1. Structure
Transglutaminase 2, also known as TGM2, tTG, TG2, TGC, and G-alpha-h (Accession numbers: Nucleotide NM_004613.2, Protein NP_004604.2; EC 2.3.2.13) has two isoforms due to alternative splicing: (a) has 13 exons encoding 687 amino acids (77kDa) and (b) has 10 exons encoding 548 amino acids (61.7kDA). The human transglutaminase 2 gene (TGM2) is located on chromosome 20q12. Transglutaminase 2 is secreted by an unknown non-conventional mechanism, since it lacks a signal sequence. Although potential glycosylation sites are present, no evidence of glycosylation has been reported. X-ray crystallography has revealed that TGM2 has four domains: an N-terminal β-sandwich domain (1-139), a transamidation core domain (140-454), and two C-terminal β-barrels (479-585 and 586-687, respectively) (Figure1). All four domains have particular roles. For example, the N-terminal domain interacts with fibronectin (Collighan RJ and Griffin M, 2009). The transamidation core domain is involved in GTP binding (Ser171 and Lys173), has an active triad site (Cys277, His335, and Asp358), and a calcium binding region. The C-terminal domain regulates transamidation activity, GTPase activity, as well as concealing the active site from contact with possible substrates. Upon calcium binding, the interaction between the active site and C-terminal domains is released, allowing transamidation enzyme activity.
Figure1.
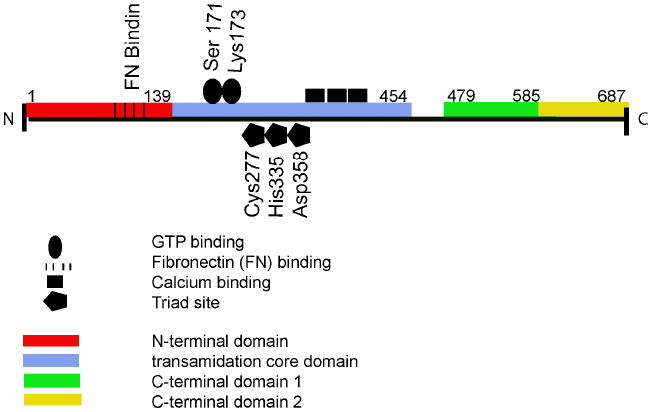
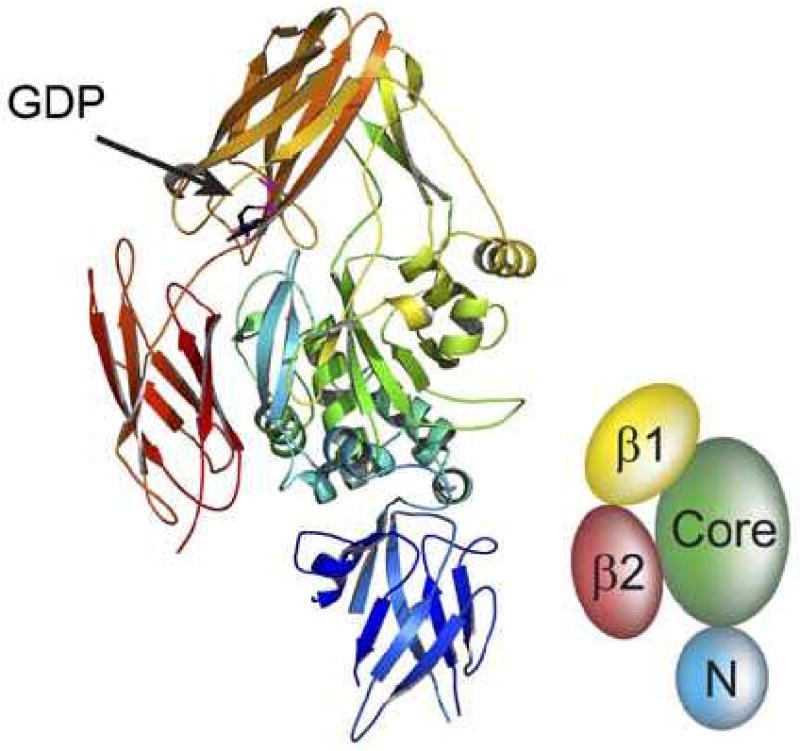
(A) The structural features of transglutaminase 2 (1-687). Red bar: N-terminal domain, 1-139. Blue bar: transamidation core domain (140-454). Green bar: C-terminal domain 1, (479-585). Yellow domain: C-terminal domain 2, (586-687). (B) 3-dimensional ribbon structure of human TGM2 showing the GDP binding site as well as the N-terminal domain (N), core domain, and C-terminal β barrels (β1 and (β2) (from Pinkas, D.M., Strop, P., Brunger, A.T., Khosla, C. 2007. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biology 5, 2788-2795).
