Abstract
Traumatic brain injury (TBI) as a consequence of exposure to blast is increasingly prevalent in military populations, with the underlying pathophysiological mechanisms mostly unknown. In the present study, we utilized an air-driven shock tube to investigate the effects of blast exposure (120 kPa) on rat brains. Immediately following exposure to blast neurological function was reduced. BBB permeability was measured using IgG antibody and evaluating its immunoreactivity in the brain. At 3 and 24 h post-exposure there was a transient significant increase in IgG staining in the cortex. At 3 days post-exposure IgG immunoreactivity returned to control levels. Quantitative immunostaining was employed to determine the temporal course of brain oxidative stress following exposure to blast. Levels of 4-hydroxynonenal (4HNE) and 3-nitrotyrosine (3NT) were significantly increased at 3 h post-exposure and returned to control levels at 24 h post-exposure. The response of microglia to blast exposure was determined by autoradiographic localization of 3H-PK11195 binding. At 5 days post-exposure increased binding was observed in the contralateral and ipsilateral dentate gyrus. These regions also displayed increased binding at 10 days post-exposure; in addition to these regions there was increased binding in the contralateral ventral hippocampus and substantia nigra at this time point. Using antibodies against CD11b/c, microglia morphology characteristic of activated microglia was observed in the hippocampus and substantia nigra of animals exposed to blast. These results indicate that BBB breakdown, oxidative stress, and microglia activation likely play a role in the neuropathology associated with TBI as a result of blast exposure.
Keywords: traumatic brain injury, oxidative stress, inflammation, blood brain barrier, PK11195
Introduction
Accumulating clinical evidence as well as experience in contemporary military operations suggests that substantial short-term and long-term neurologic deficits can be caused by blast exposure without a direct blow to the head (Trudeau et al., 1998; Cernak et al., 1999, DePalma et al., 2005, Ling et al., 2009, Elder et al., 2009). With an estimated 15% of troops serving in Iraq sustaining some level of neurological impairment due to blast exposure, TBI has become the signature injury of this war (Hoge et al., 2008). Hoge and colleagues reported a high correlation between service members who reported symptoms consistent with mild TBI and those who received exposure to blast. Although sharing common clinical features with both, brain injury caused by blast (bTBI) is clinically distinct from closed head and/or penetrating TBI (Ling et al., 2009). While the exact biophysics underlying bTBI are not completely understood, interaction with a fast moving, transient pressure wave is generally accepted as the primary cause of brain injury that results from blast exposure (Elsayed, 1997; DePalma et al., 2005; Taber et al., 2006). Experimental studies in rodents have demonstrated a correlation between memory dysfunction and distribution of neuropathological damage in the brain after exposure to blast overpressure (BOP) (Cernak et al., 2001a, 2001b).
Previous studies have also demonstrated that rats exposed to low and moderate intensity BOP (83 kPa or 112 kPa) had lowered food intake and exercise performance (Bauman et al., 1997) with similar findings being reported in sheep (Mundie et al., 2000). Rats exposed to low intensity BOP (20 kPa) displayed significant performance deficits on rotametric and grip-strength tests (Moochhala et al., 2004, Saljo et al., 2009) whereas animal studies using moderate levels of BOP (126 kPa) have shown Morris water maze performance impairment, gliosis, and fiber degeneration (Long et al., 2009). Reactive gliosis, neuronal swelling, and cytoplasmic vacuolation have also been observed in the hippocampus of rats subjected to thoracic blast injury (Cernak et al. 2001). Rhesus monkeys exposed to high BOP (207 kPa, 276 kPa, or 345 kPa) demonstrated significant, albeit transient, memory and performance deficits (Bogo et al., 1971).
TBI can result in brain microvasculature and blood-brain barrier (BBB) breakdown leading to increased BBB permeability. Disruption of the BBB following TBI results in brain edema, a primary event that affects both morbidity and mortality following TBI (Unterberg et al., 2004). Edema increases intracerebral pressure (ICP), and leads to secondary ischemic injuries by impairing cerebral perfusion and oxygenation (Unterberg et al., 2004). Following TBI, various mediators are released which enhance vasogenic and/or cytotoxic brain edema. These include glutamate, lactate, H+, K+, Ca2+, nitric oxide, arachidonic acid and its metabolites, free oxygen radicals, histamine, and kinins (Unterberg et al., 2004). BBB permeability to endogenous proteins, such as immunoglobulins (IgG), is increased following experimental TBI (Tanno et al., 1992; (Sullivan et al. 2000). An additional consequence of BBB disruption is the infiltration of leukocytes into brain tissue, activation of microglia and inflammation (Morganti-Kossmann et al., 2007). There is some indication of a widespread activation of microglia 1-14 days after exposure to blast, however, it remains unclear if this activation was a direct result of blast wave or it was caused indirectly by an increase in permeability in blood-brain barrier and transfer of inflammatory mediators from circulation (Kaur et al., 1995). Oxidative stress has been shown to play a critical role in the secondary injury process following TBI. In recent work, our colleagues demonstrated that following both controlled cortical impact and weight drop models of TBI there is a rapid increase in the lipid peroxidation marker, 4-hydroxynonenal (4-HNE), and protein nitration marker, 3-nitrotyrosine (3-NT) (<30 min post injury), which returned to control levels 24 h post-injury (Deng et al. 2007; Hall et al. 2004).
PK11195, an isoquinoline derivative, is an antagonist of the translocator protein (TPSO) (18 kDa) (Banati et al., 1997). TPSOs, previously referred to as the peripheral benzodiazepine receptor (PBR), play an important role in steriodogenesis by acting as cholesterol pores in the outer mitochondrial membrane (Scarf et al., 2009). TPSOs are absent from neurons but are expressed in high density in glial and ependymal cells (Chen and Guilarte, 2008). Increased PK11195 binding has been localized to activated microglia in several animal models of CNS injury including TBI, ischemia, and excitotoxic lesion models (Dubois et al., 1988; Gotti et al., 1990; Grossman et al., 2003). The use of TPSO autoradiographic analysis as a marker of inflammation is advantageous over other methods due to the high sensitivity and anatomical resolution of the technique (Benavides et al., 2001).
