Abstract
Changes in AMPA receptor (AMPAR) surface expression in the rodent nucleus accumbens (NAc) are produced by cocaine exposure and implicated in addiction-related behaviors. The direction of change depends on the animal’s prior drug history. However, little is known about the effect of a single exposure to cocaine on AMPAR distribution in the NAc of untreated rats. This is essential information for interpreting the literature on AMPAR trafficking after repeated cocaine exposure. In the present study, we used a protein crosslinking assay to determine the effect of a single cocaine injection on surface and intracellular AMPAR subunit levels in the rat NAc. We found increased AMPAR surface expression in the NAc 24 h, but not 30 min or 2 h, after cocaine injection. A major effect of cocaine is to increase extracellular dopamine (DA) levels, leading to DA receptor activation. Therefore, we also evaluated the effects of directly acting DA receptor agonists. In contrast to the effects of cocaine, AMPAR surface expression was significantly decreased 24 h after injection of the D2-class agonist quinpirole, whereas no significant effects were produced by the D1-class agonist SKF 81297 or the mixed DA agonist apomorphine. Our results show that the effects of a single cocaine exposure in drug- and injection-naïve rats are distinct from those previously reported after repeated cocaine administration. They further suggest that cocaine exerts these effects by influencing neuronal circuits rather than simply stimulating NAc DA transmission.
Keywords: D1 receptor, D2 receptor, glutamate, plasticity, sensitization
INTRODUCTION
It is generally accepted that alterations in glutamate transmission and receptor trafficking in the nucleus accumbens (NAc) contribute to addiction-related behaviors (Wolf et al., 2004; Thomas et al., 2008; Kalivas, 2009; Wolf and Ferrario, 2010). Studies using the behavioral sensitization model, in which repeated drug exposure results in enhancement of subsequent behavioral responses to cocaine, have found bidirectional changes in α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor (AMPAR) trafficking in the NAc. After several weeks of withdrawal, AMPAR upregulation in the NAc of sensitized rodents has been demonstrated using a protein crosslinking assay to measure cell surface receptors (Boudreau and Wolf, 2005; Boudreau et al., 2007, 2009; Ferrario et al., 2010) and electrophysiology to measure AMPA/NMDA ratios in the shell subregion (Kourrich et al. 2007). On the other hand, when sensitized animals are re-exposed to cocaine after 10-14 days of withdrawal, decreased AMPAR surface expression and a decreased AMPA/NMDA ratio are observed 24 h later (Thomas et al., 2001; Boudreau et al., 2007; Kourrich et al., 2007; Ferrario et al., 2010). Additional support for these results has been provided using behavioral approaches (Bachtell and Self, 2008) and subcellular fractionation (Ghasemzadeh et al., 2009; Schumann and Yaka, 2009). The effect of repeated cocaine exposure on the cellular distribution of AMPARs is of functional significance because it has been shown that drug seeking requires AMPAR transmission in the NAc (e.g., Cornish and Kalivas, 2000; Di Ciano and Everitt, 2001) and that enhanced AMPAR transmission in the NAc is associated with enhanced drug seeking (Suto et al., 2004; Conrad et al., 2008; Anderson et al., 2008; for review, see Wolf and Ferrario, 2010).
Surprisingly, very little is known about the effect of a single exposure to cocaine on AMPAR distribution. Using mice, Kourrich et al. (2007) found that a single exposure to cocaine had no effect on the AMPA/NMDA ratio in the shell region of the mouse NAc 24 h later. However, this result does not exclude the possibility of changes in the NAc core, proportional changes in AMPAR and NMDAR currents, more rapid changes that have subsided by the 24 h time-point, or different effects in rats. The goal of the current study was to further examine some of these possibilities by testing the effect of a single injection of cocaine on AMPAR surface expression in the NAc of drug-naïve rats at three time-points (30 min, 2 h and 24 h). In addition, because many of cocaine’s effects in the NAc are due to increased extracellular DA levels, leading to DA receptor activation, we also evaluated the ability of directly acting DA receptor agonists to alter AMPAR distribution in the NAc.
MATERIALS AND METHODS
Subjects
Male Sprague Dawley rats (Harlan), weighing 250-275 g at the start of the experiment, were housed in groups of three (12:12 h light/dark). Food and water were continually available. All treatment and testing were conducted in the light phase of the cycle. All animal procedures were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee.
Drugs
All drugs were obtained from Sigma-Aldrich (St. Louis, MO). We tested cocaine (15 and 30 mg/kg, i.p.), the D2-class agonist quinpirole (0.3 and 3 mg/kg, i.p.), the D1-class agonist SKF 81297 (0.3 and 3 mg/kg, s.c.) and the mixed D1/D2-class agonist apomorphine (3 mg/kg, s.c.). Our selection of drug doses was based on prior studies (e.g., De Vries et al., 2002; Edwards et al., 2007).
