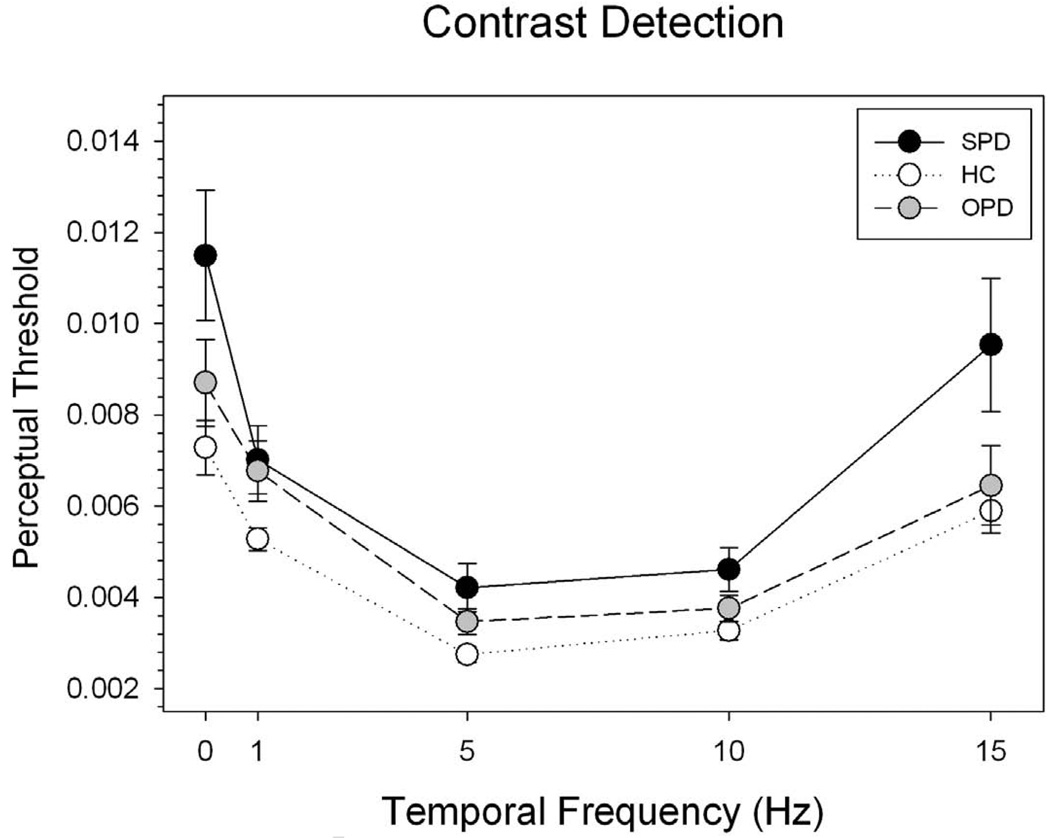
A Repeated-Measures ANOVA showed a significant effect of subject group on the contrast detection threshold (F(2, 48) = 5.358, p = .008) (Figure 1). The effect of temporal frequency was also significant (F(4, 192) = 43.286, p < 0.001). Contrast detection thresholds in all groups were markedly low for the 5 Hz condition and high for the 0 Hz (static) and 15 Hz (highly temporally modulated) conditions, a pattern consistent with findings in literature (Kelly et al., 1979; Chen et al., 1996). There was no significant interaction between subject group and temporal frequency (p = 0.462).
Fig. 1.
Summary of contrast detection thresholds in SPD, HC and OPD groups Perceptual threshold was defined as minimum difference in luminosity between the bars of the sinusoidal grating needed for subjects to perform the task at equivalent 79% accuracy level. The X axis represents temporal frequencies whereas Y axis represents the means of perceptual threshold for each group. The error bars indicate one standard error
A Turkey post-hoc test indicated a significant group difference between the SPD and the HC (p = 0.006), but not between the OPD and the HC (p = 0.438) or between the OPD and the SPD (p = 0.248). The SPD group had higher contrast detection thresholds at all frequencies tested.
Between SPD patients and controls, a repeated-measure ANOVA showed a significant group effect on the threshold (F(1, 38) = 10.856, p = 0.002). Temporal frequency also had a significant effect on detection threshold (F(4,152) = 26.885, p < 0.001). However, there was no significant interaction between subject group and temporal frequency (p = .110).
