Abstract
Supramolecular self-assembly of nanoscale filaments offers a vehicle to signal cells within dense cell aggregates such as pancreatic islets. We previously developed a heparin-binding peptide amphiphile (HBPA) that self-assembles into nanofiber gels at concentrations of 1% by weight when mixed with heparin and activates heparin-binding, angiogenic growth factors. We report here on the use of these molecules at concentrations 100 times lower to drive delivery of the nanofibers into the dense islet interior. Using fluorescent markers, HBPA molecules, heparin, and FGF2 were shown to be present in and on the surface of murine islets. The intraislet nanofibers were found to be necessary to retain FGF2 within the islet for 48 hours and to increase cell viability significantly for at least 7 days in culture. Furthermore, enhanced insulin secretion was observed with the nanofibers for 3 days in culture. Delivery of FGF2 and VEGF in conjunction with the HBPA/heparin nanofibers also induced a significant amount of islet endothelial cell sprouting from the islets into a peptide amphiphile 3-D matrix. We believe the infiltration of bioactive nanofibers in the interior of islets as an artificial ECM can improve cell viability and function in vitro and enhance their vascularization in the presence of growth factors such as FGF2 and VEGF. The approach described here may have significant impact on islet transplantation to treat type 1 diabetes.
Keywords: Angiogenesis, Cell viability, Diabetes, Heparin, Peptide, Self-assembly, Nanostructure
1. Introduction
Islet transplantation is a promising treatment for select patients with type 1 diabetes. Widespread clinical application, however, is constrained by the lack of available donors, inefficient engraftment of the transplanted tissue, and limited durability of long-term islet function [1]. Recent work suggests that early islet engraftment is compromised as a consequence of cellular injury that occurs during islet isolation from donor tissue resulting in the loss of vascular networks [2-5] and islet cell-matrix interactions [6-10]. Pancreatic islets are large cell aggregates that are densely and specifically vascularized for efficient delivery of oxygen and nutrients to islet cells and rapid secretion of hormones to the circulation [2, 11]. The extracellular matrix (ECM) surrounding native islets provides mechanical support and mediates chemical signaling among cells [7, 9, 12]. In contrast, transplanted islets must rely on the extravascular diffusion of oxygen and nutrients into the dense cell network to survive until new blood vessels can form [2, 13]. Revascularization typically requires 10 to 14 days, however islet viability is significantly decreased after only 48 hours due to lower oxygen and nutrient supply, especially in the center of the islet [14]. Recently, interest has shifted to intraislet endothelial cells, which exhibit an angiogenic capacity in vitro [4, 11, 15] and contribute to the formation of functional blood vessels within islet grafts [4, 13]. Islet endothelial cells are also believed to secrete ECM proteins that mediate insulin expression and proliferation of β-cells, the islet cells responsible for insulin production and secretion [16, 17]. Enhancing revascularization with pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF2) [3, 5, 11, 18, 19] and reestablishing islet- ECM interactions with synthetic peptides [7, 8, 10] and polymer scaffolds [6, 9, 20] have all been shown to improve islet survival and function.
Therapies that target the inner core of the islets may preserve cell function and improve survival, since this region of the islet is most at risk during culture and the initial period following transplantation. Strategies to directly target this inner zone have rarely been employed to localize and maintain high levels of biological factors within the islet. Supramolecular self-assembly of nanoscale filaments offers a biomimetic vehicle to signal cells as ECM does within the dense cell aggregates of pancreatic islets. Over the past decade, we developed self-assembling biomaterials [21-24], including bioactive peptide amphiphiles (PAs) that can self-assemble into high aspect ratio cylindrical nanofibers that mimic ECM fibrils. These PAs contain a charged amino acid sequence covalently bound to a hydrophobic alkyl segment and self-assemble in salt-containing aqueous media into micron scale nanofibers due to hydrophobic collapse of the alkyl tails and formation of β-sheet secondary structure. Electrolytes in the water promote formation of high aspect ratio nanofibers due to electrostatic screening of repulsive interactions among the charged residues [21, 22]. These nanofibers, which resemble natural ECM fibrillar proteins, can form hydrogel networks at relatively low concentrations [25]. By modifying the peptide sequence, the PA can be customized to present specific biological signals on the surface of the nanofiber to promote cell adhesion [26-28], differentiation of neural progenitor cells [29], functional recovery after spinal cord injury [30], bone [31-35] and enamel [36] biomineralization, or angiogenesis [18, 37, 38]. PAs can also be used as drug delivery vehicles in vivo [39] and magnetic resonance imaging contrast agents [40, 41].
We recently reported on a heparin-binding peptide amphiphile (HBPA) containing a consensus sequence designed to bind and display heparin to localize and activate growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF2) through their heparin-binding domains [37]. HBPA nanofiber gels have been shown to persist in tissue for up to 30 days and exhibit excellent biocompatibility in vivo [42]. The presentation of VEGF and FGF2 via the HBPA-heparin gel enhanced their angiogenic bioactivity in vitro [38] and induced an extensive angiogenic response in vivo in a rat corneal assay [37]. Delivery of VEGF and FGF2 with HBPA-heparin gels also significantly increased the vessel density in a mouse omental transplant site. When transplanted adjacent to islets in a streptozotocin-induced diabetic mouse model, this angiogenic scaffold significantly improved outcomes, suggesting the increase in blood vessel density may contribute to enhanced islet engraftment [18]. The HBPA system has been shown to bind FGF2 and delay its release [37]. The presentation of heparin bound to HBPA nanofibers may be optimal for growth factor binding and activation, resulting in the high bioactivity observed [37, 38]. In fact, heparin bound to the specific amino acid sequence LRKKLGKA in HBPA significantly increases the bioactivity of VEGF and FGF2 in vitro compared to heparin bound to a PA control with a scrambled amino acid sequence [38]. Localizing and retaining growth factors with HBPA/heparin nanostructures may enhance islet revascularization and have a significant impact on engraftment and transplant success.
We report here a method to deliver into islets artificial matrix fibrils that amplify signaling of angiogenic factors. We have studied this potential approach to enhance islet survival and function during transplantation using in vitro experiments, including the role of the synthetic matrix fibrils in the sprouting of intra-islet endothelial cells.