Drug administration and behavioral measures
Rats were allowed to acclimatize to the animal colony for 7-10 days. During this time they were handled once per day. On the behavioral testing day, each rat was placed in a rectangular plastic cage (41 × 25.5 × 2.3 cm) surrounded by a photocell frame (San Diego Instruments, San Diego, CA). Locomotor activity was recorded as the total number of beam breaks per 5 min interval. Rats were allowed 40 min to habituate to the activity monitors and were then given a single injection of saline, cocaine, quinpirole or SKF 81297 (see previous section for doses). For the 15 mg/kg and 30 mg/kg doses of cocaine, NAc tissue was collected 30 min, 2 h or 24 h after injection. For other drugs (quinpirole, SKF 81297 and apomorphine), tissue was collected 24 h after the injection. Locomotor activity was recorded throughout the habituation period and for 1.5 h after the injection, except for the 30 min groups, in which locomotor activity was monitored only for 30 min. The 30 min and 2 h groups were taken from the locomotor activity cages, 30 min or 2 h after drug injection, to a separate room where tissue was obtained. Each experimental group (i.e., rats with the same drug treatment and tissue collection time-point) had its own saline control group.
BS3 protein crosslinking and Western blotting
Levels of cell surface and intracellular AMPAR subunit proteins were determined using a protein crosslinking assay (Boudreau and Wolf, 2005). This assay uses BS3 (Pierce Biotechnology, Rockford, IL), a bi-functional chemical crosslinker that does not penetrate cell membranes. Therefore, it only crosslinks proteins expressed on the cell surface, forming high molecular weight aggregates, whereas intracellular proteins are not modified. The surface-expressed pool of a particular protein can then be separated from the intracellular pool based on molecular weight using SDS-PAGE and Western blotting. Details of the crosslinking assay, including SDS-PAGE and Western blotting, have been described previously (Boudreau and Wolf, 2005; Ferrario et al., 2010). Briefly, at the appropriate time after drug injection (see previous section), rats were decapitated and the NAc was rapidly dissected from a 2 mm coronal section obtained using a brain matrix (Activational Systems, Warren, MI). Tissue was chopped into 400μm slices using a McIllwain chopper (The Vibratome Company, St. Louis, MO) which were then incubated in artificial cerebrospinal fluid (aCSF) containing 2mM BS3 for 30 min at 4°C with gentle agitation. Crosslinking was terminated by quenching with glycine (100mM). Lysates were prepared, aliquotted and stored at −80°C for later analysis. Bilateral NAc tissue from one rat yields enough lysate for multiple proteins to be evaluated. SDS-PAGE and Western blotting were performed using Bis-Tris gradient gels (BioRad, Hercules, CA) to separate unmodified (intracellular) and crosslinked (cell surface) AMPAR subunits. Immunoblots were probed with antibodies to the AMPAR subunits GluA1 (1:1000; Chemicon, Temecula, CA, AB1504 or Affinity Bioreagents, Golden, CO, PA1-37776) or GluA2 (1:1000; Chemicon, AB1768). Proteins were detected using ECL (GE HealthCare, Piscataway, NJ) and quantified with a VersaDoc 5000 imaging system (BioRad, Hercules, CA). After analysis, membranes were stained with Ponceau S (5 min; Sigma-Aldrich) to assess total protein in the lane. Samples from an experimental group and its saline control group were run together on the smallest possible number of gels, with an approximately equal number of saline and drug samples loaded onto each of the gels.
Data Analysis
Separate saline control groups were generated for each drug dose and time-point. The N’s for each group are as follows: 15 mg/kg cocaine/30 min (N=12), saline (N=12); 15 mg/kg cocaine/2 h (N=12), saline (N=12); 15 mg/kg cocaine/24 h (N=23; two replications), saline (N=23; two replications); 30 mg/kg cocaine/30 min (N=8), saline (N=8); 30 mg/kg cocaine/2 h (N=8), saline (N=8); 30 mg/kg cocaine/24 h (N=12), saline N=11); 0.3 mg/kg SKF 81297/24 h (N=9), saline N=9; 3 mg/kg SKF 81297 (N=11), saline (N=11); 0.3 mg/kg quinpirole/24 h (N=10), saline N=21; 3 mg/kg quinpirole (N=8), saline N=12; 3 mg/kg apomorphine (N=12), saline (N=12). In some cases, one or two rats from a particular group were not included in the biochemical analysis due to problems with particular lanes. Protein band intensity measures for all experimental groups are expressed as percent of their own saline control group. Statistical analysis was performed by comparing each experimental group to its own saline group using two-way unpaired t-tests unless otherwise noted. Immunoreactivity on Western blots was analyzed using Quantity One analysis software (BioRad, Hercules, CA). Diffuse densities for surface and intracellular bands in each lane were determined. Total protein levels were determined by summing surface and intracellular values. Surface, intracellular and total protein values were then normalized to total protein in the lane as determined by Ponceau S staining. Corrected values for surface, intracellular, and total protein levels, as well as the ratio of surface to intracellular protein (S/I), were determined for each rat, and data expressed as mean ± S.E.M. Locomotor activity was evaluated using two-way repeated measures ANOVA with time × injection group as factors. A Pearson correlation analysis was conducted to compare GluA1 surface levels and S/I ratios for individual rats to the corresponding measures for GluA2 after treatment with 0.3 mg/kg quinpirole.