3.3 Cognitive measures
In addition to the contrast detection paradigm, some of the SPD patients also completed a cognitive testing session. Since not all of the patients completed the testing, we only have N-Back data for 11 of the 21 SPD patients and the other tests for 18 of 21 patients. Although the sample was too small to be definitive, the N-Back computer task showed a significant correlation with contrast detection threshold (see Table 2). In particular, poor performance on the 2-Back condition was associated with a higher contrast detection threshold at 5 Hz (r(10)= −.769, p=.003). Significant correlation was also found between SPD patients’ mean contrast detection threshold and performance on the Trail-making Test A (see Table 3). No significant correlation was found between contrast detection threshold and performance on the WAIS-R intelligence test, or the PASAT (see Table 3).
Table 2.
Correlation between visual contrast detection threshold and neuropsychological measures
| Temporal Frequency | PASAT (n=18) |
WAIS-R Vocab (n=18) |
WAIS-R Block (n=18) |
Trails A (n=18) |
Trails B (n=18) |
|---|---|---|---|---|---|
| 0 Hz | |||||
| Pearson Correlation | −.338 | .233 | .076 | .422 | .313 |
| Sig. (2-tailed) | .170 | .352 | .766 | .081 | .206 |
| 1 Hz | |||||
| Pearson Correlation | −.315 | .290 | −.521* | .638** | .300 |
| Sig. (2-tailed) | .203 | .244 | .027 | .004 | .227 |
| 5 Hz | |||||
| Pearson Correlation | −.075 | .326 | −.464 | .414 | .141 |
| Sig. (2-tailed) | .766 | .187 | .053 | .088 | .577 |
| 10 Hz | |||||
| Pearson Correlation | .165 | .237 | −.107 | .173 | −.048 |
| Sig. (2-tailed) | .513 | .344 | .672 | .493 | .850 |
| 15 Hz | |||||
| Pearson Correlation | −.278 | −.016 | .160 | .496* | .123 |
| Sig. (2-tailed) | .264 | .951 | .527 | .036 | .627 |
| Mean | |||||
| Pearson Correlation | −.302 | .223 | −.116 | −.619** | .244 |
| Sig. (2-tailed) | .224 | .374 | .646 | .006 | .329 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 3.
Correlation between contrast threshold and 2-Back performance
| Temporal Frequency | 2-Back (n=11) |
|---|---|
| 0 Hz | |
| Pearson Correlation | −.497 |
| Sig. (1-tailed) | .060 |
| 1 Hz | |
| Pearson Correlation | −.373 |
| Sig. (1-tailed) | .129 |
| 5 Hz | |
| Pearson Correlation | −.569* |
| Sig. (1-tailed) | .034 |
| 10 Hz | |
| Pearson Correlation | −.769** |
| Sig. (1-tailed) | .003 |
| 15 Hz | |
| Pearson Correlation | .014 |
| Sig. (1-tailed) | .484 |
| Mean | |
| Pearson Correlation | −.136 |
| Sig. (1-tailed) | .345 |
Correlation is significant at the 0.05 level (1-tailed).
Correlation is significant at the 0.01 level (1-tailed)
4. Discussion
This study found that the SPD patients had significantly higher visual contrast detection thresholds (lower contrast sensitivity) across a range of temporal frequencies compared to healthy controls. There was no significant group difference between the OPD patients and the HCs.
4.1 Contrast detection in SPD
SPD patients’ decreased contrast sensitivity found in this study appears to be in contrast with those found in the study by O’Donnell et al. (2006). The difference was intriguing in that both studies were similar in terms of the characteristics of SPD patients tested. In terms of experimental paradigm, however, there was a critical difference. In O’Donnell et al.’s study, subjects were asked to respond immediately after each stimulus presentation (whether the target was present or not). In our study, subjects were asked to respond after two stimulus presentations (whether the target was present in the first or the second presentation). In other words, the O’Donnell et al.’s paradigm did not require temporal integration of visual information whereas our paradigm did. The involvement of visual integration may contribute the different results yielded in the two studies. This interpretation is also in line with several previous findings in which SPD patients showed deficient performance on the visual and cognitive tasks that depend upon information integration over time (Cadenhead et al., 1999; Coleman et al., 1996; Farmer et al., 2000; Siever et al., 2002; Barch et al., 2004; Gooding et al., 2006).
Although attention is typically impaired in SPD (Roitman et al. 1997), attention is unlikely to be a confounding factor in this experiment. The contrast detection results cannot be accounted for by impaired attention as this basic perceptual task does not have a significant amount of attention. In addition, schizophrenia patients have well-documented difficulties with attention (Heinrichs and Zakzanis, 1998), but schizophrenia patients can still perform this task above, similar to, or below normal levels, suggesting that the attention problem is not a primary limiting factor (Chen et al., 2003).
