Abstract
Previous research demonstrates that men and women differ in the way that they perceive and process pain. Much of this work has been done in healthy adults with a lack of consensus in clinical pain populations. The purpose of this study was to investigate how men and women with shoulder pain differ in their experience of experimental and clinical pain and whether psychological processes differentially affect these responses. Fifty nine consecutive subjects (24 women, 35 men) seeking operative treatment for shoulder pain were enrolled in this study. Subjects completed self report questionnaires to assess clinical pain, catastrophizing, anxiety and depression and underwent a series of experimental pain tests consisting of pressure pain, thermal pain (threshold and tolerance), and thermal temporal summation. Results indicated that women experienced greater clinical pain and enhanced sensitivity to pressure pain. Age did not affect the observed sex differences. There were no sex differences in psychological association with experimental and clinical pain in this cohort. The relationship between clinical and experimental pressure pain was stronger in women as compared to men. These findings offer insight into the interactions between biological and psychosocial influences of pain and how these interactions vary by sex.
Keywords: Sex differences, pain sensitivity, quantitative sensory testing (QST), chronic pain, biopsychosocial
Introduction
A burgeoning body of literature demonstrates that men and women differ in the way that they perceive and process pain (Fillingim et al., 2009; Greenspan et al., 2007). Most studies document that women display greater sensitivity to experimental pain (Fillingim et al., 2009; Riley et al., 1998). Much of this work has been done in healthy adults using experimentally induced pain with fewer studies investigating sex differences in clinical populations. Studies that have explored sex differences in clinical pain have led to variable results including no sex related differences in clinical pain severity (Robinson et al., 2005), enhanced experimental pain sensitivity in women (George et al., 2007) and increased low back pain intensity in men (George et al., 2006). We propose that examining associations between experimental pain, clinical pain and psychological processes will help us better understand the biological and psychosocial interactions potentially leading to these incongruent findings.
Research has begun to illuminate the disparate effects of certain psychological variables on the pain experience in men and women. Several reports suggest that anxiety more strongly influences pain response in men as compared to women (Edwards et al., 2000; Fillingim et al., 2009; Robinson et al., 2005). Alternatively, several studies have demonstrated that pain related catastrophizing is more prevalent in women and mediates sex differences in reports of day to day pain (Edwards et al., 2004), pain related outcomes in osteoarthritis (Keefe et al., 2000) and experimental pain (Sullivan et al., 2000). These studies reiterate the importance of investigating how psychological processes potentially influence pain perception differentially for men and women.
The present investigation evaluated experimental and clinical pain responses in men and women with shoulder pain. To best capture pain processing differences between sexes, we utilized multiple modalities. Pressure pain thresholds have previously demonstrated the most robust differences between sexes as compared to other experimental pain modalities (Riley et al., 1998). Thermal pain tolerance was used because suprathreshold measures are believed to be more clinically relevant than threshold measures (Edwards et al., 2005). Finally, we chose to include temporal summation because this dynamic psychophysical measure is thought to better capture the pain modulatory ability of the central nervous system as compared to static measures such as threshold and tolerance that only measure a single point in the pain processing continuum (Arendt-Nielsen and Yarnitsky, 2009).
Relatively few studies have examined sex differences in both experimental and clinical pain and those that have yield little consensus (Edwards et al., 2005; Fillingim et al., 1999; Kim et al., 2004). We hypothesized that women would display enhanced experimental pain sensitivity, which would be associated with greater clinical pain. The study also sought to further investigate the potentially disparate impact of catastrophizing and anxiety on experimental and clinical pain across sexes. We hypothesized that catastrophizing would more strongly influence experimental and clinical pain ratings in women whereas anxiety would more significantly predict pain in men. Finally as an exploratory aim, we examined the relationship of experimental and clinical pain sensitivity between the sexes.