RESULTS
Effect of acute cocaine administration on AMPAR distribution in the NAc
Rats received an injection of either saline or cocaine (15 or 30 mg/kg, i.p.) and NAc tissue was collected 30 min, 2 h or 24 h later. For presentation of locomotor activity, we pooled the response of saline and cocaine groups destined for analysis at different time-points (Fig. 1). Both cocaine doses produced a significantly greater locomotor response than saline injection (Fig. 1; main effect of group F(2,822)=86.4, p<0.0001), although we did not observe a significant difference between the 15 mg/kg and 30 mg/kg cocaine groups. This may be attributable to a number of factors including significant individual variability in responding to cocaine (e.g., some rats in the 30 mg/kg group had a low response while some in the 15 mg/kg group had a high response), the presence of stereotypy in the 30 mg/kg group which limited the locomotor response, and the fact that some experiments at the 30 mg/kg dose were conducted almost a year after the initial experiments. Interestingly, the level of locomotor activity produced by a drug does not seem to be related to its effect on AMPAR distribution (see below; compare across DA receptor agonists and cocaine).
Fig. 1.
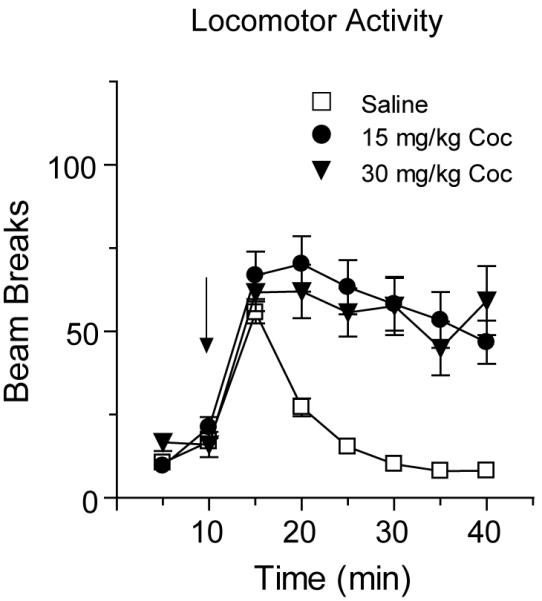
A single exposure to cocaine (15 mg/kg or 30 mg/kg) produced a significant increase in locomotor activity. Data are expressed as the average number of beam breaks per 5 min interval after saline or cocaine injection. Saline- and cocaine-injected rats destined for tissue collection at different time-points (30 min, 2 h and 24 h) were pooled for presentation of these locomotor activity data (see Methods for N values for each group) and the arrow indicates time of injection. Both cocaine-injected groups showed a significant increase in locomotor activity compared to saline-injected controls, but the locomotor response to 15 mg/kg and 30 mg/kg cocaine did not differ, probably due to the presence of stereotypy in the 30 mg/kg group as well as other variables discussed in Results.
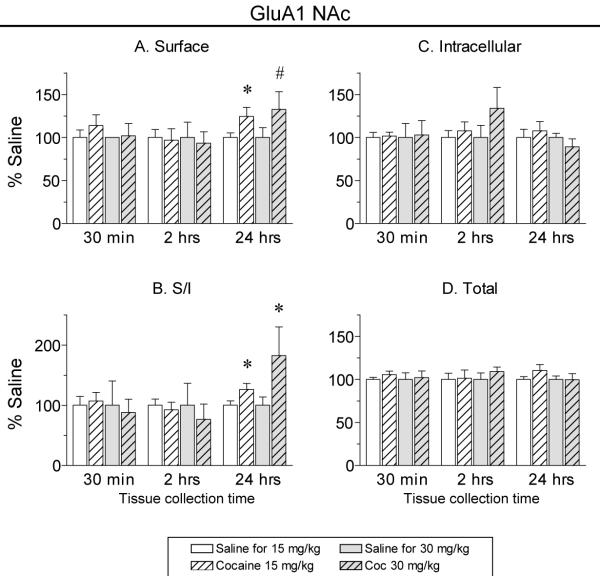
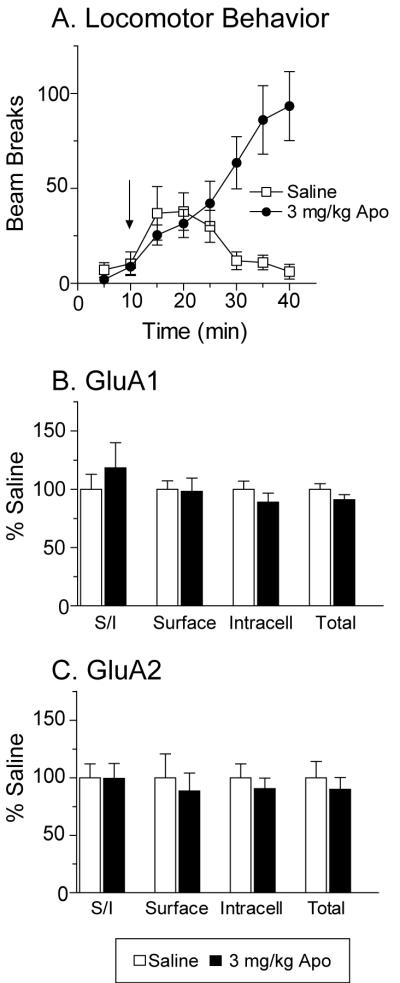
Fig. 2 shows the distribution of the AMPAR subunit GluA1 in the NAc after injection of cocaine or saline, determined using a BS3 protein crosslinking assay. Acute cocaine administration (15 mg/kg or 30 mg/kg) did not alter GluA1 distribution compared to saline controls 30 min or 2 h after injection (Fig. 2). There was a small trend towards increased surface GluA1 (and GluA2; see below) 30 min after injection of 15 mg/kg cocaine, but this trend was not evident 30 min after injection of the 30mg/kg cocaine. However, when tissue was collected 24 h after 15 mg/kg cocaine, there was a significant increase, relative to the saline control group, in surface GluA1 (Fig. 2A: t44=−2.0, p<0.05) and the GluA1 surface/intracellular ratio (S/I; Fig. 2B: t44=−2.0, p<0.05). Similar results were observed 24 h after the higher dose of cocaine (30 mg/kg). There was a significant increase