To begin to elucidate the mechanism(s) involved in bTBI neuropathology the current study was designed to assess acute changes in neurological function and to characterize acute changes in BBB permeability and oxidative damage, and the subacute inflammatory response of the brain following BOP exposure. The results demonstrate the novel findings that following exposure to a moderate BOP (120 kPa) there is significant reflex suppression, disruption of the BBB, oxidative stress, and widespread microglia activation. Taken together, these data suggest that bTBI shares mechanisms of pathobiology that are hallmarks of injury in other models of TBI, which may widen future avenues of neuroprotective interventions for blast injuries.
Materials and Methods
Animals and Exposure to Blast
Adult virus-free male Sprague-Dawley rats (250 - 300 g; n = 49) were randomly divided into blast-exposed (n = 44) and control group (n = 14). Animals were anesthetized with isoflurane and positioned in a holder that prevented secondary and tertiary blast injuries as previously described (Chavko et al., 2006). Animals were then placed into the end of the expansion chamber of an air-driven shock tube (2.5 ft compression chamber connected to a 15 ft expansion chamber) with their right side ipsilateral to the direction of the BOP. Animals assigned to exposures were subjected to a peak overpressure of 120 kPa. Control animals received anesthesia and were treated in the same way except for the exposure to BOP.
Acute Neurological Assessment
Acute neurological function was assessed using a battery of tests that are analogous to motor components of the Glasgow Coma Scale (GCS) as previously described (Dixon et al., 1987). These tests measure the duration of suppression of a response due to injury. Immediately following BOP exposure or control treatment, the duration of suppression of three reflexes were acutely evaluated. The reflexes of control animals were evaluated in order to determine the effects of anesthesia on reflex suppression. The corneal reflex was assessed by lightly touching the eye to elicit a blinking response. The paw flexion reflex was assessed by briefly applying a pinch (approximately 0.2 kg/mm2) to the hind paw to elicit a withdrawal response. The righting response was tested by placing the animal on its back and timing the return to a spontaneous upright position. Evaluation continued until all reflexes had returned.
Immunohistochemistry
For IgG immunohistochemical assessment a total of 24 animals were exposed to blast and allowed to recover for either 0.5, 3, 24, or 72 hours following BOP exposure (n=6/group). A total of 6 control animals were handled in a similar manner and did not receive any BOP exposure. At the designated survival intervals animals were anesthetized with pentobarbital (95 mg/kg body weight) and transcardially perfused with phosphate buffered saline (PBS) followed by 4 % paraformaldehyde, pH 7.4. After removal, the brains were placed in 4 % paraformaldehyde-sucrose (15%) for an additional 24 hrs. Coronal sections (50 μm) were then cut using a freezing microtome throughout the rostral caudal extent of the brain, extending through the septal area to the most posterior extent of the hippocampus. BBB permeability was assayed on every twelfth section from each animal with biotinylated anti-rat IgG antibody as previously described (Hoane et al., 2006). Sections were rinsed with PBS and incubated in 0.3% H2O2 in order to block endogenous peroxidase activity. Next sections were blocked in 10% normal goat serum with 0.2% Triton X-100 in PBS for 1 h at room temperature followed by incubation in primary antibody overnight at 4°C (1:1000; biotinylated goat anti-rat IgG, Vector Labs, Burlingame, CA). Sections were rinsed and incubated in avidin-horseradish peroxidase complex (Vectastain ABC, Burlingame, CA) for 1 hour. IgG immunoreactivity was visualized by incubation of sections in 0.05% diaminobenzidine, 0.01% H202, and 0.3% imidazole for 10 minutes. Lastly, sections were rinsed with PBS, mounted on gelatinized slides, and coverslipped with permount. IgG immunoreactivity was quantified using a gray-level-index (GLI) as previously described (Bisler et al., 2002). Quantification was performed using Image-Pro Plus software (Mediacybernetics, Bethesda, MD) such that GLI values corresponded to the area of immunoreactivity in relation to an area of interest. The GLI value for each section (∼12 sections per animal) were calculated and averaged in order to determine the percent total brain area immunoreactive for IgG. These values were then averaged for each group.
For immunohistochemical detection of brain oxidative stress levels a total of 6 animals were subjected to BOP and allowed to recover for either 3 h or 24 h following BOP exposure. A total of 3 control animals were handled in a similar manner and did not receive any BOP exposure. At the designated survival intervals animals were anesthetized with pentobarbital and perfused with PBS and 4 % paraformaldehyde. Brains were placed in 4 % paraformaldehyde-sucrose (15%) for an additional 24 hrs. Coronal sections (50 μm) were then cut using a freezing microtome throughout the rostral caudal extent of the brain, extending through the septal area to the most posterior extent of the hippocampus. Free floating sections were double-labeled for 3-NT (1:1000; mouse anti-3NT monoclonal antibody, Upstate, Lake Placid, NY) and 4-HNE (1:5000; rabbit anti-HNE polyclonal antibody, Calbiochem, San Diego, CA) and visualized by infrared immunohistochemistry as previously described with few modifications (Hawes et. al., 2005). Briefly, sections were washed three times for 5 min each in PBS, followed by adduct reduction in 0.1 M NaBH4 in 0.1 MOPS (Sigma) for 10 min. Following reduction sections were rinsed three times for 5 min each in PBS, followed by blocking in 10% normal goat serum with 0.2% Triton X-100 in PBS for 1 hour at room temperature. Sections were incubated in primary antibodies overnight at 4°C. Before incubation in secondary antibody, sections were washed three times for 5 min each in PBS. Sections were then incubated with secondary antibodies for 2 h at room temperature (1:20,000; IRDye700DX conjugated anti-mouse IgG; Gilbertsville, PA) (1:10,000; IRDye800 conjugated anti-rabbit IgG; Gilbertsville, PA). All sections were rinsed three times for 5 min each in PBS and mounted onto Fisher Superfrost Plus Slides. Slides were scanned simultaneously on a LICOR Odyssey Infrared Imager (LI-COR Biosciences). The fluorescence intensity for each section (∼12 sections per animal) was quantified using an area of similar size throughout each section. In order to obtain a value for each animal the levels of 3-NT and 4-HNE for each section were averaged. These values were then averaged for each group.
For assessment of microglia morphology antibodies against CD11b/c that recognize microglia-specific complement type 3 receptor were utilized. Briefly, endogenous peroxidase activity was blocked using 0.3% H2O2. Next sections were blocked in 10% normal goat serum with 0.2% Triton X-100 in PBS for 1 h at room temperature followed by incubation in primary antibody overnight at 4°C (1:1000; mouse anti-rat CD11b/c; BD Pharmigen, San Diego, CA). Sections were then incubated in secondary antibody for 2 h at room temperature (1:500; biotinylated goat anti-mouse; Vector Laboratories, Burlingame, CA). Sections were rinsed and incubated in avidin-horseradish peroxidase complex (Vectastain ABC, Burlingame, CA) for 1 hour. IgG immunoreactivity was visualized by incubation of sections in 0.05% diaminobenzidine, 0.01% H202, and 0.3% imidazole for 10 minutes. Lastly, sections were rinsed with PBS, mounted on gelatinized slides, and coverslipped with permount.