Methods
Subjects
Consecutive patients presenting to the University of Florida’s Orthopedics Sports Medicine Institute (OSMI) for surgical treatment of rotator cuff pathology were considered for this study. Patients meeting the following inclusion criteria were considered eligible for participation: (a) between 18 and 85 years of age, (b) complaints of pain limited to anterior, lateral, or posterior shoulder, (c) documented or suspected rotator cuff tendinopathy (evidence from clinical examination or imaging studies) including small (<1 cm), medium (1 to 3 cm), and large (3 to 5 cm) tears, (d) documented or suspected adhesive capsulitis (evidence from clinical examination or imaging studies), (e) documented or suspected labral lesion (evidence from clinical examination or imaging studies), and (f) scheduled for arthroscopic procedure.
Patients were deemed inappropriate for the study if meeting any of the following exclusion criteria: (a) current complaints of neck, elbow, hand, low back, hip, knee, or ankle pain lasting greater than the past three months, (b) massive or complete rotator cuff tear (defined as tear >5 cm), (c) documented shoulder osteoarthritis or rheumatoid arthritis, (d) prior shoulder surgery within the past year or current complaints of pain from prior shoulder surgery, (e) current shoulder fracture, tumor, or infection, (f) previously diagnosed chronic pain disorder (including but not limited to irritable bowel syndrome, fibromyalgia, tempromandibular joint disorder, and chronic low back pain), (g) current medical management for psychiatric disorder (defined as taking two or more psychiatric medications), and (h) current gastrointestinal or renal illness. Subjects deemed eligible and willing to participate provided informed consent according to guidelines set forth by the University of Florida’s Institutional Review Board for Human Subjects.
Measures
Demographics
Information regarding sex, age, self reported race, medication status, work status, marital status, and involved upper extremity was collected from each subject.
Self report questionnaires
Pain Catastrophizing Scale (PCS)
The PCS (Sullivan et al., 1995) was used to measure pain catastrophizing in the study subjects. This tool is a 13 item scale that assesses catastrophic cognitive (“I keep thinking about how much it hurts”) and affective (“I worry all the time about when the pain will end”) responses to pain. The participant is asked to recall the extent in which they have had specific catastrophic thoughts and feelings in response to a past occurrence of pain. Ratings of one (not at all) to four (always) are summed for a score range of 0 to 52 with higher scores indicating greater frequency of cognitions related to helplessness, magnification, and/or rumination. A recent study established the psychometric soundness of the PCS for use in both men and women (D’Eon et al., 2004).
State-Trait Anxiety Index (STAI)
The STAI, used to evaluate anxiety in the study subjects, consists of two 20-item scales; one which measures situational (state) anxiety and the other which assesses more general, long standing (trait) anxiety. Items on each subscale are rated on a four point likert scale, yielding a sum scare in which higher scores reflect greater levels of anxiety. This tool has been well validated (Spielberger et al., 1983) and widely used in research and clinical settings. For purposes of this investigation only the trait subscale was used as it captures the relatively stable individual differences in anxiety.
Beck Depression Inventory (BDI)
Depression levels were assessed with the BDI which is a commonly used, 21-item measure that demonstrates excellent reliability and validity (Beck et al., 1988). This self report measure asks subjects to rate the severity with which they experience symptoms or attitudes reflective of cognitive, affective, or vegetative symptoms of depression.
Brief Pain Inventory (BPI)
Clinical shoulder pain was assessed using the BPI, a tool that has broad use in various research and clinical arenas. The BPI (Cleeland and Ryan 1994) measures pain intensity on an eleven point numerical rating scale (0-10) in three conditions; worst pain in the past week, least pain in the past week, and current pain. The BPI has been deemed valid and reliable in multiple populations; recently in patients experiencing non-malignant pain (Tan et al., 2004). The BPI question asking for the subject’s current pain (“pain right now”) was used for analyses involving clinical pain severity. This aspect of the subject’s clinical pain was chosen as this rating corresponded to the timing of the experimental pain procedures and completion of psychological questionnaires.