in GluA1 S/I ratio (Fig. 2B: t21=1.7, p<.05, one-way) and a trend towards increased GluA1 surface expression (Fig. 2A: t21=1.4, p=0.08, one-way). In tissue collected 24 h after 15 mg/kg cocaine, it is interesting that small trends towards increased intracellular and total GluA1 accompanied the increase in surface GluA1 (Fig. 2C, D). This might suggest a mechanism involving increased synthesis or decreased degradation of GluA1. However, the increase in surface GluA1 observed 24 h after injection of 30 mg/kg cocaine was not accompanied by these trends. Instead, there was a slight decrease in intracellular GluA1, in the absence of a change in total GluA1 (Fig. 2C, D). This pattern is more consistent with redistribution of GluA1 from inside the cell to the cell surface. However, even though total protein did not change, a contribution of increased protein synthesis cannot be ruled out. If the S pool is smaller than the I pool [something that is difficult to determine with certainty, because an antibody may have different affinity for its crosslinked target (S) than its unmodified target (I)], it is possible that a small increase in the S pool would be detectable whereas the corresponding small increase in I might not be evident.
Fig. 2.
The GluA1 surface/intracellular ratio (S/I) in the NAc was increased 24 h after injection of 15 mg/kg or 30 mg/kg cocaine. NAc tissue was collected 30 min, 2 h or 24 h after injection of 15 mg/kg cocaine, 30 mg/kg cocaine, or saline. Data are normalized to the corresponding saline control group for each tissue collection time. The S/I ratio for GluA1 was significantly increased in both cocaine-injected groups 24 h after drug exposure (*p<0.05). Surface levels were significantly increased after 15 mg/kg cocaine, but did not reach significance for the 30 mg/kg cocaine group (#p=0.08, one-way t-test). No changes in GluA1 distribution were observed 30 min or 2 h after cocaine injection.
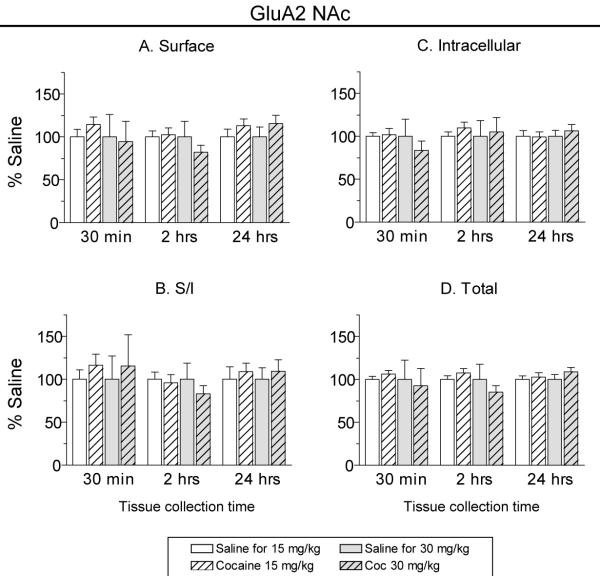
AMPARs are tetramers (Mano and Teichberg, 1998; Rosenmund et al., 1998) composed of GluA1-4 subunits (previously termed GluR1-4; see Collingridge et al., 2009). Biochemical results indicate that GluA1A2 receptors are a major population in the NAc (Reimers et al., 2007; Wolf and Ferrario, 2010), consistent with findings in hippocampus (Wenthold et al., 1996; Lu et al., 2009). Therefore, given that GluA1 surface expression was increased 24 h after cocaine injection, we expected to find a similar increase in GluA2. To test this, GluA2 immunoblotting was performed on different aliquots of the same NAc samples used to generate GluA1 results. The GluA2 data are shown in Fig. 3. Although no significant changes in GluA2 expression were found at any time-point, there was a trend towards increased GluA2 surface expression for both doses of cocaine after 24 h. Together with GluA1 data, these trends for GluA2 could indicate increased surface expression of GluA1A2-containing AMPARs, although other explanations are possible (see Discussion).
Fig. 3.