2. Materials and methods
2.1. Peptide synthesis and purification
The HBPA used in this work was provided by Nanotope, Inc. (Skokie, IL). The peptide was synthesized using methods previously reported [42] and purified using reverse phase high performance liquid chromatography (HPLC) in an acetonitrile/water gradient under acidic conditions. To improve biocompatibility of the purified HBPA, residual trifluoroacetic acetate (TFA) counter ions were exchanged by sublimation from 0.01M HCl. After lyophilization from the HCl solution, HBPA was resolubilized in deionized water and sodium hydroxide to raise the pH to 7.2. The HBPA was separated into 1 mg aliquots and lyophilized. The lyophilized HBPA aliquots were stored at -20°C until needed.
2.2. Islet isolation
Islets were isolated from adult male FVB/N strain mice (Jackson Laboratory, Bar Harbor, ME) as previously described [18, 43]. All procedures were approved by the Center for Comparative Studies at Northwestern University and followed guidelines set by the American Veterinary Medical Association. Briefly, animals were anesthetized by an intraperitoneal injection of a 220 mg/kg dose of Avertin (2,2,2-Tribromoethanol; Sigma). A midline laparotomy was performed, and the common bile duct was cannulated. A cold solution of 0.5 mg/mL collagenase (Type XI; Sigma) in Hanks' Balanced Salt Solution (HBSS; Gibco-BRL/Invitrogen) was then injected into the duct. The distended pancreas was removed and subjected to stationary digestion at 37°C for 15 minutes. The islets were purified from the digestate using a discontinuous dextran gradient (Sigma) at densities 1.111, 1.092, 1.083, and 1.039 g/mL. Islets were hand-picked from the purification media and placed in HBSS before quantifying and separating for the different experimental conditions.
2.3. Preparation of experimental conditions
Immediately after isolation and purification, islets were separated into groups of 100-150 islets per well in non-tissue culture-treated 24-well plates for suspension culture unless otherwise noted. Islets were cultured under one of four experimental conditions: (1) media only (“control”); (2) media with HBPA and heparin (“HBPA”); (3) media with HBPA, heparin, VEGF, and FGF2 (“HBPA/GF”); (4) media with VEGF and FGF2 (“GF”). Islet media for all conditions was prepared fresh using RPMI 1640 media (Cellgro, Mediatech) with 10% fetal bovine serum (HyClone), 1% l-glutamine (Invitrogen), and 1% penicillin/streptomycin (Invitrogen). HBPA and HBPA/GF samples were prepared using lyophilized HBPA reconstituted in sterile water at pH 7.4 at 1 mg/mL and porcine-derived heparin sodium salt (Sigma) dissolved in sterile phosphate buffered saline (PBS; HyClone) at 1 mg/mL. For HBPA/GF samples, recombinant human VEGF and FGF2 (both from Peprotech) were added at 1 μg/mL each to heparin in PBS. HBPA and HBPA/GF solutions were prepared by mixing equal volumes of HBPA and heparin solutions before adding the mixture to media. This HBPA solution was added to media for final concentrations of 0.1 mg/mL HBPA and 0.1 mg/mL heparin. HBPA/GF solution was also added to media for final concentrations of 0.1 mg/mL HBPA, 0.1 mg/mL heparin, and 100 ng/mL each of VEGF and FGF2. GF samples were prepared by adding VEGF and FGF2 to PBS at 1 μg/mL each, then diluting in media to a final concentration of 100 ng/mL of VEGF and FGF2 each. Islets were cultured for up to 7 days at 37°C in 5% CO2. Media was changed at 24 hours and then every 2 days, without supplementing any additional HBPA, heparin, or growth factors.
2.4. Confocal microscopy to visualize HBPA, heparin, and FGF2
Confocal microscopy was used to probe the location and retention of fluorescently-labeled HBPA, heparin, and FGF2 within and on the surface of islets. A biotinylated version of HBPA (biotin-HBPA; see Fig. S1 in Supplementary Information) was prepared to visualize HBPA within and on the surface of islets via a streptavidin-Alexa Fluor 405 conjugate (Molecular Probes, Invitrogen). As described for the HBPA sample, biotin-HBPA was reconstituted in sterile water at pH 7.4 at 1 mg/mL and mixed at equal volumes with 1 mg/mL heparin in PBS. The biotin-HBPA/heparin solution was diluted in media for a final concentration of 0.1 mg/mL biotin-HBPA and 0.1 mg/mL heparin. Islets were cultured in the biotin-HBPA supplemented media for either 1 hour or 5 days. For the 5-day sample, media was changed after 24 hours of incubation then every 2 days without supplementing the media with biotin-HBPA or heparin. Islet samples were collected into a centrifuge tube and spun down at 5000g for 5 seconds. The supernatant was carefully removed and replaced with PBS for rinsing. This process was repeated two times for a total of three PBS rinses before fixing the islets in 2% paraformaldehyde for 10 min at room temperature. Samples were then rinsed three times to remove remaining fixative. The islets were incubated in 250 μL of 20 μg/mL streptavidin-Alexa Fluor 405 conjugate (Invitrogen) for 1 hour at room temperature then rinsed three times with PBS. Islets were transferred to a glass-bottom culture dish (Mattek) for laser scanning confocal microscopy imaging. Untreated and HBPA-treated islet samples were also prepared using this method to serve as negative controls that did not stain with streptavidin-Alexa Fluor 405 conjugate.
Fluorescein-conjugated heparin (“fl-heparin”; Invitrogen) was used to visualize heparin within and on the surface of islets. The HBPA sample was prepared as describe above by mixing at equal volumes of 1 mg/mL HBPA in water with 1 mg/mL fl-heparin in PBS. A fl-heparin only sample was also prepared by supplementing media with 1mg/mL fl-heparin in PBS without HBPA to observe if HBPA is needed to localize and retain heparin. Freshly isolated islets were cultured in the media supplemented with HBPA/fl-heparin or fl-heparin only for 1 hour or 5 days. As needed, media was changed after 24 hours of incubation then every 2 days without supplementing the media with HBPA or fl-heparin. Islets were washed in PBS to remove unbound fl-heparin and placed on glass-bottom culture dishes for laser scanning confocal microscopy imaging.