Experimental Pain Sensitivity Procedures
Thermal pain threshold and tolerance
To determine participants’ pain threshold and tolerance in response to heat pain, thermal stimuli were delivered to the involved and uninvolved volar forearms. Thermal stimuli were delivered using a contact thermode and a computer controlled Medoc Neurosensory Analyzer (TSA-2001; Ramat Yishai, Israel) with a peltier element stimulator. Temperature of the thermode was increased at a rate of 0.5°C per second until the patient reported their first sensation of pain (threshold). Tolerance was determined by having subjects say “stop” when the pain from the thermode became intolerable. Between trials, the thermode was alternated between arms and shifted to avoid sensitization or habituation. Temperature of the thermode at the time subjects reported threshold and tolerance was recorded. Two trials each of threshold and tolerance were repeated and the average of these trials was used for analysis.
Thermal temporal summation
Temporal summation (TS) was measured using a contact thermode which delivered three series of five heat pulses of less than one second duration to the thenar eminence of both hands. An interstimulus interval of .33 Hz was used to ensure the development of TS. Each heat pulse rapidly fluctuated from a baseline temperature of 35°C to a maximum temperature of 47°C and 49°C followed by a return to the 35°C baseline temperature at a rate of 10°C per second. Subjects were cued to rate their delayed (second) pain intensity associated with each heat pulse using a verbal 0 (no pain) to 100 (worst pain intensity imaginable) numerical rating scale. These ratings are believed to measure the progressive increase in magnitude of C-fiber input as a result of repeated neural firing (Arendt-Nielsen and Yarnitsky, 2009). TS was quantified by subtracting the first pain rating from the last pain rating as this reflected the slope or the amount of summation obtained as a result of repeated C fiber stimulation.
Pressure pain threshold
A Fischer pressure algometer (Pain Diagnostics and Thermography Inc, Great Neck, NY) was used to assess pressure pain thresholds bilaterally at the acromion processes (PPacromion) and masseter muscle (PPmasseter). Mechanical pressure was applied at a rate of 1kg per second until the subject reported the first sensation of pain from the pressure at which point the amount of pressure being applied was recorded. This process was repeated four times bilaterally at each site and the average of the four assessments was used for subsequent analyses.
Procedures
All study procedures were approved by the University of Florida Institutional Review Board. Participants completed the 90 minute study session three to five days prior to their scheduled shoulder surgery. Following initial screening and signing of the informed consent, subjects completed the demographic and self report questionnaires. Next, participants began the experimental pain testing protocol which consisted of thermal pain threshold and tolerance, pressure pain threshold, and thermal temporal summation. Following the completion of experimental pain testing, subjects were reimbursed for their time and thanked for their participation.
Data Analysis
Data analysis was performed using SPSS, Version 17.0. Prior to analysis, the distribution of variables was tested by visual examination and with the Kolmogorov-Smirnov test. Paired t-tests showed no differences in pain sensitivity between the right and left side of the body (p>.05); therefore measurements from both sides were averaged into one score for the remainder of analyses. Descriptive statistics (means, standard deviations) were calculated for all variables.
Independent t-tests were used to determine whether there were differences in clinical pain, experimental pain, or psychological measures between sexes. Sex differences in TS were investigated using mixed model analysis of variance (ANOVA) with sex as the between subject factor and temporal summation pulse (first, third, and fifth pulse) as the within subject factor. The relationships between clinical pain, experimental pain sensitivity and psychological measures were assessed using Pearson correlation, separated by sex. For analyses of correlation with temporal summation, the slope of the pulses was used to obtain a single value of summation. The slope was calculated by subtracting the pain level of the fifth pulse from the pain level of the first pulse. To detect significant differences in Pearson coefficients between sexes, correlations were converted to Fisher Z scores. The difference between the male and female Fisher Z scores was compared to determine whether the difference between the two was significant.
To account for multiple comparisons, we adopted an alpha level of .01 to control for type I error inflation. A Bonferroni correction would have called for a more stringent alpha correction that would have risked the dismissal of certain relationships and variables as potentially relevant for future research considerations. Adopting an alpha level of .01 balanced the need to adjust for numerous analyses with our desire to capture potentially important relationships that might inform future investigations. We did not perform an a priori power calculation for sex differences given that this was a secondary analysis (George et al., 2008). With the sample size recruited for the primary study, analyses were powered to detect moderate and large sex differences, but not small sex differences.