GluA2 distribution was not significantly altered by 15 or 30 mg/kg cocaine at any time-point, although modest trends towards increased surface GluA2 were observed 24 h after cocaine injection. NAc tissue was collected 30 min, 2 h or 24 h after injection of 15 mg/kg cocaine, 30 mg/kg cocaine, or saline. Data are normalized to the corresponding saline control group at each tissue collection time. S/I, surface/intracellular.
Effect of acute DA receptor agonist administration on AMPAR distribution in the NAc
One major effect of cocaine is to enhance extracellular DA levels resulting in stimulation of D1-class (D1 and D5) and D2-class (D2, D3, D4) DA receptors. Therefore, we examined the effect of a single injection of the D1-class agonist SKF 81297 (0.3 or 3 mg/kg, s.c.), the D2-class agonist quinpirole (0.3 or 3 mg/kg, i.p.), or the mixed D1/D2-class agonist apomorphine (3 mg/kg, s.c.) on AMPAR distribution in the NAc 24 h after drug injection.
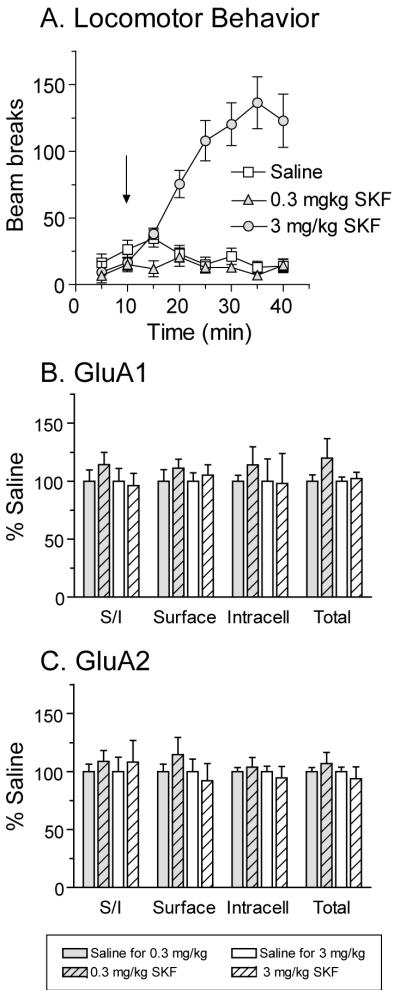
The locomotor response to a low dose of SKF 81297 (0.3 mg/kg) did not differ from saline controls whereas the higher dose of SKF 81297 (3 mg/kg) produced robust locomotor activation (Fig. 4A; 3 mg/kg SKF vs saline: main effect of group F(1,280)=79.6, p<0.0001; group × time interaction F(7,280)=18.8, p<0.0001). Tissue samples from the NAc were collected and crosslinked 24 h after injection. GluA1 and GluA2 distribution were not significantly altered by either dose of SKF 81297, although trends towards increased GluA1 levels were observed after the higher dose (Fig. 4B, C).
Fig. 4.
Administration of the D1-class agonist SKF 81297 (SKF, 0.3 or 3 mg/kg) did not alter GluA1 or GluA2 distribution in the NAc 24 h after drug injection. Panel A shows the average number of beam breaks per 5 min interval after saline or SKF injection. The arrow indicates time of injection. Only the higher dose of SKF produced a significant increase in locomotor activity compared to saline-injected controls. NAc tissue was collected 24 h after injection. No significant differences were found for GluA1 (B) or GluA2 (C) distribution in the NAc, although the lower dose of SFK produced small trends towards increases in all GluA1 measures as well as GluA2 surface expression.
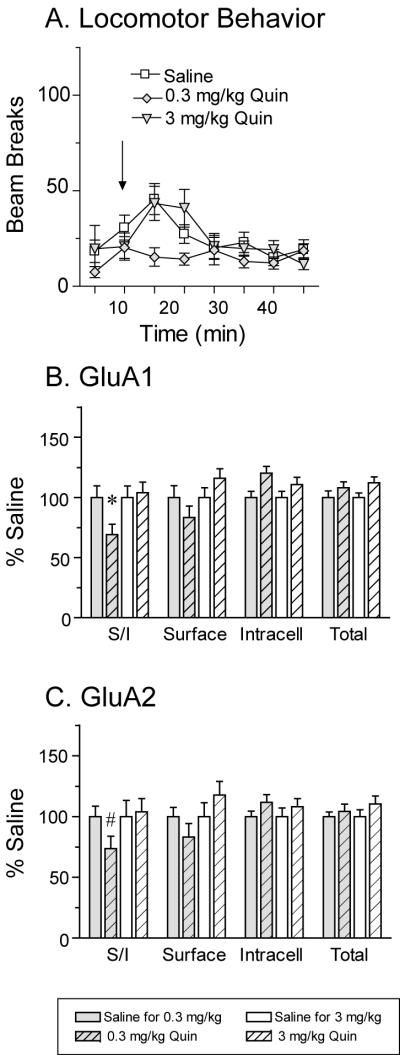
The locomotor response to quinpirole did not differ from saline controls at either dose tested (Fig. 5A). However, the higher dose of quinpirole produced a number of other behavioral responses including arching of the back, squinting of the eyes, gaping of the mouth and sporadic jumping and hopping behaviors. These behaviors were not produced by the lower dose of quinpirole. In NAc tissue obtained 24 h after injection of 0.3 mg/kg quinpirole, the S/I ratio for GluA1 was significantly decreased compared to saline controls (Fig. 5B: t28=2.04, p<0.01). This was due to a slight decrease in surface GluA1 accompanied by a slight increase in intracellular GluA1, suggesting redistribution from surface to intracellular pools (Fig. 5B). A trend towards a decrease in the GluA2 S/I ratio was also observed (Fig. 5C: t29=1.86, p=0.07). Decreased S/I ratios for both GluA1 and GluA2 suggest that GluA1A2-containing AMPARs are being removed from the cell surface. In addition, both S/I ratios and surface values for GluA1 positively correlated with the corresponding values for GluA2 (Pearson correlation: S/I, r=0.88; surface r=0.99; P<0.001). In contrast, the higher quinpirole dose (3 mg/kg) did not significantly alter GluA1 or GluA2 distribution (Fig. 5B and C).