To evaluate the retention of growth factors on the surface and inside the islet, we covalently linked FGF2 to N-hydroxysuccinimide-rhodamine by an ester linkage using a commercially available kit (Pierce Biotechnology). HBPA-GF and GF samples were prepared as described in Section 2.3, but FGF2 was replaced with rhodamine-tagged FGF2. Islet samples were collected after 48 hours of culture, which included a media change after a 24-hour incubation. Islets were rinsed three times in PBS then placed on glass-bottom culture dishes for confocal imaging.
All islet samples were imaged with a Zeiss LSM 510 META laser scanning confocal microscope with the appropriate excitation and emission wavelengths for Alexa Fluor 405 (Ex=405 nm, Em=420-480 nm), fluorescein (Ex=488 nm, Em=505-530 nm), and rhodamine (Ex=543 nm, Em=560-615 nm). Image z-stacks were captured for the entire islet, and the fluorescence images shown represent an optical slice approximately halfway through the islet and are representative of all islets imaged.
2.5. Islet Viability
Islet viability and morphology was assessed on a Nikon inverted fluorescent microscope using the Live/Dead™ Viability/Cytotoxicity Kit (Invitrogen). Viability was also quantified by normalizing to DNA content using a CellTiter Blue Viability Assay (Promega) and FluoReporter Blue Fluorometric dsDNA Quantitation Kit (Invitrogen) for islets on Days 1, 3, and 7. Freshly isolated islets were also included in this experiment to serve as a standard. Islets were transferred to 96-well plates, and the media was replaced with 120 μL of CellTiter Blue reagent diluted 1:6 with islet media. The CellTiter Blue reagant contains resazurin, which is converted by viable cells to a highly fluorescent resorufin. Islets were incubated with the reagent for 4 hours before collecting 100 μL of the supernatant for fluorescence readings on a Spectramax M5 plate reader. Resorufin was excited at 560 nm, and emission was detected at 590 nm. Background fluorescence from the reagent in media was subtracted. After removal of the CellTiter Blue reagent, islets were immediately stored at -80°C until DNA quantification could be completed on all samples.
To quantify DNA, islet samples were thawed to room temperature, and 100 μL of DI water added to each well before incubating 1 hr at 37°C. Samples were returned to -80°C overnight to lyse the cells. After thawing again, 100 uL of Hoescht reagent was added and fluorescence measured (Ex=360nm, Em=460nm) with background subtraction of the Hoescht reagent and CellTiter Blue reagents. The resulting data was fit to a standard curve using 0-10 μg/mL calf thymus dsDNA (Invitrogen). Viability/DNA was calculated by dividing the viability fluorescence measurement by the corresponding DNA concentration. These values were normalized to the viability/DNA of freshly isolated islets and represented as % viability. Due to the large number of islets required per sample, these values represent data collected from two separate isolations. During one isolation, islets were separated into two wells per experimental condition for N=2. Another sample well per condition from a separate isolation was included for N=3 to represent three independent experiments with 100-150 islets per N.
2.6. Glucose-stimulated insulin release
Islets were seeded into cell culture inserts (MilliCell) placed in 24-well plates at a density of 50 islets/insert under each culture condition (N=3) for 1, 3, and 7 days. Cell culture inserts were used for easy transfer of islets into glucose solutions. All glucose solutions used were warmed to at 37°C prior to use with islets. At each timepoint, inserts were drained of media on sterile gauze and placed in 50 mg/dL glucose in Krebs buffer for 30 minute at 37°C in 5% CO2 to wash islets. Islets were washed again by draining and transferring to 50 mg/dL glucose solution before incubating in 50 mg/dL glucose for 1 hour at 37°C in 5% CO2 to stimulate insulin release at low glucose concentration. Finally, islets were incubated in 500 mg/dL glucose for 1 hour at 37°C in 5% CO2 to stimulate insulin release at high glucose concentration. The supernatant was collected and stored at -20°C until insulin could be measured for all samples using a radioimmunoassay kit (Millipore) that labels insulin with 125I. Insulin release was quantified based on a rat insulin standard and represented as the “stimulation index”, the ratio of release in a high-glucose as compared to a low-glucose state.
2.7. Islet sprouting assay
Previous work culturing islets embedded in fibrin gel or Matrigel matrices showed that islet endothelial cells formed branched cords or tube-like structures when stimulated with growth factors such as FGF2 and VEGF [11, 15]. This sprouting assay provides a method to determine what conditions activate the angiogenic potential of islet endothelial cells and/or their progenitors.
For this study, we used a synthetic, non-heparin binding PA gel to provide a three dimensional matrix to support sprouting. The PA gel was prepared by mixing 20 μL of a 10 mg/mL solution of a PA with the sequence VVAAEE (see Fig. S2 in Supplementary Information) in water with 20 μL of islet media into each well in a 96 well plate and allowed to gel for 1 hour at 37°C. After 24 hours of culture, fifty islets were added on top of the gels, and 20 μL of 10 mg/mL VVAAEE PA and 20 μL of media were added to encapsulate the islets within the gel. The samples were incubated for 30 minutes at 37°C to allow the gel to form before adding 200 μL of media on top. Media was changed every 2 days, and islets were observed daily by phase microscopy for sprouting. Sprouts were defined as cord-like, linear cell extensions out of the islet and were confirmed to be endothelial cells at all time points in all groups, as described in section 2.8. At 1, 3, and 7 day time points after encapsulation in the gel, islets were stained with Live/Dead™ Viability/Cytotoxicity Kit to visualize sprouts due to the large number of islets being counted. Islet sprouting was quantified as a percentage of the total number of islets. The data shown is representative of two islet isolations to demonstrate reproducibility. As noted in section 2.5, islets were separated into groups of 100-150 per well into two wells per experimental condition for N=2 from one isolation. The sample size was increased to N=3 by repeating the experiment with another sample well per condition from a separate isolation.