Results
Sample characteristics
A total of 59 participants were included the study. Descriptive statistics for the demographic, clinical and psychological measures from the sample are summarized in Table I. In a preliminary analysis female participants were significantly older than male participants [t(56)=2.13; p=.038] so we evaluated the effects of age on our hypotheses. Using regression to control for age while examining the influence of sex on clinical pain, experimental pain and psychological measures, we found that age had minimal influence on these variables (p’s >.05). To examine the influence of age on sex differences in temporal summation, we included age as a covariate in the mixed model ANOVA. Again, age had minimal influence on temporal summation at 47°C and 49°C (p’s >.05). Overall we concluded that the age differences between the sexes had minimal influence and therefore present our results unadjusted for age.
Table I.
Descriptive data (n=59)
| Variable | Mean or Value | % or SD | |
|---|---|---|---|
| Sex | |||
| Male | 35 | 59.3% | |
| Female | 24 | 40.7% | |
| Age (years) | |||
| Male | 47.0 | 14.6 | |
| Female | 55.2 | 14.4 | |
| No medication | 29 | 49.2% | |
| Pain medication | 27 | 48.2% | |
| Involved arm | |||
| Right | 21 | 35.6% | |
| Left | 38 | 64.4% | |
| Clinical pain (0-10) | 3.9 | 2.5 | |
| STAI | 36.6 | 11.8 | |
| PCS | 13.4 | 9.6 | |
| BDI | 7.1 | 5.6 | |
| Pain tolerance (°C) | 48.0 | 2.2 | |
| Pain threshold (°C) | 44.3 | 2.9 | |
| TS (slope 47°C) | −1.5 | 21.0 | |
| TS (slope 49°C) | 1.8 | 12.1 | |
| PPacromion (kg) | 3.4 | 1.7 | |
| PPmasseter (kg) | 1.7 | 0.7 |
Differences in clinical pain, experimental pain, and psychological measures between sexes
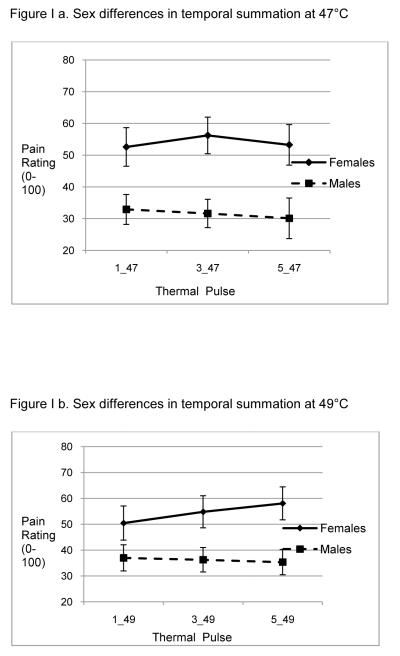
Sex differences in clinical pain, experimental pain sensitivity and psychological measures are presented in Table II. Women experienced greater clinical pain (p = 0.005), had lower thermal pain tolerance (p<.001) and lower pressure pain thresholds for PPacromion (p <.001) and PPmasseter (p = .001). Sex differences in TS varied by stimulus temperature (Figure 1). The interaction term between sex and TS (Figure 1a) was non-significant at 47°C [F(2,44) = 0.56; p= 0.57]. There was a significant main effect for sex [F(1,46) = 9.78; p= 0.003], such that women had a higher overall pain ratings for each pulse. There was no significant main effect for TS pulse [F(2,44) = 0.45; p= 0.64]. The interaction term between sex and TS (Figure 1b) was significant at 49°C [F(2,44) = 5.12, p= 0.01]. Contrasts revealed that females had higher pain ratings for the third [F(1,44) = 5.66, p= 0.02] and fifth pulse [F(1,44) = 7.99, p= 0.01], but not the first pulse (p > .05). Men and women did not differ on measures of catastrophizing (p=0.388), trait anxiety (p=0.636), or depression (p=0.434).