[3H]PK11195 Autoradiography
For autoradiography studies a total of 14 animals were subjected to BOP and allowed to recover for either 5 or 10 days following exposure (n=7/group). A total of 5 control animals were given the same treatment except for BOP exposure. Animals were euthanized and their brains were immediately removed and frozen in isopentane on dry ice. Brains were sectioned (16 μm) on a cryostat and mounted on Fisher Superfrost Plus Slides. TPSO autoradiography was performed using 1 nM [3H]PK11195 ligand (PerkinElmer, Boston, MA, specific activity=85.5Ci/mmol) as previously described (Kelso et al., 2009). Brain sections were preincubated in 50 mM Tris- HCl, pH 7.4 at 4°C for 15 minutes, followed by incubation with radioactive ligand at 4°C for 2 hours. The binding was terminated by washing sections in 50 mM Tris- HCl, pH 7.4 (3 × 3 min), followed by dipping in ice cold water. The sections were exposed to Kodax BioMax Autoradiography Film (Kodak, Rochester, NY) for 55 days. All films were developed using a Kodak D-19 developer and analyzed using NIH image v1.59 on a Power Macintosh connected to a Sony XC-77 CCD camera via a Scion LG-3 frame-grabber (Scion, Inc., Frederick, MD). Autoradiograph data was quantified by densitometry.
Statistics
Individual reflexes (corneal, paw flexion, and righting) were analyzed by one-tailed t-test. Data from immunohistochemical assays were analyzed by one-way ANOVA followed by Student Newman-Keuls (SNK) post-hoc analysis. [3H]PK11195 autoradiography results were analyzed by two-way repeated measures (hemisphere) ANOVA followed by Bonferroni post-hoc analysis. Significance was accepted as p<0.05. All values are presented as means ± SEM.
Results
Effect of blast overpressure on acute neurological function and brain morphology
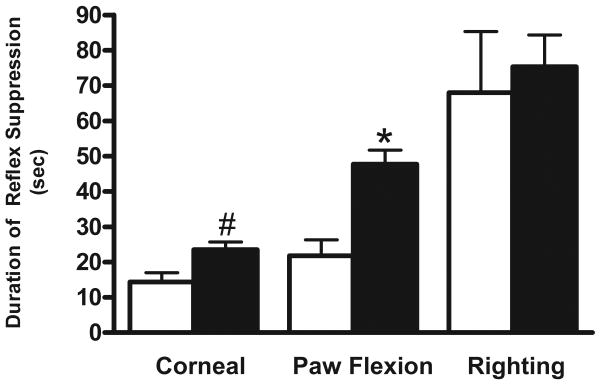
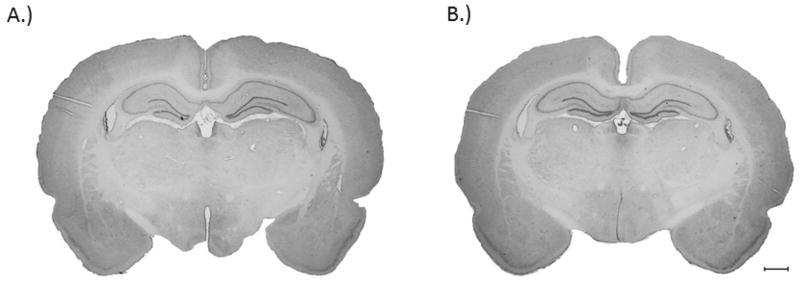
Recently Hamm (2001) demonstrated that reflex suppression is a sensitive predictor of injury effect, therefore acute neurological deficits as a result of BOP exposure were assessed using a battery of reflexes which test somatomotor function. The survival rate of animals following BOP exposure was 98%. The return of the corneal reflex in BOP exposed animals (23.6 ± 2.2 sec) was significantly delayed compared to control animals (14.33 ± 6.6 sec) (p<0.05). Additionally, BOP exposure resulted in a significant suppression of the paw flexion reflex (47.7 ± 4.0 sec) compared to control animals (21.8 ± 4.5 sec) (p<0.01) (Fig. 1). However, no significant differences were measured between the righting reflexes of either group. There was no evidence of tissue disruption or tissue loss in BOP exposed animals (Fig. 2).
Fig. 1.
Effect of BOP on acute neurological functioning immediately following exposure. Exposure to blast (full columns) significantly delayed the return of corneal and paw flexion reflexes compared to control (open columns). The data represents the mean duration of suppression ± SEM. Significance (t-test) is denoted as follows: #p<0.05, *p<0.01 vs. control.
Fig. 2.
Representative images of H & E stained brain sections of control (A) and at 3 days post blast 120 kPa (B). There were no overt signs of tissue disruption or cell loss. Scale bar denotes 1 mm.