2.8. Endothelial cell staining
We verified the sprouting observed in the islet sprouting assay was composed of endothelial cells at all timepoints for each group using fluorescein isothiocyanate-lectin from Bandeiraea simplicifiolia (Griffonia simplicifolia) (FITC-lectin; Sigma), a specific marker for microvascular endothelial cells. Islets embedded in PA gels were rinsed three times in PBS then fixed in 2% paraformaldehyde for 30 minutes at room temperature. Samples were rinsed three more times in PBS then incubated in 200 μL of 20 μg/mL FITC-lectin for 1 hour at room temperature. After labeling, samples were washed three times for 10 minutes per wash in PBS to remove any unbound FITC lectin then stored in PBS until imaging. Prior to imaging, islets in PA gels were placed on a glass slide then flattened with a glass cover slip to facilitate imaging with confocal microscopy. FITC was excited at 488 nm, and the emission light was collected between 505 and 530 nm.
2.9. Statistics
Mean values for islet viability, glucose-stimulated insulin release, and islet sprouting were determined based on N=3 where each N represents a population of islets specified above. All error bars represent the standard error of the mean. Differences between groups were determined using a one-way analysis of variance (ANOVA) with a Bonferonni multiple comparisons post hoc test. Significance between groups was established for p<0.05, p<0.01, and p<0.001.
3. Results and Discussion
Previous work in our laboratory showed that delivery of growth factors via the HBPA-heparin nanostructures induces a significant angiogenic response in vitro [38] and in vivo [18, 37]. The positively charged HBPA nanofibers bind the negatively charged heparin macromolecules and therefore localize and present angiogenic growth factors through their heparin-binding domains. This in turn may assist in displaying the signaling domains of these growth factors to their corresponding receptors to promote bioactivity, thus inducing the extensive angiogenesis seen previously [37, 38]. In this study, HBPA and heparin were mixed prior to addition to media to allow interaction between the two molecules and were used at concentrations 100 times lower than that required to create a nanofiber gel network. This was done to promote formation and diffusion of the heparin-HBPA nanoscale fibrils into the interior of the dense islet cell aggregate. Cryogenic transmission electron microscopy (cryoTEM) confirmed HBPA forms nanofibers in the presence of heparin at the concentrations used (see Fig. S4 in Supplementary Information). Visualization of HBPA nanofibers within islets via conventional electron microscopy is challenging due to the low PA concentrations and morphological similarities between PA nanofibers and native ECM. Therefore we used biotin-HBPA labeled with a streptavidin-Alexa Fluor 405 conjugate, fluorescein-conjugated heparin, and rhodamine-tagged FGF2 and imaged the islets using confocal laser scanning microscopy. While this approach cannot confirm the nanoscale ultrastructure of the fibrils directly, in combination with prior TEM studies it demonstrates both nanofiber formation and their association with the islet.
Islets were incubated in media containing biotin-HBPA/heparin to visualize biotin-HBPA by labeling with streptavidin-Alexa Fluor 405. Confocal microscopy showed HBPA covers the islet surface and is present inside the islet after 1 hour (Fig. 1a). After media changes and rigorous washes, HBPA remains associated with the islet for at least 5 days (Fig. 1b). HBPA may be immobilized to the islet surface through interactions with cell membranes. Amphiphilic molecules, such as poly(ethylene glycol)-phospholipid conjugates [44] and poly(vinyl alcohol) derivatives with alkyl side chains [45], have been previously shown to immobilize to islet surfaces through interactions between hydrophobic alkyl groups and the lipid bilayer. The positive charge of the HBPA may also be attracted to the negatively charged cell membrane. In addition, our laboratory has shown cells encapsulated in PA gels can internalize PA nanofibers via endocytosis [25].
Figure 1.
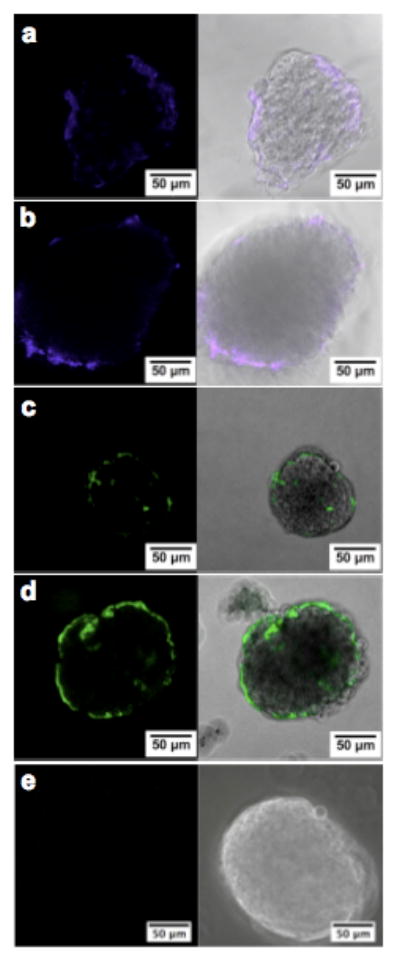
Confocal laser scanning microscopy images of murine islets showing the presence of HBPA and heparin inside and on the surface of islets. Islets were cultured in media supplemented with biotin-HBPA and heparin for (a) 1 hour and (b) 5 days before labeling biotin-HBPA with streptavidin-Alexa Fluor 405 (blue) or in media supplemented with HBPA and fluorescein-tagged heparin (green) for (c) 1 hour and (d) 5 days. (e) Islets cultured in media supplemented with fluorescein-tagged heparin without HBPA did not retain heparin at 5 days, illustrating that HBPA is needed to localize heparin to the islets.
For islets treated with HBPA/fluorescein-heparin, heparin was present on the surface and inside the islets after 1 hour of incubation (Fig. 1c) and at 5 days (Fig. 1d). Without HBPA, however, heparin was not detectable at 5 days (Fig. 1e), supporting the presence of the bioactive forms of HBPA nanofibers within the islets. Furthermore, both the positively charged HBPA and heparin are visualized in similar locations within and on the islets, and thus may be bound to each other and immobilized to the islet. In the context of in vivo transplantation, the coating of islet surfaces with heparin would provide an additional benefit by protecting islets from innate immune and inflammatory reactions to islet grafts [46]. When islets come in contact with blood, inflammatory signals trigger coagulation and a cascade of events called the instant blood-mediated inflammatory reaction (IBMIR), which is believed to be a major cause of poor islet survival and function as well as thrombosis in the portal vein during clinical islet transplantation [45, 46]. Systemic administration of heparin or other inhibitors of IBMIR is typically associated with significantly increased risk of bleeding and other side effects. As an alternative, heparinization of islets was shown to inhibit coagulation and platelet adhesion in vitro and provide protection for islets from IBMIR in vivo [46].