Table II.
Sex differences in clinical pain, experimental pain and psychological factors
| Measure | Female | Male | Effect Size | T statistic (df) | P value |
|---|---|---|---|---|---|
| Clinical Pain | 5.00 (2.78) | 3.l7 (2.00) | 0.77 | 2.94 (57) | 0.005 |
|
| |||||
| Experimental pain modalities | |||||
|
| |||||
| Threshold (°C) | 43.58 (3.62) | 44.89 (2.24) | 0.45 | −l.67 (55) | 0.l00 |
| Tolerance (°C) | 46.68 (2.59) | 49.0l (l.20) | l.23 | −4.54 (55) | <0.001 |
| PPacromion (kg) | 2.35 (l.l3) | 4.05 (l.67) | l.2l | −4.35 (57) | <0.001 |
| PPmasseter (kg) | l.33 (0.62) | l.9l (0.64) | 0.92 | −3.38 (56) | 0.001 |
|
| |||||
| Psychological measures | |||||
|
| |||||
| PCS | 14.75 (l0.98) | l2.54 (8.49) | 0.23 | .87 (57) | 0.388 |
| STAI | 35.67 (11.05) | 37.l7 (12.47) | 0.l3 | −.48 (57) | 0.636 |
| BDI | 7.83 (4.86) | 6.65 (6.l3) | 0.2l | .79 (56) | 0.434 |
Statistically significant values (p<.0l) are bolded.
Clinical Pain measured by the Brief Pain Inventory using a numerical rating scale (0-l0).
PP: Pressure pain (measured in Kg); PCS: Pain Catastrophizing Scale; STAI: State Trait Anxiety Inventory; BDI: Beck Depression Inventory; df: degrees of freedom
Figure 1.
In a series of five heat pulses delivered to the thenar eminence at 47°C (a) women display significantly greater pain ratings to all heat pulses but showed no difference in degree of temporal summation. For heat pulses delivered at 49°C (b) women demonstrated significantly greater temporal summation to the third and fifth heat pulses as compared to men.
Correlations among experimental, clinical pain and psychological measures
None of the psychological variables were significantly correlated with the clinical or experimental pain measures, nor did these relationships significantly differ as a function of sex (Table III). Pain catastrophizing was negatively associated with PPmasseter in women (indicating that increased levels of catastrophizing were associated with decreased pressure pain thresholds, or enhanced sensitivity to pressure pain) whereas pain catastrophizing was positively correlated with PPmasseter in men (increased levels of catastrophizing were associated with increased pressure pain thresholds), although this sex difference in association (p=0.015) did not reach statistical significance set at p<.01 for the Bonferroni correction. Table IV summarizes sex differences in the Pearson correlations between clinical and experimental pain. Sex differences were found only in the relationship between PPmasseter and clinical pain (p=.005) revealing that pressure pain in the masseter is more strongly associated with clinical pain in women as compared to men.
Table III.
Pearson correlations among experimental and clinical pain and psychological variables
| C.Pain | TS (slope 47°C) |
TS (slope 49°C) |
Threshold | Tolerance | PPacromion | PPmasseter | |
|---|---|---|---|---|---|---|---|
| PCS Female | 0.511 | 0.095 | 0.146 | −0.031 | −0.014 | −0.231 | −0.371 |
| Male | 0.390 | −0.157 | −0.148 | −0.055 | −0.138 | −0.217 | 0.220 |
| Sex differences | p = 0.295 | p = 0.216 | p = 0.186 | p = 0.380 | p = 0.326 | p= 0.485 | p= 0.015 |
|
| |||||||
| STAI Female | 0.307 | −0.231 | 0.228 | −0.214 | −0.138 | −0.315 | −0.112 |
| Male | 0.208 | −0.045 | −0.235 | −0.071 | −0.104 | −0.245 | −0.013 |
| Sex differences | p= 0.351 | p = 0.193 | p = .077 | p = 0.302 | p = 0.442 | p = 0.393 | p = 0.360 |
|
| |||||||
| BDI Female | 0.203 | −0.246 | 0.309 | −0.368 | −0.200 | −0.407 | −0.257 |
| Male | 0.343 | 0.192 | −0.004 | 0.022 | −0.152 | −0.247 | −0.144 |
| Sex differences | p = 0.300 | p = 0.435 | p = 0.164 | p = 0.072 | p = 0.428 | p = 0.260 | p = 0.332 |
None of the correlations were significant at the .01 level.
