Summary
Background
The serine-threonine kinase Akt plays an important role in regulating platelet activation. Stimulation of platelets with various agonists results in Akt activation as indicated by Akt phosphorylation. However, the mechanisms of Akt phosphorylation in platelets are not completely understood.
Objectives and Methods
We used P2Y12 knockout mice to address the role of P2Y12 in Akt phosphorylation in response to thrombin receptors in platelets.
Results
Thrombin or the PAR4 thrombin receptor peptide AYPGKF at high concentrations stimulated substantial phosphorylation of Akt residues thr308 and Ser473 in P2Y12 deficient platelets. AYPGKF-induced Akt phosphorylation is enhanced by expression of recombinant human PAR4 cDNA in Chinese hamster ovary (CHO) cells. P2Y12-independent Akt phosphorylation was not inhibited by integrin inhibitor peptide RGDS or integrin β3 deficiency. Akt phosphorylation induced by thrombin or AYPGKF in P2Y12 deficient platelets was inhibited by the calcium chelator dimethyl-BAPTA, the Src family kinase inhibitor PP2, and PI3K inhibitors, respectively.
Conclusions
Our results reveal a novel P2Y12-independent signaling pathway mediating Akt phosphorylation in response to thrombin receptors.
Introduction
Platelets play a central role in hemostasis and thrombosis. Upon vascular injury, platelets are activated by various soluble and immobilized agonists. The signaling associated with platelet activation includes a series of rapid positive feedback loops that greatly amplify the activation signals and enable robust platelet recruitment at the site of vascular injury. Akt is a serine/threonine protein kinase [1]. Three isoforms of Akt have been identified in both human and mouse cells, including Akt 1, Akt 2, and Akt 3 [2, 3]. Akt 1 and Akt 2 occur in blood platelets [4–6]. Both Akt 1 and Akt 2 play important roles in platelet activation [5–8]. Akt regulates platelet function, in part by phosphorylating and inhibiting GSK beta [9].
Activation of Akt is a consequence of phosphorylation of residues Thr308 in the activation loop and Ser473 in the hydrophobic phosphorylation motif [10]. In platelets, Akt is phosphorylated upon stimulation with various platelet agonists [4–6, 11–15]. The ADP receptor P2Y12 plays an important role in Akt phosphorylation not only in response to ADP, but also in response to other platelet agonists, such as U46619 and thrombin [12–14]. However, it is controversial whether Akt phosphorylation induced by thrombin depends on the Gi pathway activated by secreted ADP. Kim et al. [13] have suggested that thrombin-induced Akt phosphorylation is mainly P2Y12 dependent, and is potentiated by the G12/13 pathway [16]. The lack of Akt phosphorylation in Gq deficient platelets [5] was explained by a defect in platelet secretion of ADP [13]. In contrast, Resendiz, et al. [14] have shown that thrombin can elicit Akt phosphorylation through a P2Y12-independent mechanism. All these conclusions are based on experiments using the ADP receptor P2Y12 antagonist, AR-C69931MX, which has recently been shown to increase intracellular cAMP levels and inhibit platelet activation through a P2Y12-independent mechanism [17]. Therefore, the role of P2Y12 in Akt phosphorylation needs to be re-evaluated. The work described below resolves this issue using P2Y12 deficient platelets rather than the P2Y12 antagonist AR-C69931MX.
In this study, we present data documenting a previously undescribed mechanism that mediates Akt phosphorylation in platelets. The data presented here demonstrate that thrombin or AYPGKF at high concentrations stimulates Akt phosphorylation via both ADP/P2Y12/Gi-dependent and ADP/P2Y12/Gi-independent mechanisms. Furthermore, the data demonstrate that the thrombin-induced Akt phosphorylation evident in the P2Y12 deficient platelets is Gq, Ca2+, Src family kinase and PI3K-dependent. These results characterize a P2Y12-independent signaling pathway that elicits Akt phosphorylation in response to thrombin stimulation.
Materials and Methods
Materials
α-Thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). PAR 4 peptide AYPGKF was custom-synthesized at Biomatik USA, LLC (Wilmington, DE). ADP and the P2Y12 receptor antagonist 2MeSAMP were from Sigma. AR-C69931MX was from the Medicines Company (Parsippany, NJ). Luciferase/luciferin reagent was from Chrono-log (Havertown, PA). The Akt inhibitors Akt IV and SH-6, the PI3K inhibitors LY294002 and wortmannin, the Src family kinase inhibitor PP2, the PKC inhibitors Ro-31-8220 and Gö6976, the PKC activator PMA, the TXA2 analog U46619, and forskolin were purchased from Calbiochem (San Diego, CA). Calcium chelator dimethyl-BAPTA, Fura-2/AM, and Pluronic F-127 were from Invitrogen. Calcium ionophore A23187 was from Fisher Scientific. A rabbit polyclonal antibody against a recombinant human Akt 1 fragment (amino acid residues 345–480) and a rabbit anti-PAR4 polyclonal antibody were purchased from Santa Cruz Biotechnology Inc., and rabbit monoclonal antibodies against phosphorylated Ser473 or Thr308 residues of Akt and phosphorylated Tyr416 of Src were from Cell Signaling Technology (Beverly, MA). cAMP ELISA kit was from Amersham Biosciences.