General eye dysfunction is also unlikely to be a significant confound. All subjects had been screened for known eye diseases before participation. In addition, the magnitudes of the contrast detection deficit in SPD subjects (figure 1) were not uniform across temporal frequency, a pattern of result not consistent with a general eye pathology problem.
4.2 Schizotypal vs. other personality disorders
Unlike in SPD, contrast sensitivity in OPD does not appear to differ significantly from that in healthy controls. No significant group difference in most stimulus conditions discounts the notion that a personality disorder is a general factor that intrinsically adversely affects contrast detection in SPD. This aspect of the result is thus consistent with the notion that the contrast detection abnormality is specific to SPD among the personality disorders.
4.3 Implications for dopamine activation in the schizophrenia spectrum disorder
The superior visual contrast detection found in unmedicated schizophrenia patients relative to HC is hypothesized to be due to hyperdopaminergic activity in the retina and is consistent with Bleuler’s observation that patients with schizophrenia exhibit a hypersensitivity to sensory stimuli (Bleuler, 1950). The reason why typical antipsychotics have been found to reduce contrast sensitivity more than the atypical agents may be because the former bind more tightly and for a longer duration to D2 receptors, including the D2 receptors in the retina (Chen et al., 2003).
The results of our study converge with other studies (Abi-Dargham et al., 2004; Mitropoulou et al., 2004) in suggesting that dopaminergic activity is not increased in SPD patients compared to controls to the extent it is in patients with schizophrenia, and may even be reduced in comparison to healthy controls in some dopamine systems such as the visual contrast system as it appears to be in the prefrontal cortex (Siever & Davis, 2004).
Reduced dopaminergic activity in the prefrontal cortex has also been shown to negatively impact neuropsychological tests, particularly the N-Back (Abi-Dargham, 2003). If reduced contrast sensitivity is caused by decreased dopamine, we would expect to find a correlation between a high detection threshold and poor performance on neuropsychological tasks. Our data show that the most salient correlations occur between N-Back performance and contrast threshold at 5 Hz, the same temporal frequency at which Chen et al. (2003) found clear distinctions between schizophrenia patients on different antipsychotic medications. In addition, we found that higher contrast detection thresholds were correlated with poorer performance on the Trails A task. These pieces of evidence provide further support for the idea that the reduced dopamine activity in the PFC in SPD may extend into the retina.
The data of this study should not be presented without a few caveats. We only have neuropsychological data for a subset of the patients and we have too little N-Back data to draw definitive conclusions. However, the results of the N-Back test do show a significant difference in the expected direction. The connection between Trails A and contrast sensitivity is less clear; as the similar Trails B test does not show a correlation. Although we did not collect neuropsychological data for control subjects, a number of previous studies have shown group deficits in attention, working memory, and context-processing (McClure et al., 2008; Mitropoulou et al., 2005; Roitman et al., 1997). Without more extensive neuropsychological data, we cannot draw definitive conclusions about the cognitive deficits associated with contrast detection.
It should also be noted that although a few studies have indicated retinal dysfunction in schizophrenia (Balogh et al., 2008; Hébert et al., 2010), no study has directly demonstrated altered dopamine processes at the retinal level. Using a visual task paradigm associated with retinal and cortical functions, this study provides behavioral evidence that supports the dopamine abnormality hypothesis.
Reduced contrast sensitivity in SPD is consistent with findings that SPD patients appear to have reduced cortical dopaminergic activity and variable subcortical dopaminergic activity compared to healthy controls. In contrast, their dopamine activity is consistently reduced in comparison to schizophrenia patients (Abi-Dargham et al., 2004; Mitropoulou et al., 2004). While an increase in dopamine activity may be related to superior contrast detection in schizophrenia patients, reduced dopamine activity may be associated with poorer contrast detection in SPD patients. These results highlight possible differences in dopaminergic activity and psychophysiology between SPD and schizophrenia patients in contrast to the numerous similarities between the two spectrum disorders (Siever and Davis, 2004). The difference in contrast detection between SPD and schizophrenia patients suggests that dopamine functioning in the early visual system is related to the brain mechanisms that prevent SPD from the emergence of severe psychosis. Still, further research is needed to identify and explain the differences between these two disorders both on the level of neurotransmission and behavior.