Effect of blast overpressure on IgG immunoreactivity
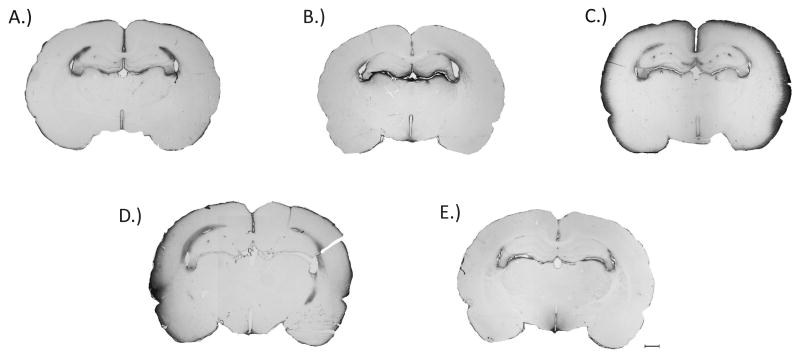
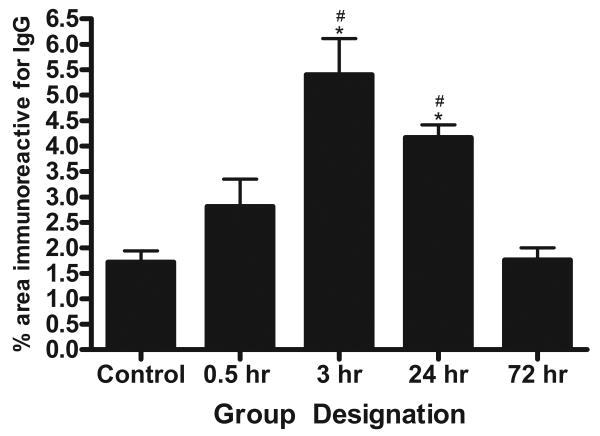
The spatial extravasation of endogenous IgG immunoreactivity was used as an index for BBB breakdown following BOP exposure. BBB disruption is a well-established hallmark following brain injury and has been assessed in several models of TBI. Qualitative assessment of IgG extravasation revealed that at 3 and 24 h following BOP exposure there was a conspicuous increase in IgG immunoreactivity in the outer most layer of the cortex. An ANOVA revealed a significant group difference (F[4,22]= 12.77, p<0.0001) in IgG immunoreactivity following BOP exposure. Following BOP exposure, post-hoc comparisons revealed a significant increase in IgG immunoreactivity at 3 (p<0.001) and 24 h (p<0.01) post BOP exposure (Fig. 3 & 4). Although not significant there was a trend towards increased IgG immunoreactivity at 0.5 h post injury surrounding the lateral ventricles.
Fig. 3.
Effect of 120 kPa BOP on brain IgG immunoreactivity. The images are representative for A - Control animals; B - 0.5 h post blast; C - 3 h post blast; D - 24 h post blast; E - 3 days post blast. Increased IgG immunoreactivity in layer I of the cortex was observed at 3 and 24 h following exposure. Although not significant there was increased staining in the area surrounding the lateral ventricles (4 out of 6 brains) at 0.5 h post blast. There was no difference in IgG staining in brains 3 days post exposure compared with controls. Scale bar denotes 1 mm.
Fig. 4.
Quantification of IgG immunoreactivity in brain sections after exposure to blast. Significant increases in IgG staining were observed at 3 and 24 h after exposure compared to control. The data represents the percentage of brain area stained for IgG ± SEM. Significance with ANOVA followed by post hoc analysis (SNK) as follows: *p<0.01 vs. control; #p<0.05 vs. 72 hr.
Effect of blast overpressure on oxidative stress
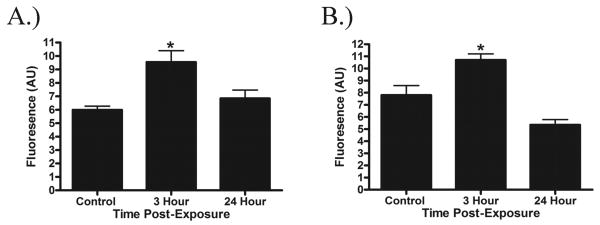
Oxidative stress is known to play a role in the secondary injury cascade following TBI; in the present study we determined 3-NT and 4-HNE levels following BOP exposure using quantitative immunohistochemistry. An ANOVA revealed a significant group difference (F[2,6]= 11.24, p<0.01) in 4-HNE levels following BOP exposure at 3 h post-exposure (p<0.01). 4-HNE levels returned to control values at 24 h post-exposure. Similarly, there was a significant group difference (F[2,6]= 11.55, p<0.01) in 3-NT levels at 3 h following exposure (p<0.05) which returned to control values at 24 h post-exposure (Fig. 5).
Fig. 5.
Quantification of 4HNE (A) and 3NT (B) levels in brain sections after exposure to blast. Significant increases in 3NT and 4HNE levels were observed at 3 h after exposure compared to control. At 24 h after exposure, 3 NT and 4HNE levels returned to control values. The data represents the mean fluorescence ± SEM. Significance with ANOVA followed by post hoc analysis (SNK) as follows: *p<0.05.
Effect of blast overpressure on TPSO binding and microglia morphology
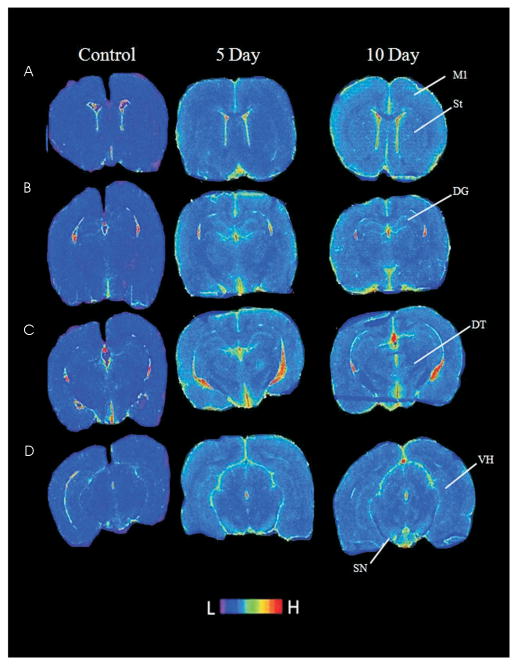
Inflammation has been demonstrated in multiple models of TBI; in the present study microglial activation was determined using TPSO autoradiographic localization. The effects of blast exposure on TPSO density were evaluated using [3H]PK11195 autoradiography in several brain regions at 5 and 10 days post blast (Fig. 6). A two-way ANOVA was significant for effect of BOP exposure (F[2, 16] = 2.287, p<0.001), but not for hemisphere (F[1, 16] = 11.20, p>0.05) (Table 1). No significant interaction between BOP exposure and hemisphere existed (F[2, 16] = 1.494, p>0.05). At 5 days post blast, post-hoc testing revealed a significant increase in the density of [3H]PK11195 binding in the ipsilateral dentate gyrus (p<0.001) and contralateral dentate gyrus (p<0.05). At 10 days post blast, post-hoc comparisons revealed a significant increase in [3H]PK11195 binding in the contralateral dentate gyrus (p<0.001), ipsilateral dentate gyrus (p<0.001), contralateral substantia nigra (p<0.01), and contralateral ventral hippocampus (p<0.05).
Fig. 6.