Animals
Mice deficient in Gαq [18], P2Y12 [19], and integrin β3 [20] were generated as described previously. Littermate wild type mice from heterozygous breeding were used as controls. Mice were bred and maintained in the University of Kentucky Animal Care Facility following institutional and National Institutes of Health guidelines after approval by the Animal Care Committee.
Expression of human PAR4 cDNA or P2Y12 constructs in Chinese hamster ovary (CHO) Cells
Human PAR4 and P2Y12 cDNA were cloned by RT-PCR using human platelet mRNA as template and verified by sequencing. PAR4 or P2Y12 was subcloned in the pcDNA3.1/Zeo(−) vector and transfected into CHO cells using LipofectAMINE plus (Life Technologies, Inc.). PAR4 stable cell lines were established by selection with 0.4 mg/ml zeocin containing culture medium and flow cytometric cell sorting with a polyclonal anti-human PAR4 antibody that recognizes the extracellular domain of PAR4. Expression of PAR4 was further assessed by Western blotting. P2Y12 stable cell lines were established by selection with 0.4 mg/ml zeocin containing culture medium and screened by examining their ability to inhibit forskolin-induced cAMP production.
Preparation of mouse platelets
Washed platelets from knockout mice and wild type controls were prepared as described previously [12]. Briefly, blood was collected from the abdominal aorta of isofluorane-anesthetized mice (8–10 weeks) using 1/7 volume of ACD as anticoagulant. For each experiment, blood was pooled from five to six mice of each genotype. Platelets were then washed twice with CGS (0.12 M sodium chloride, 0.0129 M trisodium citrate, 0.03 M D-glucose, pH 6.5), resuspended in modified Tyrode’s buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 10 mM HEPES, pH 7.4) at 3 × 108/ml, and incubated for 1 h at 22°C before use.
Western blot analysis of Akt and Src phosphorylation
Washed platelets were stimulated with various concentrations of thrombin or AYPGKF in a platelet aggregometer at 37° for 5 min and then solubilized in SDS-PAGE sample buffer. Platelets were pre-incubated with various inhibitors at the indicated concentrations or vehicle control at 37° for 5 min prior addition of the platelet agonists to examine the effects of inhibitors on Akt phosphorylation. To examine Akt phosphorylation in CHO cells, cells expressing vector, PAR4, or P2Y12 were resuspended in Tyrode’s buffer (5 × 106/ml) and incubated with ADP (10 μM) or AYPGKF (500 μM) at 37° for 2 or 5 min. Platelet or cell lysates were analyzed by SDS-PAGE on 4–15% gradient gels and immunoblotted using a polyclonal anti-Akt antibody, rabbit monoclonal antibodies specific for the phosphorylated Akt residues Ser473 or Thr308, or phosphorylated Src residue Tyr416 (Cell Signaling Technology, Beverly, MA),
Determination of intracellular cAMP levels
Washed platelets from P2Y12 deficient or wild type mice were pre-incubated with platelet agonists for 5 min at 37°C. Platelets were then added with forskolin and incubated for additional 5 min at 37°C. The reaction was stopped by addition of equal volumes of ice-cold 12% (wt/vol) trichloroacetic acid. cAMP levels were measured using a cAMP enzyme immunoassay kit (Amersham Biosciences) [21].
Calcium Mobilization
Intraplatelet calcium was measured using Fura-2/AM as described previously [22]. Briefly, washed mouse platelets were incubated with 12.5 μM Fura-2/AM/0.2% Pluronic F-127 (Invitrogen) for 45 min at 37°C. After washing with CGS [12] once more, platelets were resuspended to 3 × 108/ml in Tyrode’s solution. Continuous fluorescent measurements were analyzed by excitation at 340 nm and 380 nm, and emission was measured at 509 nm using a model LS55 Luminescence Spectrometer (Perkin-Elmer Cetus). The intracellular Ca2+ level was expressed as relative fluorescence calculated based on the ratio of emissions simultaneously using FL Win Lab 4.0 software (Perkin-Elmer Cetus).
Results
Thrombin or AYPGKF induces Akt phosphorylation in P2Y12 deficient platelets
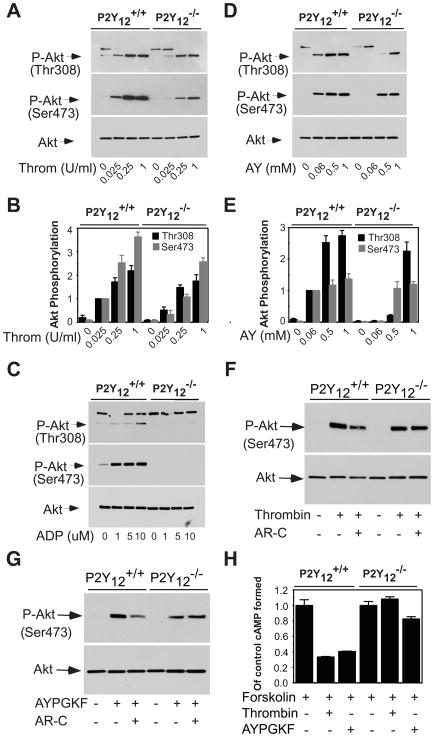
The mechanisms of Akt phosphorylation elicited by thrombin or thrombin receptor peptide stimulation of platelets were re-evaluated using P2Y12 deficient platelets. We examined Akt phosphorylation in response to various concentrations of thrombin in P2Y12 deficient platelets to determine whether or not P2Y12 is required for thrombin-stimulated Akt phosphorylation. As demonstrated in Fig. 1A and 1B, stimulation of platelets with increasing concentrations of thrombin led to a dose-dependent increase of Akt phosphorylation in the washed platelets from wild type mice. Surprisingly, while Akt phosphorylation induced by a low concentration of thrombin (0.025U/ml) was abolished in P2Y12 deficient platelets, thrombin at high concentrations induced substantial Akt phosphorylation in the P2Y12 deficient platelets, demonstrating a P2Y12-independent mechanism mediating thrombin induced Akt phosphorylation. Thrombin-induced Akt phosphorylation in the P2Y12 deficient platelets is unlikely to be dependent on secreted ADP, because ADP induced Akt phosphorylation was abolished in the P2Y12 deficient platelets (Fig. 1C).