Since heparin was found to be present on the surface and within the islets, we expect growth factors to be bound by their heparin-binding domains and localized with heparin. Figure 2 shows that HBPA/heparin structures localize and retain rhodamine-tagged FGF2 for at least 48 hours. When free rhodamine-tagged FGF2 was introduced into islets without HBPA and heparin, it was no longer detectable by confocal microscopy after 48 hours (Fig. 2b). Localizing VEGF and FGF2 within the islet the heparin-displaying HBPA nanostructures may facilitate revascularization and engraftment into the host tissue, as observed in our prior studies at other tissue sites [18, 37]. Other heparin-binding growth factors, such as hepatocyte growth factor shown to have a synergistic effect with VEGF and promote insulin release and β-cell proliferation [47] could also be incorporated to improve islet engraftment and function.
Figure 2.
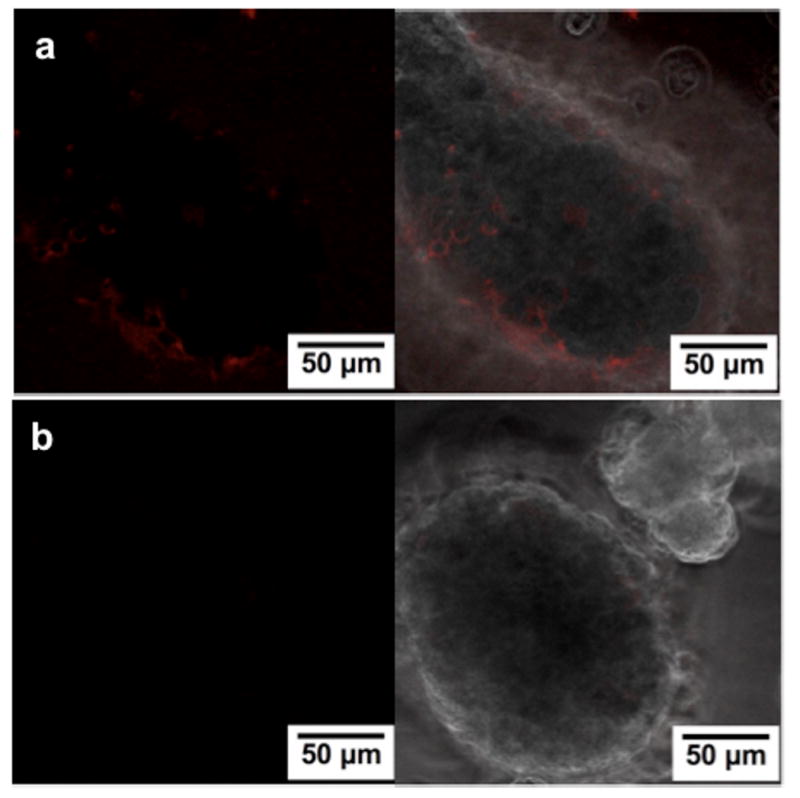
Confocal laser scanning microscopy images of murine islets showing retention of rhodamine-FGF2 inside and on the surface after 48 hours of culture in (a) the presence of HBPA/heparin system compared to in (b) media supplemented with rhodamine FGF2 only.
In vitro viability was studied to observe any effect of HBPA, heparin, and growth factors on the surface and within islets. As described in the Materials and Methods section, islets were cultured under one of four experimental conditions: (1) media only (“control”); (2) media with HBPA and heparin (“HBPA”); (3) media with HBPA, heparin, VEGF, and FGF2 (“HBPA/GF”); (4) media with VEGF and FGF2 (“GF”). Interestingly, we found that at day 7 the presence of the HBPA-heparin nanostructures seemed to improve the overall viability of the islets (Fig. 3). HBPA and HBPA/GF containing islets compared to control and GF islets appeared to have more live cells and maintained normal rounded morphology seen in islets after isolation on Day 0 (Fig. S5). Control and GF islets lost their normal morphology, and islet cells dissociated from the aggregate. The center or core of control and GF islets also contained more dead cells indicating poor oxygen and nutrient diffusion into the dense aggregate. Differences in islet size are attributed to maintenance of the normal islet morphology with HBPA and HBPA-GF islets compared to the dissociated control and GF islets that appeared larger. The enhanced viability was confirmed using a quantitative method normalizing viability to DNA to illustrate the viability of all islet cells regardless of size.
Figure 3.
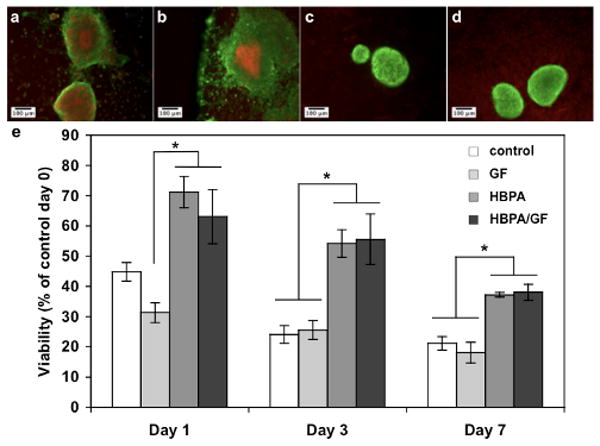
Fluorescence microscopy images of murine islets at Day 7 showing live (green) and dead (red) cells after culture in (a) media only (control) or media supplemented with (b) VEGF and FGF2 (GF), (c) HBPA and heparin (HBPA), and (d) HBPA, heparin, VEGF, and FGF2 (HBPA-GF). Islets in (c) and (d) appeared to have more live cells and maintained their normal rounded morphology. (e) Viability quantified using a fluorometric assay and normalized to DNA content. Viability is represented as percent of freshly isolated islets on day 0. Overall islet viability is significantly enhanced when islets are cultured in HBPA or HBPA-GF compared to GF at Day 1 and compared to control and GF at Days 3 and 7 (* p<0.05).