Effect of exposure to 120 kPa BOP on TPSO expression. Pseudocolored autoradiogram of brain sections labeled with [3H]PK11195 in control and in 5 or 10 days post exposure animals. Four different levels are shown: A - anterior striatum; B - dorsal hippocampus; C - dorsolateral thalamus; D - ventral hippocampus. Brain regions abreviated as follows: primary motor cortex (M1), striatum (St), dentate gyrus (DG), dorsolateral thalamus (DT), ventral hippocampus (VH), and substantia nigra (SN). Prominent areas of high TPSO density in control animals are restricted to ventricular/choroid plexus regions. High density areas of TPSO in exposed animals are the dentate gyrus and substantia nigra. The scale shown at bottom shows the range from low to high expression.
Table 1.
Quantification of TPSO expression in selected brain regions. There was a significant increase in PK11195 binding in the dentate gyrus at both survival intervals. In addition the TPSO expression was increased in both the contralateral substantia nigra and ventral hippocampus 10 days after blast. The data represents the mean density of [3H]PK11195 binding (arbitrary optical units) ± SEM. Significance from control is denoted as follows: *p<0.001; **p<0.05.
| Region | 5 Day | 10 Day | |||
|---|---|---|---|---|---|
| Control | Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | |
| Primary motor cortex | 41.25 (6.8) | 41.53 (5.5) | 43.79 (3.6) | 44.89 (1.9) | 48.32 (3.0) |
| Striatum | 38.22 (4.9) | 37.29 (7.3) | 38.47 (5.8) | 42.68 (6.1) | 43.99 (3.7) |
| Dentate gyrus | 37.07 (5.4) | 43.95 (4.4)* | 45.34 (3.3)** | 48.04 (3.4)* | 47.68 (2.5)* |
| Dorsolateral thalamus | 37.69 (11.0) | 39.15 (6.5) | 39.88 (7.2) | 41.84 (3.1) | 42.09 (3.0) |
| Substantia nigra | 39.28 (8.0) | 42.80 (6.0) | 45.64 (3.5) | 45.70 (3.5) | 49.74 (3.1)** |
| Ventral hippocampus | 40.41 (11.3) | 42.06 (3.4) | 43.45 (4.9) | 42.97 (4.7) | 48.28 (3.5)** |
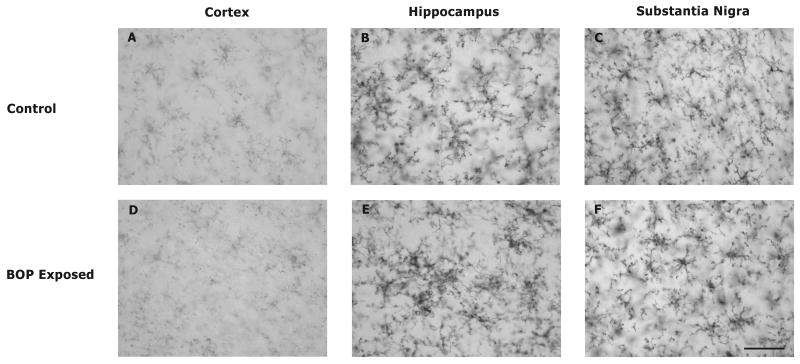
In order to further demonstrate the response of microglia to BOP exposure antibodies against CD11b/c were used to visualize microglia morphology. In the cortex, hippocampus, and substantia nigra of control animals microglia demonstrated a resting morphology characterized by highly ramified processes (Fig. 7). In contrast microglia in the hippocampus from animals exposed to blast displayed an activated morphology characterized by clustering. In addition to this microglia in the substantia nigra of BOP exposed animals revealed activated morphology characterized by rounded cell bodies and fewer ramifications.
Fig. 7.
Effect of 120 kPa BOP on microglia morphology in selected brain loci. In order to confirm PK11195 autoradiographic data demonstrating microglial activation in the dentate gyrus and substantia nigra, antibodies against CD11b/c were employed to visualize microglial morphology. In sham animals all regions that were evaluated contained microglia with resting morphology characterized by highly ramified processes (A-C). In the cortex of animals exposed to blast microglia also displayed resting morphology (D). Exposure to blast resulted in activated microglial morphology characterized by microglial clustering in the hippocampus (dentate gyrus, E). In addition activated microglia morphology in the substantia nigra is revealed by microglia with rounded cell bodies and reduced ramifications (F). Scale bar = 50 μm.
Discussion
Brain injury in rats as a result of exposure to BOP using an air-driven shock tube was used to study the progression of neuropathological processes that could be relevant to blast exposed populations. The systemic effects of the level of blast (∼120 kPa) used in the present study were previously characterized and it was shown to induce moderate pulmonary damage with an acute inflammatory responses that were resolved by 8 days (Chavko et al., 2006). The exposure to this level of BOP does not produce lethal damage and the survival rate of rats at 120 kPa under isoflurane anesthesia is more than 90%. In the present study we looked for some basic neurological and neuropathological changes in the brain and demonstrated that 120 kPa BOP exposure produced acute reflex suppression in rats which was similar to suppression observed in other models of diffuse brain injury (Denny-Brown and Russell, 1940; Dixon et al., 1987; Fijalkowski et al., 2007). This extent of reflex suppression is consistent with concussion and mild TBI which closely resembles the clinical manifestation of bTBI (Jones et al., 2007; Bruns and Jagoda, 2009). Previous studies have demonstrated a dose response relationship between the extent of acute neurological impairment and overall cognitive outcome following experimental diffuse brain injury (Beaumont et. al., 1999). Long and colleagues (2009) reported that following 126 kPa BOP exposure there is transient cognitive dysfunction; the observed reflex suppression in the present study predicts these findings by Long. The results also demonstrate increased IgG immunoreactivity in the brain 3 and 24 h after exposure indicating increased early permeability of BBB followed by microglia activation at 5 and10 days post-exposures.
BBB breakdown has been shown to occur early after TBI; it is mostly transient and the time course of the BBB opening varies in different models. In a fluid percussion model of injury, BBB damage in the rat brain was most pronounced within the first hour after TBI and was reestablished by 6 h after injury (Tanno et al., 1992). Others also reported that the BBB sealed within a few hours after fluid-percussion injury (Enters et al., 1992). We observed significant BBB breakdown following blast which was limited to layer I of the cortex and was considerably smaller in comparison to diffuse fluid percussion brain injury, where overt BBB breakdown was observed throughout the neocortex, hippocampus, and thalamus (Kelley et al., 2007). Similarly to other TBI models BBB disruption was transient and appeared recovered by 3 days.