Figure 1. Akt phosphorylation in response to thrombin or AYPGKF in P2Y12 deficient platelets.
(A–E), Washed platelets from P2Y12 deficient mice and littermate wild type controls were incubated with increasing concentrations of thrombin (Throm) (A), ADP (C), or AYPGKF (AY) (D) at 37°C in a platelet aggregometer for 5 min, and solubilized with SDS-PAGE sample buffer. Phosphorylation of Akt was detected by Western blotting with rabbit monoclonal antibodies specifically recognizing the phosphorylated Akt residues Ser473 or Thr308. A rabbit polyclonal antibody against total Akt was used to verify equal loading. Immunoblotting results as in experiments described in panels (A) and (D) were scanned and quantitated. Values were normalized with respect to wild type platelets stimulated with lowest concentration of thrombin (B) or AYPGKF (E) for each immunoblot and are expressed as relative phosphorylation (mean ± SD from 3 separate experiments). (F–G) Washed platelets from P2Y12 deficient mice and littermate wild type controls were incubated with AR-C69931MX (1 μM) for 5 min, and then added with thrombin (F), or AYPGKF (G) at 37°C in a platelet aggregometer for 5 min, and solubilized with SDS-PAGE sample buffer. (H), Washed platelets (1 × 108/ml) from P2Y12 deficient or wild type mice were pre-incubated with thrombin (0.25 U/ml) or AYPGKF (0.5 mM) at 37 °C for 5 min. These platelets were treated for 5 min with forskolin (10 μM). cAMP concentrations were determined by using a cAMP immunoassay kit.
In mouse platelets, PAR4 is a major thrombin signaling receptor. Similar to high concentrations of thrombin, the PAR4-activation peptide, AYPGKF, stimulated Akt phosphorylation in the P2Y12 deficient platelets (Fig. 1D and E). Likewise, AR-C69931MX reduced but did not abolish Akt phosphorylation in response to thrombin and PAR4, respectively in wild type platelets (Fig. 1F and 1G). In contrast, AR-C69931MX failed to affect thrombin-induced Akt phosphorylation in P2Y12 deficient platelets. These data demonstrate that there are P2Y12-dependent and -independent pathways mediating Akt phosphorylation in response to thrombin receptors and that the effect of AR-C69931MX on Akt phosphorylation is specifically mediated by the ADP receptor P2Y12.
The thrombin receptor PAR4 couples to Gq and G12/13 pathways. Activation of the Gi pathway by thrombin in mouse platelets is dependent on secreted ADP interacting with its receptor P2Y12 [23]. Consistent with the previous report, thrombin or AYPGKF repressed approximately 60% of forskolin-stimulated cAMP production in wild type mouse platelets, but not in P2Y12 deficient platelets (Fig. 1H). Thus, thrombin- or AYPGKF-induced Akt phosphorylation in P2Y12 deficient platelets is Gi independent.
AYPGKF induces Akt phosphorylation in recombinant CHO cells expressing human PAR4 cDNA
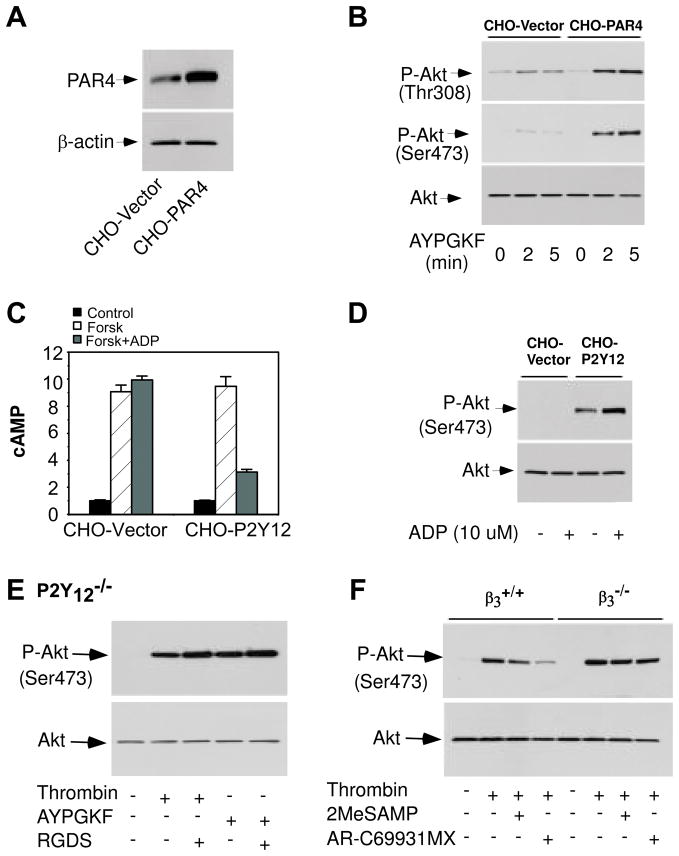
Kim et al. [13] have suggested that chemokines secreted by platelets may activate the Gi and Gz pathways, and thereby induce P2Y12-independent Akt phosphorylation. Therefore, to address this suggestion and the general role of platelet secretion in P2Y12-independent Akt activation, we established a CHO cell line expressing human PAR4 cDNA and investigated whether stimulation of thrombin receptor PAR4 could directly induce Akt phosphorylation (Fig 2A). CHO cells express endogenous PAR4 (Fig. 2A). Consequently, stimulation of CHO cells with the PAR4 peptide AYPGKF induced Akt