The limited scope of this study leaves room for a number of future experiments. For example, other research groups have suggested that impaired visual processing in schizophrenia may also be modulated by NMDA/glutamate dysregulation (Butler et al., 2005). Whether the deficient contrast detection in schizotypal personality disorder, found in this study, is also related to the glutamatergic theory is an interesting question that remains to be explored.
Another interesting question is how the contrast detection deficit in SPD influences their processing of other visual information. It was found that deficits in one visual processing domain - backward masking - appear to be related to schizotypal personality symptoms (Cadenhead et al., 1996). Thus, a comprehensive study on different levels of visual processing and their associations with clinical outcomes in SPD patients are warranted.
Acknowledgement
We are grateful for the support from grant # MO1-RR-00071 (NCRR), by a Veterans Affairs Merit Review Grant (7609-028), and grant # MH56140 from NIMH. We would also like to thank Bikki Smith for help preparing the manuscript, and Daniel Norton who provided technical guidance on the contrast sensitivity tasks. We thank the participants of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This publication was made possible by grant # MO1-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. This research was also supported in part by grant # MH56140 from the National Institute of Mental Health to Dr. Siever, and by a Veterans Affairs Merit Review Grant (7609-028).
Contributors
Larry Siever and Yue Chen designed the study. Zachary Weinstein wrote the protocol. Brendon Kent conducted the literature searches and statistical analyses. Vincent Passarelli was the clinical coordinator. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, O'Flynn K, Koenigsberg HW, Van Heertum R, Cooper T, Laruelle M, Siever LJ. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol. Psychiatry. 2004;55(10):1001–1006. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A. Probing cortical dopamine function in schizophrenia: what can D1 receptors tell us? World Psychiatry. 2003;2(3):166–171. [PMC free article] [PubMed] [Google Scholar]
- Balogh Z, Benedek G, Kéri S. Retinal dysfunctions in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):297–300. doi: 10.1016/j.pnpbp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J. Abnorm.Psychol. 2004;113(4):556–568. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. first ed. New York: International Universities Press; 1950. [Google Scholar]
- Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson's disease patients. Trends Neurosci. 1990;13(7):296–302. doi: 10.1016/0166-2236(90)90113-o. [DOI] [PubMed] [Google Scholar]
- Boumghar L, Marois A, Jolicoeur FJ, Casanova C. Apomorphine modifies the visual responses of cells in the rabbit's lateral geniculate nucleus. Can. J. Physiol. Pharmacol. 1997;75(7):853–858. [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am. J. Psychiatry. 2001;158(7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62(5):495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Braff DL. The relationship of information processing deficits and clinical symptoms in schizotypal personality disorder. Biol. Psychiatry. 1996;40(9):853–858. doi: 10.1016/0006-3223(95)00547-1. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Shafer K, Braff DL. Cognitive functions in schizotypal personality disorder. Schizophr Res. 1999;37(2):123–132. doi: 10.1016/s0920-9964(98)00147-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bedell HE, Frishman LJ. Temporal-contrast discrimination and its neural correlates. Perception. 1996;25(5):505–522. doi: 10.1068/p250505. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Nakayama K, Matthysse S, Holzman PS. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am. J. Psychiatry. 2003;160(10):1795–1801. doi: 10.1176/appi.ajp.160.10.1795. [DOI] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch. Gen. Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrenia. Biol. Psychiatry. 2004;55(8):834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Levy DL, Lenzenweger MF, Holzman PS. Thought disorder, perceptual aberrations, and schizotypy. J. Abnorm. Psychol. 1996;105(3):469–473. doi: 10.1037//0021-843x.105.3.469. [DOI] [PubMed] [Google Scholar]
- Corbe C, Arnaud F, Brault Y, Janiak-Bolzinger C. Effect of a dopaminergic agonist, piribedil (Trivastal 50 mg LP), on visual and spatial integration in elderly subjects. J. Neurol. 1992;239 Suppl 1:22–27. doi: 10.1007/BF00819563. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Diamond A, Herzberg C. Impaired sensitivity to visual contrast in children treated early and continuously for phenylketonuria. Brain. 1996;119(2):523–538. doi: 10.1093/brain/119.2.523. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Hankins MW, Hirano J, Archer SN. Neurobiology of retinal dopamine in relation to degenerative states of tissue. Vision Res. 1997;37(24):3509–3529. doi: 10.1016/S0042-6989(97)00129-6. [DOI] [PubMed] [Google Scholar]