C. Pain: Clinical pain; TS: Temporal summation; PP: Pressure pain; PCS: Pain Catastrophizing Scale; STAI: State Trait Anxiety Inventory (trait anxiety scale score shown); BDI: Beck Depression Inventory
Table IV.
Pearson correlations among clinical and experimental pain
| TS (slope at 47°C) |
TS (slope at 49°C) |
Threshold (°C) |
Tolerance (°C) |
PPacromion (kg) |
PPmasseter (kg) |
|
|---|---|---|---|---|---|---|
| C.Pain Female | 0.313 | 0.163 | −0.326 | −0.288 | −0.461 | −0.504 |
| Male | 0.073 | 0.055 | −0.153 | −0.159 | −0.064 | 0.168 |
| Sex differences | p = 0.221 | p = 0.371 | p = 0.251 | p = 0.312 | p = 0.059 | p = 0.005 |
Statistically significant values (p<.01) are bolded.
C. Pain: Clinical pain; TS: Temporal summation; PP: Pressure pain
Clinical Pain measured by the Brief Pain Inventory using a numerical rating scale (0-10).
Discussion
The purpose of this study was to investigate how the sexes differ in their experience of experimental and clinical pain and whether psychological processes differentially affect these responses. This study adds to the literature by examining these factors in a clinical population and investigating differences in association between experimental pain, clinical pain, and psychological processes. We found that, as hypothesized, females with shoulder pain displayed enhanced sensitivity to experimental pain and reported higher levels of clinical pain intensity. Associations between psychological processes, clinical shoulder pain and experimental pain ratings did not vary by sex. Finally, this study found that women with shoulder pain demonstrated a more robust relationship between pressure pain sensitivity and clinical pain ratings.
This study extends findings from healthy populations to demonstrate that females with shoulder pain display greater sensitivity to experimental pain modalities. A finding of particular interest was that women had a greater rate of TS at 49°C. Sex differences for TS have been demonstrated in healthy individuals (Fillingim et al., 1998; Maixner et al., 1998; Robinson et al., 2004; Sarlani et al., 2004), patients with chronic low back pain (George et al., 2007) and patients with tempromandibular disorders (Sarlani et al., 2007). This study replicates these findings in a novel population; patients with shoulder pain. The fact that a dynamic psychophysical modality with a strong C-fiber component elicits robust sex differences in healthy and clinical populations adds evidence to a sex mediated differences in processing of C-fiber mediated pain (Fillingim et al., 1998). Enhanced summation in response to repetitive C fiber input is one mechanism impacting the development of central sensitization and might be one factor, among many, leading to an over-representation of women in chronic pain disorders.
Findings from studies investigating clinical pain severity in musculoskeletal pain conditions have ranged from women experiencing more intense pain (Bingefors and Isacson, 2004; George et al., 2007), to an equal pain experience between sexes (Robinson et al., 2005), to men experiencing more severe pain (George et al., 2006), to women and men differing on some but not all aspects of the pain experience (Rustoen et al., 2004). The current study found that women reported greater pain intensity produced by their shoulder condition. This finding could be explained, in part, by the pain processing differences revealed using experimental pain modalities. In addition to increased rates of temporal summation, women experienced enhanced pressure pain which is proposed to measure the sensitivity of deep tissue afferents (Staahl et al., 2009), a relevant mechanism likely producing pain in this specific population. Along with potential biological differences in pain processing, a number of other explanations have been proposed to account for sex differences in pain severity including sex differences in musculature (Rollman and Lautenbacher, 2001), hormonal influences on pain perception (Fillingim and Ness, 2000) and psychological influences (Edwards et al., 2004). It is most likely that a complex combination of biological, social, and psychological variables interacts to produce disparate clinical pain severities between men and women. For this reason the current study investigated whether psychological processes might contribute to these sex differences in clinical pain.