phosphorylation. Expression of recombinant PAR4 dramatically enhanced AYPGKF-stimulated Akt phosphorylation in comparison with vector-transfected CHO cells (Fig. 2B), demonstrating that stimulation of thrombin receptor PAR4 is able to elicit Akt phosphorylation in the absence of platelet secretion. It is unlikely that PAR4-induced Akt phosphorylation in CHO cells involves the P2Y12 pathway, because CHO cells apparently do not express functional P2Y12. This conclusion is supported by the observation that ADP failed to stimulate Akt phosphorylation and inhibit forskolin-induced cAMP production in CHO cells (Fig. 2C and 2D). As a positive control, ADP stimulated Akt phosphorylation and inhibited forskolin-induced cAMP production in a recombinant CHO cell line expressing human P2Y12 cDNA (Fig. 2C and 2D). Interestingly, Akt was phosphorylated in the CHO cell line expressing human P2Y12 cDNA even without ADP stimulation, suggesting that P2Y12 is partially activated when expressed in CHO cells.
Figure 2. Akt phosphorylation in recombinant CHO cells and the role of integrin outside-in signaling in P2Y12-independent Akt phosphorylation.
(A), CHO cells were transfected with human PAR4 cDNA or pCDNA 3.1/Zeocin (−) (Vector). Expression of PAR4 was shown by Western blotting using an anti-human PAR4 polyclonal antibody. (B), Vector- or PAR4-transfected CHO cells were incubated with buffer or AYPGKF (0.5 mM) at 37 °C for 2 or 5 min. Phosphorylation of Akt was detected by Western blot. (C) CHO cells were transfected with human P2Y12 cDNA or pCDNA 3.1/Zeocin (−) (Vector). CHO cells expressing vector or P2Y12 were incubated with ADP (10 μM) for 5 min. The cells were then incubated with forskolin (10 μM) for 5 min. cAMP concentrations were determined by using a cAMP immunoassay kit. (D), Vector- or P2Y12-transfected CHO cells were incubated with buffer or ADP (10 μM) at 37 °C for 5 min. Phosphorylation of Akt was detected by Western blot. (E), Washed platelets from P2Y12 deficient mice or wild type controls were pre-incubated with RGDS peptide (2 mM) for 5 min, and then stimulated with thrombin (0.25 U/ml) or AYPGKF (0.5 mM) at 37°C in a platelet aggregometer for 5 min. (F), Washed platelets from β3 deficient mice or wild type controls were pre-incubated with 2MeSAMP or AR-C69931MX, and then stimulated with thrombin at 37°C in a platelet aggregometer for 5 min.
Thrombin-induced Akt phosphorylation in P2Y12 deficient platelets is independent of integrin αIIβ3 outside-in signaling
It has been reported that integrin αIIbβ3 outside-in signaling stimulates PKB/Akt phosphorylation [24, 25]. Therefore, we examined the effect of the integrin inhibitor RGDS on thrombin-induced Akt phosphorylation in the P2Y12 deficient platelets to investigate this question. Thrombin-induced Akt phosphorylation was not diminished, rather it was enhanced by RGDS treatment in the P2Y12 deficient platelets (Fig. 2E). Furthermore, thrombin-induced Akt phosphorylation was also enhanced, not inhibited in the integrin β3 deficient platelets, compared to the wild type controls (Fig. 2F). More importantly, in the presence of P2Y12 antagonists, which blocked the P2Y12 pathway, thrombin-induced Akt phosphorylation was higher in β3 deficient platelets than that in wild type platelets. Together, these data demonstrate unequivocally that integrin αIIbβ3 outside-in signaling is not required for, but negatively regulates, thrombin-induced, P2Y12-independent, Akt phosphorylation.