Fig. 5.
Administration of a low dose of the D2-class agonist quinpirole (Quin, 0.3 mg/kg) significantly decreased the GluA1 surface/intracellular (S/I) ratio 24 h after drug injection and produced the same trend for GluA2. Panel A shows the average number of beam breaks per 5 min interval after saline or quinpirole injection. The arrow indicates time of injection. The locomotor response to Quin did not differ significantly from the response to saline, although some behavioral changes were observed in response to the higher drug dose (see Results). A significant decrease in the S/I ratio 24 h after injection of 0.3 mg/kg Quin was observed for GluA1 (*p<0.05) and a strong trend was observed for GluA2 (C; #p=0.07). Trends towards decreased surface levels of GluA1 and GluA2 were also observed (B and C). No significant changes were found after injection of the higher dose of quinpirole (Quin, 3 mg/kg, B and C).
The mixed D1/D2-class agonist apomorphine (3 mg/kg) significantly increased locomotor activity compared to saline controls (Fig. 6A: main effect of group F(1,154)=8.1, p<0.01; group × time interaction F(7,154)=12.1, p<0.0001). However, no significant change in GluA1 or GluA2 distribution in the NAc was found 24 h after the injection (Fig. 6B, C).
Fig. 6.
Administration of the mixed D1/D2-class agonist apomorphine (Apo, 3 mg/kg) did not alter GluA1 or GluA2 distribution in the NAc 24 h after drug injection. Panel A shows the average number of beam breaks per 5 min interval after saline or Apo injection. The arrow indicates time of injection. The locomotor response to Apo was significantly greater than the response to saline injection. No significant differences in GluA1 (B) or GluA2 (C) distribution were found.
DISCUSSION
Cocaine and directly acting DA receptor agonists exert different effects on AMPAR distribution
Our results show that GluA1 surface expression is increased 24 h but not 30 min or 2 h after a cocaine injection (15 mg/kg or 30 mg/kg) is given to drug-naïve rats. The delayed increase in AMPAR surface expression could indicate a requirement for neuronal processes that develop gradually over time, although the fact that cocaine is still “on board” at the earlier times complicates interpretation of the time course. It was surprising to us that the effect of cocaine was not mimicked by acute administration of directly acting DA agonists. Administration of the D1-class agonist SKF 81297 produced no significant effect, while the D2-class agonist quinpirole caused GluA1 and GluA2 to redistribute from surface to intracellular pools. The mixed DA agonist apomorphine did not significantly alter GluA1 or GluA2 surface expression. These results suggest that cocaine alters AMPAR distribution in the NAc of drug-naïve rats through a mechanism more complicated than enhancement of DA transmission in the NAc. For example, cocaine may increase levels of DA and other monoamines in the prefrontal cortex (PFC), leading to altered modulation of PFC-NAc projection neurons and altered glutamate release in the NAc; this in turn may trigger postsynaptic AMPAR plasticity. Consistent with this possibility, Bachtell and Self (2008) found that cocaine-induced glutamate release was responsible for decreased NAc AMPAR sensitivity in cocaine-sensitized rats challenged with cocaine. There are several possible reasons why this circuitry might not be similarly activated by administration of directly acting DA agonists. For example, cocaine’s effects on serotonin or norepinephrine may be critical. Alternatively, the temporal and spatial pattern of endogenous DA accumulation triggered by cocaine may lead to effects that are not reproduced by systemic administration of SKF 81297, quinpirole or apomorphine.
We have previously shown that treatment with a D1-class agonist can increase GluA1 surface expression in NAc neurons through a protein kinase A (PKA)-dependent pathway. These results were obtained in cultured neurons after 5-15 min of D1 agonist exposure (Chao et al., 2001; Mangivacchi and Wolf, 2004; Sun et al., 2008), and are therefore difficult to compare to the effect we observed 24 h after systemic injection of SKF 81297 to adult rats. Local infusions of D1 agonist would make the results more comparable to our prior work in neuronal cultures, but this would not be relevant to the overall goal of our study, which is to understand the effects of systemic cocaine injection on AMPARs in the intact NAc. However, it is interesting that a trend towards an increase in total GluA1 levels was produced by the low dose of SFK (Fig. 4B) in light of a report that D1 receptor stimulation can increase surface GluA1 levels in cultured hippocampal neurons through a mechanism requiring local protein synthesis (Smith et al., 2005).