The increase in viability was expected to be higher at Day 7 for HBPA and HBPA-GF islets compared to control and GF islets based on the Live/Dead images. The lower quantified viability may result from dead cells that were not visible during imaging but contribute to the DNA content. The presence of HBPA and heparin, with or without growth factors, however, significantly increased viability at Day 1 compared the GF group and at Days 3 and 7 compared to the control and GF samples (Fig. 3e). The HBPA-heparin nanostructures may be providing an ECM-like scaffold both on the surface and within the islets to improve viability. The natural ECM surrounding native islets is known to provide mechanical support and mediate cell signaling, and the majority of β-cells in native islets are in contact with a specific type of ECM, known as the perivascular basement membrane (BM), associated with the endothelial cells [12, 16]. Loss of the BM proteins during isolation may lead to islet cell death due to disruption of islet cell-BM interactions [9, 12]. Infiltrating the islet with HBPA/heparin nanostructures may provide a temporary “artificial BM” to maintain islet architecture and improve viability. By Day 7, however, viability continues to decrease in the HBPA and HBPA-GF groups. Fig. 1 illustrates that HBPA and heparin are still present at Day 5, but these nanostructures may diffuse out or degrade by Day 7. Viability may be further improved if the media is resupplemented with HBPA/heparin nanofibers during media changes.
Insulin secretion in response to glucose was also measured to assess any changes in islet function in the presence of HBPA, heparin, and growth factors. Insulin secretion is represented as a glucose stimulation index determined by normalizing the insulin secretion at high glucose levels to insulin secretion at low glucose levels to account for differences in islet size and β-cell number. Indices equal to 1 indicate the β-cells are not secreting insulin in response to higher glucose levels and suggests loss of function. Indices greater than 1 indicate that the β-cells secrete more insulin when exposed to higher glucose levels and therefore are functional. We saw a significant increase in the glucose stimulation index for the HBPA and HBPA-GF samples compared to control and GF islets at Day 1 and to control at Day 3. The presence of the HBPA-heparin fibrils appears to preserve β-cell function as evidenced by higher insulin secretion. Despite the enhanced viability at Day 7, we did not see a significant improvement in the insulin secretion at Day 7 in the HBPA or HBPA-GF groups. Figure 4 shows that the insulin secretion decreases for all samples by Day 3 and is maintained at Day 7. The decrease in insulin secretion was expected since islet function is known to decline in culture [8, 48]. This may be caused by a reduction in insulin production by Day 7 for all islet groups. The glucose stimulation index also reflects the function of all remaining viable β-cells at each timepoint. Since the HBPA and HBPA-GF groups had a significantly higher percentage of viable islets, the total number of islets that maintained function may also be higher than in the other groups. This suggests that the HBPA and HBPA-GF treatment may improve islet function in vitro compared to control and GF groups by promoting viability.
Figure 4.
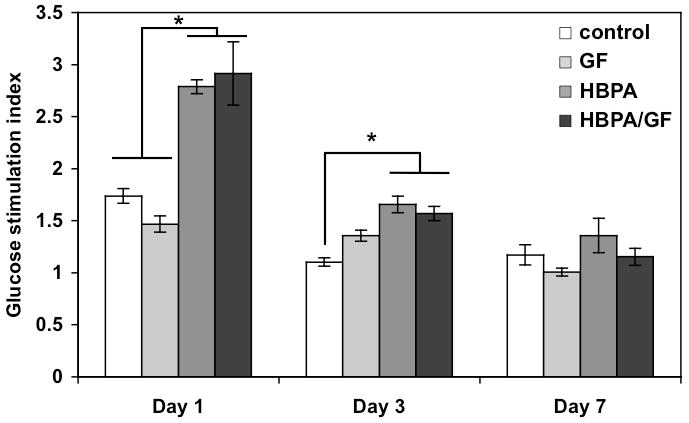
Insulin secretion from islets represented by glucose stimulation index shows a significant difference at day 1 between HBPA and HBPA-GF islets compared to the control and GF islets and at Day 3 between HBPA and HBPA-GF compared to the control (*p<0.05). A significant difference was not observed at Day 7.
Although delivery of VEGF and FGF2 via the HBPA-heparin nanostructures did not significantly improve viability or function, it stimulated an angiogenic response in the islets. Islet endothelial cells have a proliferative capacity and can form capillary-like sprouts out of the islet in response to angiogenic factors such as VEGF and FGF2 [11, 15]. Figure 5 shows a phase contrast image of an HBPA-GF islet at Day 3 with cells sprouting from the islet into a 3D matrix formed by a non-heparin binding PA. These capillary-like sprouts were confirmed to be endothelial cells using FITC-labeled lectin in order to specifically stain for endothelial cells sprouting from islets after 7 days (Fig. 6). At Day 7, we also see an extensive number of endothelial cells sprouting from the islets into the surrounding PA hydrogel compared to Day 3 (Fig. 5). The number of endothelial cell sproutings per islet also appeared to increase, which may further enhance engraftment. The confocal microscopy image in Fig. 6 also shows regions within the islets stained positive for FITC-lectin, suggesting possible maintenance or regeneration of the vasculature within the islets.
Figure 5.
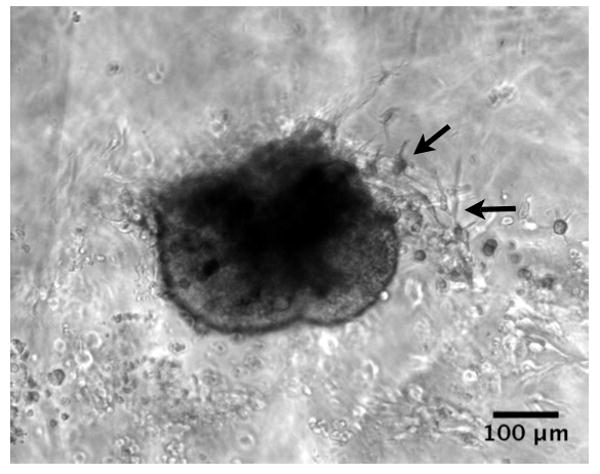
Phase contrast microscopy image of islet encapsulated in gel on Day 3. Arrows indicate sprouting extending from the islet.