ADP receptor P2Y12-independent Akt phosphorylation requires Gq-induced increases of Ca2+ concentration
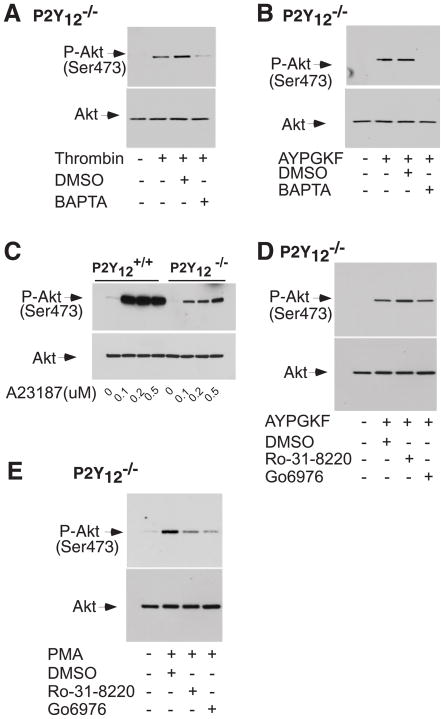
Platelet secretion elicited by thrombin requires Gq signaling [18]. Platelet secretion from dense granules is completely absent in thrombin-stimulated Gq deficient platelets as indicated by ATP release. Akt phosphorylation induced by thrombin is abolished in the Gq deficient platelets (data not shown) [5], suggesting a requirement for Gq signaling in P2Y12-independent Akt phosphorylation induced by thrombin. Two major signaling effects resulting from activation of phospholipase C by Gq signaling are an increase of intracellular Ca2+ concentration and PKC activation. Therefore, we examined the effect of dimethyl-BAPTA on Akt phosphorylation in P2Y12 deficient platelets to investigate the role of intracellular Ca2+ in Gi-independent Akt phosphorylation in response to thrombin stimulation. As shown in Fig. 3, dimethyl-BAPTA markedly inhibited Akt phosphorylation stimulated by thrombin (Fig. 3A) and AYPGKF (Fig. 3B), respectively in the P2Y12 deficient platelets. Thus, an increase of intracellular calcium concentration is required for P2Y12-independent Akt phosphorylation in response to thrombin.
Figure 3. The role of Ca2+ and PKC in P2Y12-independent Akt phosphorylation induced by thrombin or AYPGKF.
(A–B), Washed platelets from P2Y12 deficient mice were pre-incubated with dimethyl-BAPTA (10 μM) or DMSO for 5 min and followed by treated with 0.25 U/ml thrombin (A) or 0.5 mM AYPGKF (B) for 5 min at 37°C in a platelet aggregometer. (C), Washed platelets from wild type or P2Y12 deficient mice were stimulated with increasing concentrations of A23187. (D–E), Washed platelets from P2Y12 deficient mice were pre-incubated with PKC inhibitors Ro-31-8220 (1 μM), Gö6976 (1 μM) for 5 min and followed by treatment with AYPGKF (D) or PMA (100 ng/ml) (E) for 5 min at 37°C in a platelet aggregometer.
The ability of the calcium ionophore, A23187, to induce Akt phosphorylation in P2Y12 deficient platelets was examined to determine whether or not an increase of intracellular calcium concentrations is sufficient to induce Akt phosphorylation. A23187 indeed stimulated Akt phosphorylation in P2Y12 deficient platelets, although Akt phosphorylation elicited by A23187 is weaker in the P2Y12 deficient platelets than in wild type platelets (Fig. 3C).
Next, we evaluated the role of PKC in P2Y12-independent Akt phosphorylation. The PKC inhibitor Ro-31-8220 abolished platelet secretion in P2Y12 deficient platelets induced by AYPGKF (data not shown), but did not significantly affect Akt phosphorylation induced by AYPGKF (Fig. 3D). Also, Akt phosphorylation induced by AYPGKF was not inhibited by Gö6976, a selective inhibitor for conventional PKC. In contrast, Ro-31-8220 and Gö6976, respectively inhibited Akt phosphorylation induced by a PKC activator PMA in the P2Y12 deficient platelets (Fig. 3E), indicating that the PKC inhibitors are effective in inhibiting PKC activity in platelets. These results confirm that Akt phosphorylation induced by thrombin receptors in the P2Y12 deficient platelets is independent of platelet secretion and that PKC is not required for P2Y12-independent Akt phosphorylation stimulated by thrombin receptors.
TXA2-induced Akt phosphorylation is largely dependent on P2Y12 signaling
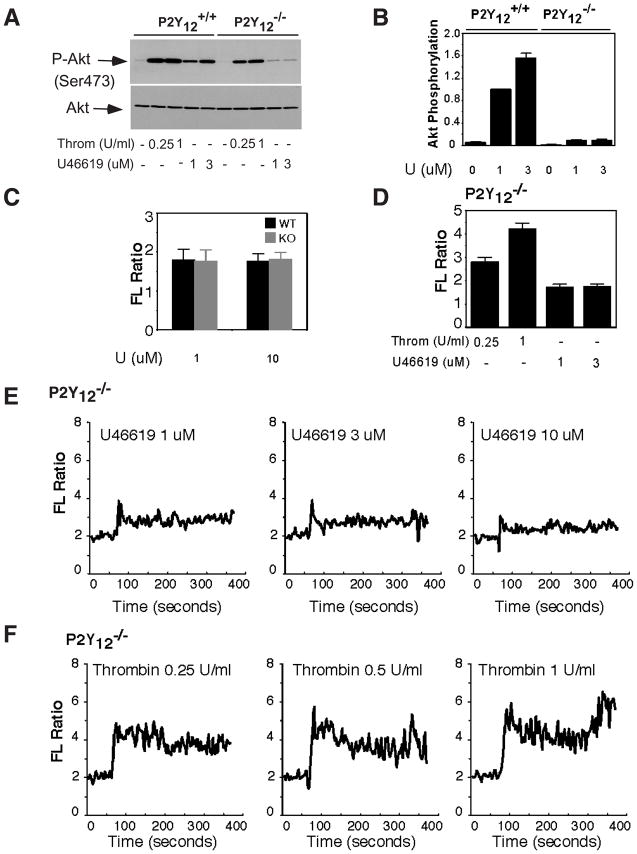
A physiological platelet agonist, TXA2, activates platelets through its G-protein coupled receptor TP. TP couples to both Gq and G12/13. Therefore, we hypothesized that a TXA2 analog, U46619, should be able to induce Akt phosphorylation in P2Y12 deficient platelets. U46619, indeed, induced Akt phosphorylation in the P2Y12 deficient platelets. However, unlike thrombin, U46619-induced Akt phosphorylation is dramatically decreased by P2Y12 deficiency (Fig. 4A–B), suggesting that TXA2-induced Akt phosphorylation is largely P2Y12 dependent. Since we have shown that P2Y12-independent Akt phosphorylation requires Ca2+, we reasoned that U46619 might elicit a low level of Ca2+ mobilization. U46619-induced Ca2+ mobilization was comparable between wild type and P2Y12 deficient platelets (Fig. 4C), indicating that secreted ADP does not significantly contribute to TXA2 elicited Ca2+ mobilization. As expected, U46619-induced Ca2+ levels in P2Y12 deficient platelets were much lower than those elicited by thrombin (Fig. 4D–F). Also, the Ca2+ elicited by U46619 quickly returned to basal level after it reached the peak. In contrast, in thrombin-treated platelets, Ca2+ maintained high levels after it reached the peak. These results demonstrate that compared to thrombin receptor, TXA2 receptor is a weak activator for Gq.