It is generally thought that the BBB opening is a major factor contributing to the presence of brain edema observed in TBI. However, in some TBI models, edema formation clearly does not correspond with BBB opening (Beaumont et al., 2000). Instead, cytotoxic mechanism is thought as the major mechanism of edema formation in TBI. Despite this a permeable BBB can provide a route for passive fluid movement and thus worsening the cytotoxic cell edema. Recently, Saljo et al (2009) reported a blast dose-dependent rise in ICP in rats exposed repetitively 3 times to blast, and an increasing time delay in elevation with decreasing intensity of exposure. While the initial elevation took place within 30 min after exposure to 60 kPa, it did not appear until after 2 h and 6 h after exposure at 30 and 10 kPa, respectively. The delayed BBB opening and similar time course in ICP increase may indicate a contribution of vasogenic component to brain damage after exposure to blast.
BBB breakdown in TBI can be a result from the initial insult or the product of secondary injuries, such as accumulation of inflammatory cytokines. Whalen et al. (1997) found a pattern of transient BBB opening that was independent of inflammation; the BBB was leaky within the first 30 minutes to 4 hours after controlled cortical impact in rats whereas white blood cell accumulation peaked at 24 hours. It was concluded that BBB damage was not related to the white blood cell accumulation and inflammation. It was previously shown that exposure to BOP produced early (1-3 h post exposure) recruitment of polymorphonuclear leukocytes (PMNs) in peripheral blood (Gorbunov et al., 2004) at the level of blast in the present study. Therefore, contribution of inflammation to the BBB opening cannot be ruled out and needs further investigation.
In virtually all pathological conditions of the CNS, accumulation and activation of microglia precedes or is concomitant to neuronal and glial cell damage. Microglia as the principal immune cells in the CNS are known as a source of highly cytotoxic substances including proinflammatory cytokines, reactive oxygen intermediates, proteinases, and complement proteins (Rieske et al., 1989; McGeer et al., 1993; Giulian et al., 1995). Microglia derived oxygen free radicals as well as subsequent reaction products, hydrogen peroxide and peroxynitrite, have the potential to harm cells and have been implicated in contributing to oxidative damage and neurodegeneration in neurological diseases. Our findings show the inflammatory response in the brain long after initial impact and with the absence of detectable cell loss. It has been suggested that microglial activation in TBI cannot be exclusively attributable to the infiltration of blood-borne macrophages or molecules from the circulating blood. This implies that the microglial activation occurring is caused largely by intrinsic mechanisms within brain tissue, e.g. as a response to diffuse axonal injury. Several recent reviews have suggested that inflammation may serve as a biomarker for bTBI (Agoston et al., 2009; Svetlov et al., 2009). Following a single severe blast, Kaur and colleagues previously demonstrated that there is widespread microglial activation up to 14 days post blast (Kaur et al., 1995). In addition, ultrastructural findings by Cernak and colleagues demonstrated glial reaction in the hippocampus of rats exposed to high levels of BOP (Cernak et al., 2001). Recently Long and colleagues reported that following moderate levels of BOP (126 kPa) there was widespread gliosis throughout all levels of the brain. However it was unclear whether microglia and/or astrocytes were activated and the regional pattern of inflammation remained uncertain.
It was recently shown that increased PK11195 binding in some brain areas corresponds with microglial activation following TBI (Benavides J, 1990; Raghavendra Rao et al., 2000). In order to provide higher anatomical resolution of regional microglial activation following a moderate level of BOP we selected PK11195 binding as a surrogate marker of microglial activation. We demonstrated increased PK11195 binding in the dentate gyrus, substantia nigra, and ventral hippocampus 5 days and 10 days after exposure to blast. At 10 days the inflammatory response appeared to be evolving further as increased binding was observed compared to 5 days. While at 5 days after exposure the distribution of lesions was localized bilaterally in both hemispheres, latter at 10 days increased binding density increase was distributed more contralaterally to the site of impact. This distribution of microglia has some analogy with previously published data on the vasospasm distribution after exposure to blast. Unlike conventional TBI, traumatic cerebral vasospasm (TCV) often occurred in vessels far distal to the area of fragment penetration in the hemisphere contralateral to the injury. It was suggested that, in addition to the fragment injury, propagation of the blast wave through the tissue could have caused TCV (Armonda et al., 2006). Another possibility is migration of toxic components of microglia activation products from the site of injury into distant regions (Gong et al., 2000).
The results presented clearly illustrate the selective vulnerability of the hippocampus and substantia nigra to BOP exposure. The high density of NMDA receptors present in the hippocampus may be partially responsible for the vulnerability of the hippocampus to excitotoxic insults (Butler et al., 2009). It was shown by positron electron tomography (PET) that PK11195 can visualize excitotoxic lesions in the living human brain (Groom et al., 1995). High vulnerability to oxidative stress of dopaminergic neurons in the substantia nigra and resultant mitochondrial dysfunction may contribute to the observed increase in microglia activation in substantia nigra (Hastings et al., 2009).
Cernak and colleagues demonstrated that high levels of BOP result in an increase in brain nitric oxide production and lipid peroxidation as early as 3 h post-exposure that resolves by 5 days post-exposure. However, prior to the current study, oxidative stress following a moderate level of blast remained undetermined. Here we report that as early as 3 h post-exposure there is a significant increase in 3-NT and 4-HNE levels. At 24 h, 4-HNE and 3-NT levels had returned to control values. This indicates that there is a rapid increase in oxidative stress following moderate BOP exposure and that oxidative stress is at least partially occurring concomitantly with BBB breakdown. It has been postulated that vascular abnormalities following TBI are dependent on the production of oxygen radicals (Pun et. al, 2009; Kontos et. al., 1986). Therefore antioxidants may represent one potential neuroprotective target for therapeutic intervention following bTBI, which may in turn reduce other downstream pathological processes (i.e. BBB breakdown).
In summary, our data demonstrate that bTBI likely share some common mechanisms of injury with other TBI models. In this study we demonstrated that inflammation likely plays a role in the neuropathology associated with bTBI. We report significant BBB breakdown that resolved by 3 days post exposure. Oxidative damage markers increased rapidly and resolved by 24 h post exposure. Taken together our results clearly show that the brain is susceptible to BOP exposure and that specific brain regions are more susceptible to blast than others.
Acknowledgments
This work was supported by the Office of Naval Research Work Unit # 601153N.04508.5180.A0805 and National Institute of Health (5T32 DA022738).