Figure 6.
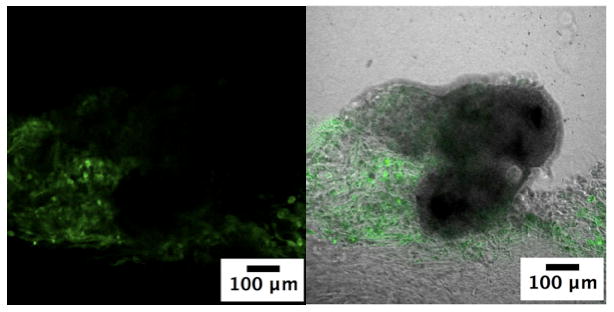
Confocal laser scanning microscopy image of endothelial cells sprouting out of HBPA-GF treated islets into the surrounding non-heparin binding PA hydrogel on Day 7. Samples were stained with FITC-tagged lectin (green) to label endothelial cells.
We stained the sproutings with FITC-lectin to confirm endothelial cell phenotype but quantified the number of islets containing these sproutings using calcein (see Fig. S3 in Supplementary Information) rather than the specific markers due to difficulties with staining islets embedded in a hydrogel and the large number of islets being observed. The number of islets with capillary-like sproutings was compared to the total number of islets per sample. The percentage of islets sprouting at Day 1 was significantly higher for GF islets compared to control and for HBPA-GF islets compared to all groups (Fig. 7). At Days 3 and 7, HBPA-GF islets were shown to have a significantly higher percentage of islets sprouting than all groups. In particular, the average percentage of islets sprouting at Day 7 in the HBPA-GF group was 47.9% compared to 15.8% in the GF group. This result suggests presence of HBPA and heparin may enhance the biological activity of VEGF and FGF2. The improvement of islet survival by HBPA-heparin nanostructures may also contribute to the dramatic increase in sprouting islets. Encouraging rapid islet endothelial cell sprouting following transplantation could help promote engraftment when islets are most vulnerable, instead of relying solely on revascularization from the surrounding tissue, which can take 1-2 weeks [12, 14]. Reestablishing the islet microvasculature may also improve insulin output, which is known to be affected by vascular alterations in islets [19].
Figure 7.
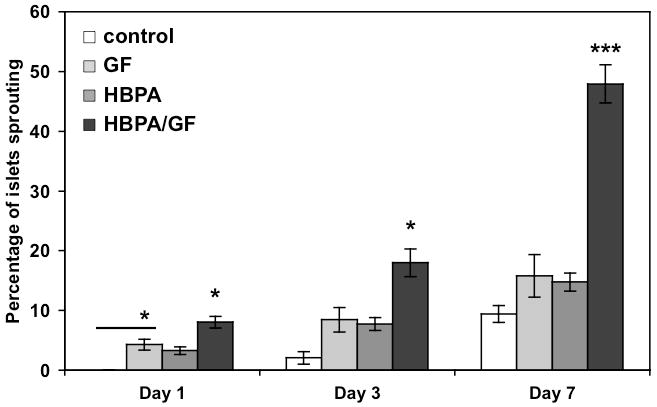
Islet sprouting assay showing percentage of islets with endothelial cell sprouting of total number of islets. Islet sprouting percentage was significantly increased for GF islets compared to control at Day 1 and at all timepoints for HBPA-GF samples (*p<0.05, ***p<0.001).
Improving viability and function of islets in vitro is particularly important since islets typically have low survival rates in culture. Clinically, islets are cultured prior to transplantation, which may improve purity and reduce immunogenecity [20]. Recent clinical work by Shapiro, et al. shows that transplanting freshly isolated islets improves initial results, but long-term insulin independence is not sustainable [48]. The poor long-term outcome may result from increased immunogenicity from the excess pancreatic tissue. This strictly controlled trial is also not ideal for widespread application when a large number of islets, typically from two to three donors, are required for successful reversal of diabetes for a single patient in clinical islet transplantation. With the shortage of donors, it is increasingly necessary to be able to culture and maintain a sufficient number of islets prior to transplantation. We propose here that the HBPA system could be used to improve current culturing methods by enhancing islet viability and function in vitro and introducing angiogenic factors to subsequently enhance revascularization and engraftment in vivo for therapeutic applications of pancreatic islets.
4. Conclusions
We have described the use of self-assembling bioactive nanoscale fibers as an artificial matrix to deliver factors into isolated pancreatic islets. The heparin-binding nanostructures with heparin displayed on their surfaces increase islet survival and insulin secretion and, in conjunction with angiogenic growth factors, lead to enhanced levels of islet endothelial cell sprouting emanating from the islets. The use of these bioactive artificial islet matrices may be useful to improve outcomes in islet transplantation to treat type 1 diabetes.