- Farmer CM, O'Donnell BF, Niznikiewicz MA, Voglmaier MM, McCarley RW, Shenton ME. Visual perception and working memory in schizotypal personality disorder. Am. J. Psychiatry. 2000;157(5):781–788. doi: 10.1176/appi.ajp.157.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Personality Disorders, (SCID-II) Washington, DC: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87(3):128–143. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Matts CW, Rollmann EA. Sustained attention deficits in relation to psychometrically identified schizotypy: evaluating a potential endophenotypic marker. Schizophr Res. 2006;82(1):27–37. doi: 10.1016/j.schres.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Gronwall D. Paced auditory serial addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Harris JP, Calvert JE, Leendertz JA, Phillipson OT. The influence of dopamine on spatial vision. Eye. 1990;4(part 6):806–812. doi: 10.1038/eye.1990.127. [DOI] [PubMed] [Google Scholar]
- Hébert M, Gagné AM, Paradis ME, Jomphe V, Roy MA, Mérette C, Maziade M. Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry. 2010;67(3):270–274. doi: 10.1016/j.biopsych.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive Deficit in Schizophrenia: A Quantitative Review of the Evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Motion and vision. II. Stabilized spatio-temporal threshold surface. J. Opt. Soc. Am. 1979;69(10):1340–1349. doi: 10.1364/josa.69.001340. [DOI] [PubMed] [Google Scholar]
- Kéri S, Antal A, Szekeres G, Benedek G, Janka Z. Transient visual channel functions in schizophrenia. Int. J. Psychophysiol. 1998;30:170. [Google Scholar]
- Kéri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2002;14(2):190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- Kirrane RM, Mitropoulou V, Nunn M, New AS, Harvey PD, Schopick F, Silverman J, Siever LJ. Effects of amphetamine on visuospatial working memory performance in schizophrenia spectrum personality disorder. Neuropsychopharmacology. 2000;22(1):14–18. doi: 10.1016/S0893-133X(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Kirrane RM, Siever LJ. New perspectives on schizotypal personality disorder. Curr. Psychiatry Rep. 2000;2(1):62–66. doi: 10.1007/s11920-000-0044-0. [DOI] [PubMed] [Google Scholar]
- Masson G, Mestre D, Blin O. Dopaminergic modulation of visual sensitivity in man. Fundam Clin. Pharmacol. 1993;7(8):449–463. doi: 10.1111/j.1472-8206.1993.tb01041.x. [DOI] [PubMed] [Google Scholar]
- McClure MM, Barch DM, Flory JD, Harvey PD, Siever LJ. Context processing in schizotypal personality disorder: Evidence of specificity of impairment to the schizophrenia spectrum. Journal of Abnormal Psychology. 2008;117(2):342–354. doi: 10.1037/0021-843X.117.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MM, Koenigsberg HW, Reynolds D, Goodman M, New A, Trestman R, Silverman J, Harvey PD, Siever LJ. The effects of risperidone on the cognitive performance of individuals with schizotypal personality disorder. J Clin Psychopharmacol. 2009;29(4):396–398. doi: 10.1097/JCP.0b013e3181accfd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Maldari LA, Moriarty PJ, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biological Psychiatry. 2002;52(12):1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Goodman M, Sevy S, Elman I, New AS, Iskander EG, Silverman JM, Breier A, Siever LJ. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizotypal personality disorder. Schizophr. Res. 2004;70(1):27–31. doi: 10.1016/j.schres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. Am J Psychiatry. 2005;162(10):1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Bismark A, Hetrick WP, Bodkins M, Vohs JL, Shekhar A. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2006;86(13):89–98. doi: 10.1016/j.schres.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indication of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Roitman SE, Cornblatt BA, Bergman A, Obuchowski M, Mitropoulou V, Keefe RS, Silverman JM, Siever LJ. Attentional functioning in schizotypal personality disorder. American Journal of Psychiatry. 1997;154:655–660. doi: 10.1176/ajp.154.5.655. [DOI] [PubMed] [Google Scholar]
- Siever LJ. Biologic factors in schizotypal personal disorders. Acta Psychiatr. Scand. 1994;384 Suppl.:45–50. doi: 10.1111/j.1600-0447.1994.tb05890.x. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am. J. Psychiatry. 2004;161(3):398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, Goodman M, Buchsbaum M. Cognitive and brain function in schizotypal personality disorder. Schizophr. Res. 2002;54(1–2):157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol. 1998;107(1):49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Supèr H, Spekreijse H, Lamme VA. A neural correlate of working memory in the monkey primary visual cortex. Science. 2001;293(5527):120–124. doi: 10.1126/science.1060496. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R: Manual: Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]