Footnotes
The experiments reported herein were conducted according to the principles set forth in the “Guide for the Care and Use of Laboratory Animals”, Institute of Laboratory Animal Resources, National Research Council, National Academy Press, 1996 and was approved by WRAIR/NMRC IACUC Committee. The opinions expressed in this presentation are those of the authors and do not reflect the official policy of the Department of Navy, Department of Defense, or the U. S. Government.
References
- Agoston DV, Gyorgy A, Eidelman O, Pollard HB. Proteomic biomarkers for blast neurotrauma: targeting cerebral edema, inflammation, and neuronal death cascades. Journal of Neurotrauma. 2009;26:901–911. doi: 10.1089/neu.2008.0724. [DOI] [PubMed] [Google Scholar]
- Armonda RA, Bell RS, Vo AH, Ling G, DeGraba TJ, Crandall B, Ecklund J, Campbell WW. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215–1225. doi: 10.1227/01.NEU.0000249190.46033.94. discussion 1225. [DOI] [PubMed] [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. Journal of Neurocyto. 1997;26:77–82. doi: 10.1023/a:1018567510105. [DOI] [PubMed] [Google Scholar]
- Bauman RA, Elsayed N, Petras JM, Widholm J. Exposure to sublethal blast overpressure reduces the food intake and exercise performance of rats. Toxicology. 1997;121:65–79. doi: 10.1016/s0300-483x(97)03656-1. [DOI] [PubMed] [Google Scholar]
- Beaumont A, Marmarou A, Hayasaki K, Barzo P, Fatouros P, Corwin F, Marmarou C, Dunbar J. The permissive nature of blood brain barrier (BBB) opening in edema formation following traumatic brain injury. Acta Neurochir Suppl. 2000;76:125–129. doi: 10.1007/978-3-7091-6346-7_26. [DOI] [PubMed] [Google Scholar]
- Beaumont A, Marmarou A, Czigner A, Yamamoto M, Demetriadou K, Shirotani T, Marmarou C, Dunbar J. The impact-acceleration model of head injury: injury severity predicts motor and cognitive performance after trauma. Neurol Res. 1999;21(8):742–754. doi: 10.1080/01616412.1999.11741008. [DOI] [PubMed] [Google Scholar]
- Benavides J, Dubois A, Scatton B. Peripheral type benzodiazepine binding sites as a tool for the detection and quantification of CNS injury. Curr Protoc Neurosci. 2001;Chapter 7(Unit7 16) doi: 10.1002/0471142301.ns0716s09. [DOI] [PubMed] [Google Scholar]
- Benavides J, S A, Duval D, Bourdiol F, Toulmond S, Scatton B. Autoradiographic detection and quantification of traumatic brain lesions in the rat by using ω3 sites radioligands. In: Krieglstein J, O H, editors. Pharmacology of Cerebral Ischemia. CRC, press; Stuttgart: 1990. pp. 39–45. [Google Scholar]
- Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K, Staiger JF. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J Chem Neuroanat. 2002;23:187–198. doi: 10.1016/s0891-0618(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Bogo V, Hutton RA, Bruner A. Technical Progress Report on Contract No DA-49-146-XZ-372) DASA 2659. Washington, DC: Defense Nuclear Agency; 1971. The effects of airblast on discriminated avoidance behavior in rhesus monkeys; pp. 1–32. [Google Scholar]
- Bruns JJ, Jr, Jagoda AS. Mild traumatic brain injury. Mt Sinai J Med. 2009;76:129–137. doi: 10.1002/msj.20101. [DOI] [PubMed] [Google Scholar]
- Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, Pauly JR, Mulholland PJ, Prendergast MA. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-d-asparate-type glutamate receptors. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. The Journal of trauma. 2001a;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001b;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Cernak I, Savic J, Ignjatovic D, Jevtic M. Blast injury from explosive munitions. The Journal of trauma. 1999c;47:96–103. doi: 10.1097/00005373-199907000-00021. discussion 103-104. [DOI] [PubMed] [Google Scholar]
- Chavko M, Prusaczyk WK, McCarron RM. Lung injury and recovery after exposure to blast overpressure. The Journal of trauma. 2006;61:933–942. doi: 10.1097/01.ta.0000233742.75450.47. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Experimental neurology. 2007;205(1):154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D, Russell WR. Experimental cerebral concussion. J Physiol. 1940;99:153. doi: 10.1113/jphysiol.1940.sp003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePalma RG, Burris DG, Champion HR, Hodgson MJ. Blast injuries. The New England journal of medicine. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. Journal of neurosurgery. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Dubois A, Benavides J, Peny B, Duverger D, Fage D, Gotti B, MacKenzie ET, Scatton B. Imaging of primary and remote ischaemic and excitotoxic brain lesions. An autoradiographic study of peripheral type benzodiazepine binding sites in the rat and cat. Brain Res. 1988;445:77–90. doi: 10.1016/0006-8993(88)91076-1. [DOI] [PubMed] [Google Scholar]
- Elder GA, Cristian A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med. 2009;76:111–118. doi: 10.1002/msj.20098. [DOI] [PubMed] [Google Scholar]
- Elsayed NM, Gorbunov NV. Pulmonary biochemical and histological alterations after repeated low-level blast overpressure exposures. Toxicol Sci. 2007;95:289–296. doi: 10.1093/toxsci/kfl138. [DOI] [PubMed] [Google Scholar]
- Elsayed NM. Toxicology of blast overpressure. Toxicology. 1997;121:1–15. doi: 10.1016/s0300-483x(97)03651-2. [DOI] [PubMed] [Google Scholar]
- Enters EK, Pascua JR, McDowell KP, Kapasi MZ, Povlishock JT, Robinson SE. Blockade of acute hypertensive response does not prevent changes in behavior or in CSF acetylcholine (ACH) content following traumatic brain injury (TBI) Brain Res. 1992;576:271–276. doi: 10.1016/0006-8993(92)90690-b. [DOI] [PubMed] [Google Scholar]
- Fijalkowski RJ, Stemper BD, Pintar FA, Yoganandan N, Crowe MJ, Gennarelli TA. New rat model for diffuse brain injury using coronal plane angular acceleration. Journal of neurotrauma. 2007;24:1387–1398. doi: 10.1089/neu.2007.0268. [DOI] [PubMed] [Google Scholar]
- Finkel MF. The neurological consequences of explosives. Journal of the neurological sciences. 2006;249:63–67. doi: 10.1016/j.jns.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871(1):57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- Gorbunov NV, McFaul SJ, Van Albert S, Morrissette C, Zaucha GM, Nath J. Assessment of inflammatory response and sequestration of blood iron transferrin complexes in a rat model of lung injury resulting from exposure to low-frequency shock waves. Crit Care Med. 2004;32:1028–1034. doi: 10.1097/01.ccm.0000120051.79520.b6. [DOI] [PubMed] [Google Scholar]