Figure 4. TXA2-induced Akt phosphorylation and calcium mobilization.
(A–B), Washed platelets from P2Y12 deficient mice and littermate wild type controls were incubated with increasing concentrations of thrombin or U46619 at 37°C in a platelet aggregometer for 5 min (A). Immunoblotting results were scanned and quantitated. Values were normalized with respect to wild type platelets stimulated with lowest concentration of U46619 (B) for each immunoblot and are expressed as relative phosphorylation (mean ± SD from 3 separate experiments). (C), Washed platelets from P2Y12 deficient or wild type mice were labeled with 12.5 μM Fura-2/AM/0.2% Pluronic F-127 and resuspended in Tyrode’s solution. Platelets were then stimulated with increasing concentration of U46619. Changes in the intracellular free calcium level were measured every 2 s and expressed as a ratio of fluorescence (FL) detected at 509 nm emission with an excitation wavelength of 340 nm and 380 nm. Statistical data from three experiments are shown. (D–F), Washed platelets from P2Y12 deficient mice were labeled with 12.5 μM Fura-2/AM/0.2% Pluronic F-127. Platelets were then stimulated with increasing concentration of U46619 (E) or thrombin (F). Statistical data from three experiments are shown in (D), and representative data are shown (E and F).
The role of PI3K in P2Y12-independent Akt phosphorylation induced by thrombin receptors
Akt is downstream of PI3K-dependent pathways [4, 5, 12, 13]. Akt phosphorylation induced by either AYPGKF or thrombin in P2Y12 deficient platelets is abolished by the PI3K inhibitors LY294002 and wortmannin, respectively (Fig. 5A and 5B), demonstrating that P2Y12-independent Akt phosphorylation induced by thrombin receptors is PI3K dependent. Consistently, A23187-induced Akt phosphorylation in P2Y12 deficient platelets was also inhibited by LY294002 and wortmannin, respectively (Fig. 5C).
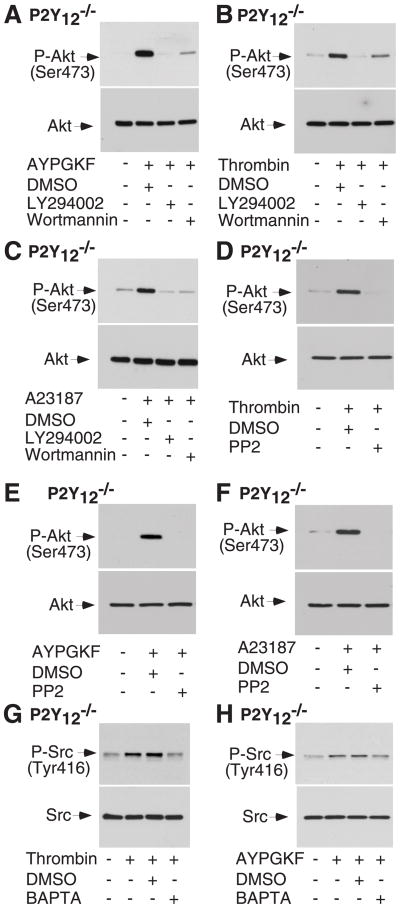
Figure 5. The role of PI3K and Src family kinase in P2Y12-independent Akt phosphorylation in response to thrombin, AYPGKF, or A23187.
(A–C), Washed platelets from P2Y12 deficient mice were pre-incubated with LY294002 (20 μM) or wortmannin (100 nM), stimulated with AYPGKF (0.5 mM) (A), thrombin (0.25 U/ml) (B), or A23187 (0.5 μM) (C). (D–F), Washed platelets from P2Y12 deficient mice were pre-incubated with PP2 (10 μM) or DMSO, stimulated with thrombin (D), AYPGKF (E), or A23187 (F). G and H, Washed platelets from P2Y12 deficient mice were pre-incubated with BAPTA (10 μM) or DMSO, stimulated thrombin (G), or AYPGKF (H). Src phosphorylation was detected by Western blotting with a rabbit monoclonal antibody specifically recognizing the phosphorylated Src residue Tyr416.