Supplementary Material
Acknowledgments
This work was funded by the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health (1R01EB003806) and the Institute for BioNanotechnology in Medicine (IBNAM) at Northwestern University. We thank Dr. James Hulvat at Nanotope, Inc (Skokie, IL) for supplying the heparin-binding peptide amphiphile material. The authors thank Dr. James Hulvat and Dr. Liam Palmer for useful discussions, Matthew Webber for help with statistical analysis, and Dr. Eugene T. Pashuck for performing cryogenic TEM. We also thank the following facilities at Northwestern University—Center for Comparative Medicine, the Cell Imaging facility, and IBNAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robertson RP. Medical progress: islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350(7):694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 2.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53(4):963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 3.Olsson R, Maxhuni A, Carlsson PO. Revascularization of transplanted pancreatic islets following culture with stimulators of angiogenesis. Transplantation. 2006;82(3):340–347. doi: 10.1097/01.tp.0000229418.60236.87. [DOI] [PubMed] [Google Scholar]
- 4.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54(8):2287–2293. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- 5.Lai Y, Schneider D, Kidszun A, Hauck-Schmalenberger I, Breier G, Brandhorst D, et al. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation. 2005;79(11):1530–1536. doi: 10.1097/01.tp.0000163506.40189.65. [DOI] [PubMed] [Google Scholar]
- 6.Blomeier H, Zhang XM, Rives C, Brissova M, Hughes E, Baker M, et al. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163(2):181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 8.Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, et al. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplantation. 2008;17(1-2):111–119. doi: 10.3727/000000008783907125. [DOI] [PubMed] [Google Scholar]
- 9.Salvay DM, Rives CB, Zhang XM, Chen F, Kaufman DB, Lowe WL, et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Y, Cong C, Zhang J, Wei L, Li S, Chen Y, et al. Self-assembling peptide nanofiber as potential substrates in islet transplantation. Transplant Proc. 2008;40(8):2571–2574. doi: 10.1016/j.transproceed.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Kidszun A, Schneider D, Erb D, Hertl G, Schmidt V, Eckhard M, et al. Isolated pancreatic islets in three-dimensional matrices are responsive to stimulators and inhibitors of angiogenesis. Cell Transplantation. 2006;15(6):489–497. doi: 10.3727/000000006783981774. [DOI] [PubMed] [Google Scholar]
- 12.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplantation. 2009;18(1):1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–1325. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 14.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacological Reviews. 2006;58(2):194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 15.Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, et al. Angiogenic capacity of endothelial cells in islets of langerhans. Faseb Journal. 2003;17(3) doi: 10.1096/fj.02-0615fje. [DOI] [PubMed] [Google Scholar]
- 16.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, et al. The vascular basement membrane: A niche for insulin gene expression and beta cell proliferation. Developmental Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Olsson R, Carlsson PO. The pancreatic islet endothelial cell: Emerging roles in islet function and disease. International Journal of Biochemistry & Cell Biology. 2006;38(5-6):710–714. doi: 10.1016/j.biocel.2006.02.004. Reprint vol 38, pg 492-497, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 20.Chun S, Huang Y, Xie WJ, Hou Y, Huang RP, Song YM, et al. Adhesive growth of pancreatic islet cells on a polyglycolic acid fibrous scaffold. Transplant Proc. 2008;40(5):1658–1663. doi: 10.1016/j.transproceed.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 21.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 22.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci U S A. 2002;99(8):5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang JJ, Iyer SN, Li LS, Claussen R, Harrington DA, Stupp SI. Self-assembling biomaterials: Liquid crystal phases of cholesteryl oligo(L-lactic acid) and their interactions with cells. Proc Natl Acad Sci U S A. 2002;99(15):9662–9667. doi: 10.1073/pnas.152667399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klok HA, Hwang JJ, Hartgerink JD, Stupp SI. Self-assembling biomaterials: L-lysine-dendron-substituted cholesteryl-(L-lactic acid)(n)over-bar. Macromolecules. 2002;35(16):6101–6111. [Google Scholar]
- 25.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomaterialia. 2005;1(4):387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006;7(6):1855–1863. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, et al. Supramolecular crafting of cell adhesion. Biomaterials. 2007;28(31):4608–4618. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Webber MJ, Tongers J, Renault MA, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomaterialia. 2009;6(1):3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 30.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spoerke ED, Murray NG, Li HL, Brinson LC, Dunand DC, Stupp SI. A bioactive titanium foam scaffold for bone repair. Acta Biomaterialia. 2005;1(5):523–533. doi: 10.1016/j.actbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Sargeant TD, Guler MO, Oppenheimer SM, Mata A, Satcher RL, Dunand DC, et al. Hybrid bone implants: self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials. 2008;29(2):161–171. doi: 10.1016/j.biomaterials.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Sargeant TD, Rao MS, Koh CY, Stupp SI. Covalent functionalization of NiTi surfaces with bioactive peptide amphiphile nanofibers. Biomaterials. 2008;29(8):1085–1098. doi: 10.1016/j.biomaterials.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sargeant TD, Oppenheimer SM, Dunand DC, Stupp SI. Titanium foam-bioactive nanofiber hybrids for bone regeneration. J Tissue Eng Regen Med. 2008;2(8):455–462. doi: 10.1002/term.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spoerke ED, Anthony SG, Stupp SI. Enzyme directed templating of artificial bone mineral. Adv Mater. 2009;21(4):425–+. doi: 10.1002/adma.200802242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Sargeant TD, Hulvat JF, Mata A, Bringas P, Koh CY, et al. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Res. 2008;23(12):1995–2006. doi: 10.1359/JBMR.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajangam K, Behanna HA, Hui MJ, Han XQ, Hulvat JF, Lomasney JW, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6(9):2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 38.Rajangam K, Arnold MS, Rocco MA, Stupp SI. Peptide amphiphile nanostructure-heparin interactions and their relationship to bioactivity. Biomaterials. 2008;29(23):3298–3305. doi: 10.1016/j.biomaterials.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Hrabie JA, Murar J, et al. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. 61st Annual Meeting of the Society-for-Vascular-Surgery; 2007 Jun 06-10; Baltimore, MD: Mosby-Elsevier; 2007. pp. 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Self-assembled peptide amphiphile nanofibers conjugated to MRI contrast agents. Nano Lett. 2005;5(1):1–4. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]
- 41.Bull SR, Guler MO, Bras RE, Venkatasubramanian PN, Stupp SI, Meade TJ. Magnetic resonance imaging of self-assembled biomaterial scaffolds. Bioconjugate Chemistry. 2005;16(6):1343–1348. doi: 10.1021/bc050153h. [DOI] [PubMed] [Google Scholar]
- 42.Ghanaati S, Webber MJ, Unger RE, Orth C, Hulvat JF, Kiehna SE, et al. Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials. 2009;30(31):6202–6212. doi: 10.1016/j.biomaterials.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen XJ, Zhang XM, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81(10):1421–1427. doi: 10.1097/01.tp.0000206109.71181.bf. [DOI] [PubMed] [Google Scholar]
- 44.Miura S, Teramura Y, Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 2006;27(34):5828–5835. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 45.Totani T, Teramura Y, Iwata H. Immobilization of urokinase on the islet surface by amphiphilic poly(vinyl alcohol) that carries alkyl side chains. Biomaterials. 2008;29(19):2878–2883. doi: 10.1016/j.biomaterials.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 46.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Kallen R, et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Ocana A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. Journal of Biological Chemistry. 2000;275(2):1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson P, et al. International trial of the edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