The effect of D2 receptor stimulation on AMPAR distribution in the NAc has not been previously examined. However, systemic quinpirole injection in mice decreased GluA1 phosphorylation at serine 845, a PKA phosphorylation site, in striatal tissue 15 min after injection (Hakansson et al., 2006). Dephosphorylation at this site is associated with the removal of GluA1-containing AMPARs from the cell surface (Lee et al., 1998, 2000; Ehlers, 2000). Thus, the ability of D2-class receptors to inhibit PKA (Neve et al., 2004) could explain our observation that quinpirole decreased GluA1 and GluA2 surface/intracellular ratios. On the other hand, D2-class agonists decrease AMPAR surface expression in cultured PFC neurons through mechanisms that are independent of PKA inhibition (Sun et al., 2005). Regardless of the mechanism involved, the fact that we observed a lasting (24 h) decrease in AMPAR surface expression after systemic quinpirole administration has potential clinical significance in light of evidence that D2 receptor-mediated AMPAR internalization contributes to protective effects of D2 agonists in excitotoxicity models (see Zou et al., 2005).
Our observation that apomorphine had no effect, while quinpirole decreased and SKF 81297 did not alter AMPAR surface expression, indicates that stimulating both receptor classes simultaneously (apomorphine) is not necessarily equivalent to the sum of stimulating each receptor separately. This is probably attributable to differential cellular and/or subcellular localization of D1- and D2-class receptors (see Sun et al., 2005 and 2008 for discussion).
What AMPAR subtype is affected by acute cocaine?
The subtype of AMPAR involved in the acute response to cocaine is not clear, as we observed significant changes for GluA1 but only trends for GluA2. It is possible that GluA1 and GluA2 redistribute proportionately (in the same heteromeric receptor) but that it is more difficult to detect redistribution of GluA2. Large pools of unassembled GluA2 inside the cell (Greger et al., 2003) could make it harder to detect a change in intracellular levels, and thus limit the ability to detect a shift in the S/I ratio for GluA2. In addition, changes in surface GluA2 could be diluted if GluA2A3 receptors do not respond to cocaine. Alternatively, the smaller effect for GluA2 could indicate that GluA2-lacking AMPAR are involved in the NAc response to acute cocaine, as has been shown previously in the VTA (Bellone and Luscher, 2006; Argilli et al., 2008). However, in the VTA, cocaine influences AMPAR subunit composition by regulating local GluA2 synthesis (Mameli et al., 2007). Our data are more consistent with redistribution of GluA1-containing AMPARs (above) or local increases in GluA1 synthesis that do not produce a detectable change in total GluA1 levels (see Results for ideas about how this might occur). Electrophysiological studies are needed to determine whether GluA2-lacking AMPAR are involved in our observed effects.
Cocaine’s effects on AMPAR distribution depend on prior treatment history
Our results add to evidence that cocaine’s effect on AMPAR distribution in the NAc varies markedly depending on the animal’s prior history, as summarized in Table I. Briefly, surface AMPARs increase after withdrawal from cocaine sensitization but then decrease in sensitized rats 24 h after challenge. In repeated saline treated rats, we also found that surface AMPAR decrease 24 h after cocaine challenge (Ferrario et al., 2010). Thus it was surprising to see that surface AMPARs increased after acute cocaine administration in drug- and injection-naïve rats. We speculate that repeated exposure to the injection procedure and injection environment in the saline-pretreated group (Ferrario et al., 2010) may explain their different response compared to drug-naïve rats in the present study, who are receiving cocaine in a novel environment. It has been shown previously that novelty alters drug effects on c-fos expression patterns (Uslaner et al., 2001), suggesting that different circuits are activated when cocaine is administered in a novel environment, perhaps leading to different AMPAR plasticity.
Table 1.
The effect of cocaine on AMPAR surface expression in the NAc depends on timing and prior treatment history
| Treatment | GluR1 S/I ratio (NAc core and shell) | AMPA/NMDA ratio (NAc shell only) |
|---|---|---|
| Drug-naïve, single coc injection |
Not changed (30 min or 2 h)1 | N.D. (30 min or 2 h) |
| Increased (24 h) 1 | Not changed (24 h) 2 | |
| Repeated saline, single coc injection, WD10-14 |
Decreased (24 h) 3 | Not changed (24 h) 2 |
| Repeated cocaine, Non-sens or sensitized, WD1 |
Not changed 4 | Decreased 2 |
| Repeated cocaine, Sens, WD ≥ 1 week |
Increased 3-6 | Increased 2 |
| Repeated cocaine, Non-sens, WD ≥ 1 week |
Not changed 4-6 | N.D. |
| Repeated cocaine, Sens, Coc chal WD10-14 |
Not changed from elevated WD level (30 min) 3 | N.D. (30 min) |
| Decreased from elevated WD level (24 h) 3,5 | Decreased from elevated WD level (24 h) 2,7 | |
| Repeated cocaine, Sens, Sal chal WD10-14 |
Higher than rats challenged with coc (24 h) 3 | Not changed from elevated WD level (24 h) 2 |
| Decreased from elevated WD level (24 h) 5 | ||
| Repeated cocaine, Non-sens, Coc chal WD14 |
Not changed (24 h) 5 | N.D. |
| Repeated cocaine, Non-sens, Sal chal WD14 |
Not changed (24 h) 5 | N.D. |
The number in superscript corresponds to these citation: present study;
2The number in superscript corresponds to these citation: Kourrich et al., 2007;
3The number in superscript corresponds to these citation: Ferrario et al., 2010;
4The number in superscript corresponds to these citation: Boudreau and Wolf, 2005;
5The number in superscript corresponds to these citation: Boudreau et al., 2007;
6The number in superscript corresponds to these citation: Boudreau et al., 2009;
7The number in superscript corresponds to these citation: Thomas et al 2001
The time in parentheses indicates the interval between the challenge injection and harvesting of tissue. Challenge injections were 15 mg/kg cocaine.