The role of Src family kinases in P2Y12-independent Akt phosphorylation induced by thrombin receptors
Src family kinases (SFKs), especially Lyn kinase, have been shown to play a role in regulating Akt phosphorylation induced by thrombin [26]. The effect of the selective SFK inhibitor PP2 on Akt phosphorylation induced by either A23187 or thrombin was examined in P2Y12 deficient platelets to investigate the role of SFKs in P2Y12-independent Akt phosphorylation. Akt phosphorylation stimulated by thrombin or AYPGKF was also inhibited by pre-treatment of platelets with PP2 (Fig. 5D and 5E), demonstrating an important role of SFKs in P2Y12-independent Akt phosphorylation in response to thrombin. PP2 also markedly inhibited Akt phosphorylation induced by A23187 (Fig. 5F).
Activation of SFKs can be monitored by phosphorylation of residue Tyr416. In P2Y12 deficient platelets, thrombin stimulates phosphorylation of Tyr416, which was inhibited by pre-treatment of the platelets with dimethyl-BAPTA (Fig. 5G and 5H), suggesting that thrombin-induced activation of SFKs is downstream from Ca2+ signaling.
Discussion
In this study, we present evidence documenting the function of a P2Y12-independent mechanism for thrombin-stimulated Akt phosphorylation in platelets. The data demonstrate that Gq-dependent calcium mobilization plays a critical role in P2Y12-independent Akt phosphorylation (Fig. 3), and that thrombin-induced, P2Y12-independent, Akt phosphorylation is mediated through the Src/PI3K pathway (Fig. 5).
Almost all platelet agonists stimulate Akt phosphorylation in both human and mouse platelets [4–6, 11–13]. However, the mechanisms for Akt phosphorylation in response to platelet agonists have not been completely elucidated. The role of Gq signaling in Akt phosphorylation induced by thrombin is controversial. Woulfe et al. showed that Akt phosphorylation in response to thrombin stimulation is downstream of Gq-dependent PI3K activation [5]. This conclusion was questioned by some later studies [13, 16]. Kim et al reported that thrombin and AYPGKF respectively failed to stimulate significant Akt phosphorylation in platelets pretreated with the P2Y12 antagonist AR-C69931MX or in the platelets from clopidogrel treated mice [13]. Therefore, it was concluded that Akt phosphorylation induced by thrombin receptors depends on the Gi activation induced primarily by secreted ADP through its receptor P2Y12 [13]. In this model, the absence of Akt phosphorylation in Gq deficient platelets is due to the lack of ADP secretion [13, 16].
Because of the new results that raise questions of the specificity of AR-C69931MX [17], we examined thrombin-stimulated Akt phosphorylation in P2Y12 deficient platelets. Our results remove the ambiguity concerning the mechanism of thrombin-induced Akt phosphorylation resulting from the work of Srinivasan et al. [17]. Data from our studies also confirm the important role of the ADP receptor P2Y12 in thrombin-induced Akt phosphorylation, and demonstrate a P2Y12-independent pathway mediating Akt phosphorylation at high concentrations of thrombin. These findings confirm and extend a previous report that thrombin can induce P2Y12-independent Akt phosphorylation in human platelets [14]. A recent report that GPVI-mediated signaling can also induce P2Y12-independent Akt phosphorylation [15] demonstrates the wide distribution of this means of Akt activation in platelets.
The data presented here support the conclusion that P2Y12-independent Akt phosphorylation in response to thrombin requires Gq but not Gi signaling. This conclusion is drawn from the following data: (1) P2Y12-independent Akt phosphorylation induced by thrombin or AYPGKF is inhibited by dimethyl-BAPTA, demonstrating that Ca2+ mobilization, which is downstream from Gq signaling, is required for the P2Y12-independent Akt phosphorylation; (2) Akt phosphorylation stimulated by thrombin or AYPGKF is absent in the Gq deficient platelets (data not shown) [5], demonstrating that Gq, but not G12/13 [27], is required for the Gi-independent Akt phosphorylation; and (3) AYPGKF-stimulated Akt phosphorylation occurs in CHO cells expressing human PAR4 cDNA in the absence of platelet secretion, suggesting that Gi is not required for PAR4-induced Akt phosphorylation.
Akt is one of the major downstream effectors of phosphoinositide 3-kinase (PI3K). It has been shown that Akt phosphorylation downstream of P2Y12 requires the PI3K signaling [12, 13]. Our data indicate that Gi-independent Akt phosphorylation is also PI3K dependent (Fig. 5A–C). Src family kinases play an important role in the Gi-mediated Akt phosphorylation [16]. Our data demonstrate that Gq/Ca2+-dependent Akt phosphorylation also requires the SFKs. That is evident because (1) SFKs are activated by thrombin in P2Y12 deficient platelets, as evidenced by phosphorylation of SFKs at residue Tyr416 in P2Y12 deficient platelets; (2) Akt phosphorylation induced by thrombin or AYPGKF in the P2Y12 deficient platelets was abolished by PP2; (3) A23187-stimulated Akt phosphorylation in P2Y12 deficient platelets was inhibited by PP2.