- Gotti B, Benavides J, MacKenzie ET, Scatton B. The pharmacotherapy of focal cortical ischaemia in the mouse. Brain Res. 1990;522:290–307. doi: 10.1016/0006-8993(90)91473-t. [DOI] [PubMed] [Google Scholar]
- Groom GN, Junck L, Foster NL, Frey KA, Kuhl DE. PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer's disease. J Nucl Med. 1995;36:2207–2210. [PubMed] [Google Scholar]
- Grossman R, Shohami E, Alexandrovich A, Yatsiv I, Kloog Y, Biegon A. Increase in peripheral benzodiazepine receptors and loss of glutamate NMDA receptors in a mouse model of closed head injury: a quantitative autoradiographic study. NeuroImage. 2003;20:1971–1981. doi: 10.1016/j.neuroimage.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. Journal of neurotrauma. 2004;21(1):9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. Journal of neurotrauma. 2001;18(11):1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Hastings TG. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson's disease. J Bioenerg Biomembr. 2009 doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Wynick D, Zachariou V, Picciotto MR. GalR1, but not GalR2 or GalR3, levels are regulated by galanin signaling in the locus coeruleus through a cyclic AMP-dependent mechanism. J Neurochem. 2005;93(5):1168–1176. doi: 10.1111/j.1471-4159.2005.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. The New England journal of medicine. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Jones E, Fear NT, Wessely S. Shell shock and mild traumatic brain injury: a historical review. The American journal of psychiatry. 2007;164:1641–1645. doi: 10.1176/appi.ajp.2007.07071180. [DOI] [PubMed] [Google Scholar]
- Kaur C, Singh J, Lim MK, Ng BL, Yap EP, Ling EA. The response of neurons and microglia to blast injury in the rat brain. Neuropathol Appl Neurobiol. 1995;21:369–377. doi: 10.1111/j.1365-2990.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. Journal of neuropathology and experimental neurology. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Scheff SW, Pauly JR, Loftin CD. Effects of genetic deficiency of cyclooxygenase-1 or cyclooxygenase-2 on functional and histological outcomes following traumatic brain injury in mice. BMC Neurosci. 2009;10:108. doi: 10.1186/1471-2202-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos HA, Povlishock JT. Oxygen radicals in brain injury. Cent Nerv Syst Trauma. 1986;3(4):257–263. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J. Explosive blast neurotrauma. Journal of neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: recreating a battlefield injury in the laboratory. Journal of neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Moochhala SM, Md S, Lu J, Teng CH, Greengrass C. Neuroprotective role of aminoguanidine in behavioral changes after blast injury. The Journal of trauma. 2004;56:393–403. doi: 10.1097/01.TA.0000066181.50879.7A. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Mundie TG, Dodd KT, Lagutchik MS, Morris JR, Martin D. Effects of blast exposure on exercise performance in sheep. The Journal of trauma. 2000;48:1115–1121. doi: 10.1097/00005373-200006000-00019. [DOI] [PubMed] [Google Scholar]
- Murthy JM, Chopra JS, Gulati DR. Subdural hematoma in an adult following a blast injury. Case report. Journal of neurosurgery. 1979;50:260–261. doi: 10.3171/jns.1979.50.2.0260. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, Pochet R. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 2009;1301:152–162. doi: 10.1016/j.brainres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43(4):348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Experimental neurology. 2000;161:102–114. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Rieske E, Graeber MB, Tetzlaff W, Czlonkowska A, Streit WJ, Kreutzberg GW. Microglia and microglia-derived brain macrophages in culture: generation from axotomized rat facial nuclei, identification and characterization in vitro. Brain Res. 1989;492:1–14. doi: 10.1016/0006-8993(89)90883-4. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljo A, Bao F, Haglid KG, Hansson HA. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. Journal of neurotrauma. 2000;17:719–726. doi: 10.1089/089771500415454. [DOI] [PubMed] [Google Scholar]
- Saljo A, Arrhen F, Bolouri H, Mayorga M, Hamberger A. Neuropathology and pressure in the pig brain resulting from low-impulse noise exposure. Journal of neurotrauma. 2008;25:1397–1406. doi: 10.1089/neu.2008.0602. [DOI] [PubMed] [Google Scholar]
- Saljo A, Svensson B, Mayorga M, Hamberger A, Bolouri H. Low levels of blast raises intracranial pressure and impairs cognitive function in rats. Journal of neurotrauma. 2009 doi: 10.1089/neu.2009.1053. [DOI] [PubMed] [Google Scholar]
- Scarf AM, Ittner LM, Kassiou M. The translocator protein (18 kDa): central nervous system disease and drug design. J Med Chem. 2009;52:581–592. doi: 10.1021/jm8011678. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Hicks RR, Gibson TR, Fletcher-Turner A, Scheff SW. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000;101:289–295. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Svetlov SI, Larner SF, Kirk DR, Atkinson J, Hayes RL, Wang KK. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. Journal of neurotrauma. 2009;26:913–921. doi: 10.1089/neu.2008.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Warden DL, Hurley RA. Blast-related traumatic brain injury: what is known? The Journal of neuropsychiatry and clinical neurosciences. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: Distribution and time course of protein extravasation. Journal of neurotrauma. 1992;9:21–32. doi: 10.1089/neu.1992.9.21. [DOI] [PubMed] [Google Scholar]
- Trudeau DL, Anderson J, Hansen LM, Shagalov DN, Schmoller J, Nugent S, Barton S. Findings of mild traumatic brain injury in combat veterans with PTSD and a history of blast concussion. The Journal of neuropsychiatry and clinical neurosciences. 1998;10:308–313. doi: 10.1176/jnp.10.3.308. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–10. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Brink BP, de Vries HE, van der Valk P, Bo L. The blood-brain barrier in cortical multiple sclerosis lesions. Journal of neuropathology and experimental neurology. 2007;66:321–328. doi: 10.1097/nen.0b013e318040b2de. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Wisniewski SR, Clark RS, Mellick JA, Marion DW, Kochanek PM. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit Care Med. 2000;28:3710–3717. doi: 10.1097/00003246-200011000-00029. [DOI] [PubMed] [Google Scholar]