In citations 4-6, a cocaine regimen was used that produces sensitization in about half the rats, so these studies yielded sensitized and non-sensitized subgroups. In citations 1 and 3, we used a different regimen in which all injections are administered in the test environment, leading to sensitization in all cocaine-injected rats. Similarly, in citations 2 and 7, mice were not separated into “sensitized” and “non-sensitized” subgroups. Please note that subtle trends (non-significant) in the data are not included here.
S/I, surface/intracellular; Sal, saline, Coc, cocaine; Non-sens, failed to sensitize; WD, withdrawal day; chal, challenge injection; N.D., not determined
Using electrophysiological methods, Kourrich et al. (2007) found that a single injection of cocaine given to mice that were handled but drug- and injection-naive did not alter the AMPA/NMDA ratio in the NAc shell 24 h after injection (contrasting with our present findings). They also found no significant effect of cocaine challenge in repeated saline-treated mice given cocaine for the first time (contrasting with results in Ferrario et al., 2010), although there is a small trend towards a decrease in their results (Kourrich et al., 2007, Fig. 5D; also see Thomas et al., 2001). A possible explanation is that cocaine alters AMPAR trafficking in the core but not shell of drug-naïve rats, which would explain a significant effect of cocaine in our studies, which used a mixed core/shell dissection, but not those of Kourrich et al. (2007). For more discussion of this possibility, see Wolf and Ferrario (2010). Another possibility is that our protein crosslinking studies are detecting insertion of AMPARs at extrasynaptic sites after a single cocaine injection. This would not have been detected by Kourrich et al. (2007) because the AMPA/NMDA ratio is derived from measuring synaptic currents. However, our results suggest that the protein crosslinking assay is mainly detecting synaptic AMPARs (Boudreau and Wolf, 2005).
A single injection of amphetamine given to handled but injection-naïve rats also leads to increased GluA1 surface expression in the NAc, but the time-course differs. Whereas cocaine produced an increase only after 24 h, surface GluA1 was increased 2 h, but not 24 h, after amphetamine injection (Nelson et al., 2009). No significant changes were observed when the same tissue was probed with a GluA2/3 antibody. These results indicate that cocaine and amphetamine utilize different mechanisms to alter AMPAR distribution in drug-naïve rats, consistent with marked differences in AMPAR plasticity in the NAc after withdrawal from repeated administration of each drug (Nelson et al., 2009). Furthermore, both cocaine and amphetamine increase extracellular DA levels and yet differently affect AMPAR surface expression; this supports the idea that glutamate rather than DA may be the primary transmitter driving differential AMPAR adaptations (Nelson et al., 2009). The present results provide further support for the conclusion that cocaine’s effects on AMPAR distribution cannot be explained by increased DA receptor stimulation.
ACKNOWLEDGEMENTS
This work was supported by USPHS grants DA009621 and DA00453 to MEW. CRF was supported by postdoctoral National Research Service Award DA024502. We thank Kerstin Ford for assistance with some experiments. CRF thanks the Santa Fe Institute for their hospitality while this manuscript was written.
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;5:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activity of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem. 2002;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacol. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Raasø H, Vanderschuren LJMJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacol. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacol. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacol. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacol. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neurosci. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosc. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-K, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lee H-K, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. Stimulation of N-methyl-D-aspartate receptors, AMPA receptors or metabotropic glutamate receptors leads to rapid internalization of AMPA receptors in cultured nucleus accumbens neurons. Eur J Neurosci. 2004;20:649–657. doi: 10.1111/j.1460-9568.2004.03511.x. [DOI] [PubMed] [Google Scholar]
- Mano I, Teichberg VI. A tetrameric subunit stoichiometry for a glutamate receptor-channel complex. Neuroreport. 1998;9:327–331. doi: 10.1097/00001756-199801260-00027. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Reimers JM, Boudreau AC, Wolf ME. A quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Soc Neurosci Abstr. 2007;33:815–19. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–9. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacol. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharm Suppl. 2004;1:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity after cocaine withdrawal. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.01.013. Epub Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Li L, Vukusic B, Van Tol HHM, Lee FJS, Wan Q, Liu F. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J Neurosci. 2005;25:4385–4395. doi: 10.1523/JNEUROSCI.5099-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]