Unlike human platelets that express both PAR1 and PAR4 receptors, mouse platelets express PAR3 and PAR4, and PAR3 does not transduce signals. Therefore, PAR4 is the major signaling receptor for thrombin in mouse platelets [28]. While it is controversial whether or not thrombin receptor PAR1 directly couples to Gi, PAR4 is believed to only couple to Gq and G12/13 [25, 29]. Thus, thrombin induced Gi activation in mouse platelets is totally dependent on secreted ADP/P2Y12. In conclusion, we have demonstrated a novel P2Y12-independent signaling pathway mediating Akt phosphorylation in response to thrombin receptors. The finding that there is a Gq/Ca2+-dependent, in addition to the ADP/P2Y12/Gi-dependent pathway mediating Akt activation in response to thrombin will increase our understanding of the role of the PI3K/Akt pathway in platelet activation.
Acknowledgments
This work is supported by American Heart Association Midwest affiliate Grand-in-Aid 0855698G (to Z.L.), and in part by the Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease Grant P20RR021954-01A1 from the National Institutes of Health/National Center for Research Resources. We thank Dr. Qiansheng Ren for his help with Ca2+ mobilization assay, and Drs. Sidney W. Whiteheart and Julie H. Oestreich for critical reading of the manuscript.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 2.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–5. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–29. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 4.Kroner C, Eybrechts K, Akkerman JW. Dual regulation of platelet protein kinase B. J Biol Chem. 2000;275:27790–8. doi: 10.1074/jbc.M000540200. [DOI] [PubMed] [Google Scholar]
- 5.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004;113:441–50. doi: 10.1172/JCI20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104:1703–10. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojanovic A, Marjanovic JA, Brovkovych VM, Peng X, Hay N, Skidgel RA, Du X. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem. 2006;281:16333–9. doi: 10.1074/jbc.M512378200. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Stojanovic A, Hay N, Du X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood. 2008;111:658–65. doi: 10.1182/blood-2007-04-085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D, August S, Woulfe DS. GSK3beta is a negative regulator of platelet function and thrombosis. Blood. 2008;111:3522–30. doi: 10.1182/blood-2007-09-111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 11.Banfic H, Downes CP, Rittenhouse SE. Biphasic activation of PKBalpha/Akt in platelets. Evidence for stimulation both by phosphatidylinositol 3,4-bisphosphate, produced via a novel pathway, and by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:11630–7. doi: 10.1074/jbc.273.19.11630. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Zhang G, Le Breton GC, Gao X, Malik AB, Du X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J Biol Chem. 2003;278:30725–31. doi: 10.1074/jbc.M301838200. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Jin J, Kunapuli SP. Akt activation in platelets depends on Gi signaling pathways. J Biol Chem. 2004;279:4186–95. doi: 10.1074/jbc.M306162200. [DOI] [PubMed] [Google Scholar]
- 14.Resendiz JC, Kroll MH, Lassila R. Protease-activated receptor-induced Akt activation--regulation and possible function. J Thromb Haemost. 2007;5:2484–93. doi: 10.1111/j.1538-7836.2007.02769.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Mangin P, Dangelmaier C, Lillian R, Jackson SP, Daniel JL, Kunapuli SP. The role of PI 3-K{beta} in glycoprotein VI-mediated akt activation in platelets. J Biol Chem. 2009;284:33763–72. doi: 10.1074/jbc.M109.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Jin J, Kunapuli SP. Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood. 2006;107:947–54. doi: 10.1182/blood-2005-07-3040. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan S, Mir F, Huang JS, Khasawneh FT, Lam SC, Le Breton GC. The P2Y12 antagonists, 2-methylthioadenosine 5′-monophosphate triethylammonium salt and cangrelor (ARC69931MX), can inhibit human platelet aggregation through a Gi-independent increase in cAMP levels. J Biol Chem. 2009;284:16108–17. doi: 10.1074/jbc.M809780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389:183–6. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 19.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–8. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Zhang G, Marjanovic JA, Ruan C, Du X. A platelet secretion pathway mediated by cGMP-dependent protein kinase. J Biol Chem. 2004;279:42469–75. doi: 10.1074/jbc.M401532200. [DOI] [PubMed] [Google Scholar]
- 22.Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang CC, Hong W, Whiteheart SW. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Foster C, Lecchi A, Quinton TM, Prosser DM, Jin J, Cattaneo M, Kunapuli SP. Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling. Blood. 2002;99:3629–36. doi: 10.1182/blood.v99.10.3629. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Banfic H, Straforini F, Tosi L, Volinia S, Rittenhouse SE. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source of phosphatidylinositol 3-phosphate. J Biol Chem. 1998;273:14081–4. doi: 10.1074/jbc.273.23.14081. [DOI] [PubMed] [Google Scholar]
- 25.Banfic H, Tang X, Batty IH, Downes CP, Chen C, Rittenhouse SE. A novel integrin-activated pathway forms PKB/Akt-stimulatory phosphatidylinositol 3,4-bisphosphate via phosphatidylinositol 3-phosphate in platelets. J Biol Chem. 1998;273:13–6. doi: 10.1074/jbc.273.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gamma-thrombin. Blood. 2002;99:2442–7. doi: 10.1182/blood.v99.7.2442. [DOI] [PubMed] [Google Scholar]
- 27.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–54. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–8. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 29.Faruqi TR, Weiss EJ, Shapiro MJ, Huang W, Coughlin SR. Structure-function analysis of protease-activated receptor 4 tethered ligand peptides. Determinants of specificity and utility in assays of receptor function. J Biol Chem. 2000;275:19728–34. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]