Associations between psychological processes, clinical shoulder pain and experimental pain sensitivity did not vary as a function of sex in this investigation. Catastrophizing, a psychological construct of interest in the pain literature, has been widely studied for sex differences. Sex mediated differences in the association between catastrophizing and experimental pain responses have shown some variability (Edwards et al., 2004) with one study showing an association among women but not men (Fillingim et al., 2005) and others showing a similar association between sexes (Goodin et al., 2009; Sullivan et al., 2000). The present investigation differs from the aforementioned studies by using a sub-acute clinical pain model in contrast to using experimental pain or studying patients with chronic pain. This population represents a unique group of patients with a discrete musculoskeletal condition; psychological relationships generalized from a chronic pain population might not extend to this population. A lack of sex mediated differences in these relationships could indicate a similar psychological response to sub-acute, single-site pain in men and women. Alternatively, our relatively strict exclusion criteria for psychiatric conditions may have contributed to this lack of sex differences by restricting our sample to those with minimal psychological distress. Indeed, mean levels of psychological functioning in both sexes (Table II) fell well below previously established cutoffs for the presence of catastrophizing (Sullivan et al., 1995), depression (Geisser et al., 1997), and anxiety (Spielberger, et al., 1977), indicating minimal psychological involvement in these participants. That both sexes exhibited particularly healthy levels of psychological functioning could contribute to our lack of sex differences in these psychological processes despite previous evidence of differences between men and women (Tsang, et al., 2008).
While the investigation of sex differences in experimental pain sensitivity enhances our understanding of nociceptive processing, there remains some debate as to how this translates into the experience of clinical pain (Edwards et al., 2005; Kim et al., 2004). Even less is known about how the relationship between experimental and clinical pain might vary by sex. In the current study, pressure pain demonstrated the strongest sex difference in the association between clinical and experimental pain, with stronger associations emerging among women. Rollman and Lautenbacher (2001) suggest that sex differences in pain sensitivity are most robust for pressure pain, an experimental pain modality that is especially sensitive to underlying pathology in musculoskeletal pain. Two other studies have reported that experimental pain responses were related to clinical pain in healthy females but not males (Fillingim et al., 1999) and that ischemic pain tolerance was related to treatment outcomes in women with chronic pain but not men (Edwards et al., 2003). These studies suggest that experimental pain responses could be more clinically relevant in women as compared to men, although the current study only found this to be true for pressure pain. Edwards (2005) proposes that the type of noxious stimuli used might shape the relationship between experimental pain and clinical pain response. In this sample of individuals with sub-acute shoulder pain, pressure pain may have been best suited to elicit clinically relevant sex differences in musculoskeletal pain processing.
Several limitations of the current study deserve mention. Our sample size allowed adequate power to detect moderate to large effect sizes, which, in our opinion, are the sized effects likely to have the most clinical relevance. We did not have adequate power to detect small effect sizes. The cross-sectional design of this investigation precludes determination of a temporal or causal relationship. Future prospective studies will better determine whether psychological processes and/or abnormal pain processing precede the development of chronic pain conditions. Also, hormonal status of females was not measured. Previous research demonstrates that menstrual cycle phase, menopausal status, and use of exogenous hormones may affect clinical and experimental pain responses (Fillingim et al., 2009), although the relative impact of these variables remains under debate (Sherman and LeResche, 2006). Finally, the current study included a homogenous group of subjects with shoulder pain prior to a surgical procedure and excluded individuals with an existing chronic pain condition. Specific characteristics of this group preclude generalizability to other populations with pain.
Despite these limitations, the current study potentially adds to the literature by examining sex differences in associations between experimental pain, clinical pain and psychological processes in patients with shoulder pain. There were no sex differences in psychological association with experimental and clinical pain in this cohort. This investigation reported that women experienced greater clinical pain and enhanced sensitivity to experimental pain. Furthermore, the relationship between pressure pain sensitivity and clinical pain was stronger in women as compared to men. These findings offer insight into the interactions between biological and psychosocial influences between the sexes and how this might relate to the experience of ongoing pain.
Acknowledgements
Data collection was funded by the University of Florida, Research Opportunity Incentive Fund, #56577 (SZG as PI) and by NS41670 (RBF). LLK was supported by NINDS training grant NS045551 to the University of Florida Comprehensive Center for Pain Research. SZG (PI), CV, and RBF were supported by Grant #AR055899 from NIAMS/NIH while preparing this manuscript. Warren H. Greenfield III, Michael Moser and Thomas Wright assisted with recruitment and testing protocols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain--A gender perspective. Eur J Pain. 2004;8:435–450. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- D’Eon JL, Harris CA, Ellis JA. Testing factorial validity and gender invariance of the pain catastrophizing scale. J Behav Med. 2004;27:361–372. doi: 10.1023/b:jobm.0000042410.34535.64. [DOI] [PubMed] [Google Scholar]
- Edwards R, Augustson EM, Fillingim R. Sex-specific effects of pain-related anxiety on adjustment to chronic pain. Clin J Pain. 2000;16:46–53. doi: 10.1097/00002508-200003000-00008. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Doleys DM, Lowery D, Fillingim RB. Pain tolerance as a predictor of outcome following multidisciplinary treatment for chronic pain: Differential effects as a function of sex. Pain. 2003;106:419–426. doi: 10.1016/j.pain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: Differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111:335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: Multiple domains of clinical relevance. Pain. 2005;114:315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: A comparative analysis. Clin J Pain. 13:163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- George SZ, Fritz JM, Childs JD, Brennan GP. Sex differences in predictors of outcome in selected physical therapy interventions for acute low back pain. J Orthop Sports Phys Ther. 2006;36:354–363. doi: 10.2519/jospt.2006.2270. [DOI] [PubMed] [Google Scholar]
- George SZ, Wallance MR, Wright TW, Moser MW, Greenfield WH, Sack BK, Herbstrom DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain. 2007;8:2–10. doi: 10.1016/j.jpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR. Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. J Pain. 2009;10:180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007;132:S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Zachariae R, Arendt-Nielsen L. Dispositional anxiety and the experience of pain: Gender-specific effects. Eur J Pain. 2003;7:387–395. doi: 10.1016/S1090-3801(02)00139-8. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- Kim H, Neubert JK, Rowan JS, Brahim JS, Iadarola MJ, Dionne RA. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5:377–384. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Dannecker EA, George SZ, Otis J, Atchison JW, Fillingim RB. Sex differences in the associations among psychological factors and pain report: A novel psychophysical study of patients with chronic low back pain. J Pain. 2005;6:463–470. doi: 10.1016/j.jpain.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. Journal of Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Rustoen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Gender differences in chronic pain: Findings from a population-based study of Norwegian adults. Pain Manag Nurs. 2004;5:105–117. doi: 10.1016/j.pmn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibuluar disorders. J Orofac Pain. 2007;21:309–317. [PMC free article] [PubMed] [Google Scholar]
- Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol. 2006;291:R245–256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state and trait anxiety inventory (form Y) Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Spielberger C, Gorsuch RL, Vagg PR, Jacobs GR. The state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto: [Google Scholar]
- Staahl C, Olesen AS, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers - An updated review. Br J Clin Pharmacol. 2009;68:149–168. doi: 10.1111/j.1365-2125.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (windup) J Pain. 2006;7:575–582. doi: 10.1016/j.jpain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- Sullivan MJL, Tripp DA, Santor D. Gender differences in pain and pain behavior: The role of catastrophizing. Cognit Ther Res. 2000;24:121–134. [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tsang A, VonKorff M, Lee S, Alonso J, Karam E, Anagermeyer MC, Guimaraes Borges GL, Bromet EJ, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Brown MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]