2. Function
Tranglutaminase 2 is one of the eight mammalian transglutaminase family members (TGM1-7 and Factor XIIIA) (Collighan RJ and Griffin M., 2009). Members of the TGM family are calcium-dependent enzymes involved in specific posttranslational modifications by cross-linking extracellular matrix (ECM) proteins, thus stabilizing the ECM and making the protein complex more resistant to enzymatic degradation. This enzyme modifies proteins by cross-linking epsilon-(gamma-glutamyl) lysine or (gamma-glutamyl) polyamine bonds. Within the TGM family, TGM2 is an ubiquitous enzyme, and its expression has been detected in the cytoplasm, plasma membrane and the nucleus of various cells. The membrane-bound form of TGM2 binds GTP and may function as a G protein.
3. Disease involvement
Transglutaminase 2 protein and/or enzyme activity can be upregulated in a variety of diseases resulting in enhanced accumulation of cross-linked ECM proteins. Cross-linking of these ECM substrates prevents proteolytic breakdown and thus results in decreased ECM turnover and excess ECM accumulation. Substrates of TGM2 include fibronectin, collagen, fibrinogen, osteopontin, laminin, elastin and nidogen. Transglutaminase 2 cross-linking activity has been implicated to be causative for many fibrotic diseases including pulmonary fibrosis, liver fibrosis, renal fibrosis and atherosclerosis. Studies indicate that TGM2 inhibition reduces fibrosis as well as preserves organ function in experimental chronic kidney disease and glomerular scarring.
In cancer, intracellular TGM2 has been described as both pro- and anti-apoptotic. Extracellular TGM2/fibronectin is linked to cell survival via both integrin interactions and RGD independent interactions through syndecan 4 (Collighan RJ and Griffin M, 2009). In breast cancer, increased expression of TGM2 is linked to cell survival, invasion, and motility, while TGM2 siRNA inhibited these fibronectin-mediated actions. Previous reports have shown that TGM2 is expressed in ocular tissues including the iris, sclera, ciliary muscle, ciliary processes, retina, lens, optic nerve head and the trabecular meshwork (TM). Several studies have suggested that TGM2 may play a role in the formation of cataracts. TGM2 cross-links lens proteins including α and β crystallins in cataract lenses, which leads to the formation of insoluble protein dimers or oligomers. Significantly, cataract patients express higher lens levels of TGM2 compared to non-cataract patients (Wan et al., 2002). TGF-β treatment of human lens epithelial cells increased TGM2 and the cross-linkage of fibronectin.
Studies of animal models of retinal degeneration have shown photoreceptors cells undergo apoptosis after photic injury. In two retinopathy models, photic injury of photoreceptors in Lewis rats and retinal dystrophy in RCS rats, TGM2 enzyme activity increased concomitantly with apoptosis of photoreceptor cells. However, the role of TGM2 in apoptosis is unclear, but TGM2 forms detergent insoluble, cross-linked proteins in the cell, which may prevent the release of potential damaging intracellular components.
Elevated intraocular pressure is a major risk factor for glaucoma and is due to increased aqueous humor (AH) outflow resistance in the TM. TGF-β2 levels are increased in the AH and TM of glaucoma patients. Cultured human TM cells and TM tissues express and secrete TGM2, and treatment with TGF-β2 further induces TGM2 expression (Welge-Lussen et al., 2000). We recently reported the presence of TGM2 in normal and glaucomatous TM cells and tissues. We found elevated TGM2 expression in both isolated glaucomatous TM cells and tissues. In addition, we demonstrated increased TGM2 enzymatic activity in GTM cells and tissues (Tovar-Vidales et al., 2008). These studies suggest that elevated TGF-β2 levels results in an increase in TGM2-mediated cross-linked ECM proteins in the TM. This may result in resistance to proteolytic degradation, increased ECM deposition and impaired AH outflow in the glaucomatous TM. Similarly, elevated levels of TGF-β2 in the glaucomatous optic nerve head would increase TGM2 activity, which may play a role in the pathogenic ECM remodeling that occurs during glaucomatous optic neuropathy.
4. Future Studies
Although expressed in numerous ocular tissues, the function of TGM2 in maintaining ECM homeostasis and its role in ocular disease processes has not been explored in detail. To maintain homeostasis in normal tissues, an equilibrium exists between the deposition and degradation of ECM proteins. Transglutaminase 2 functions as a matrix stabilizer, and overexpression may be involved in fibrotic ocular diseases. It has been suggested that TGM2 may play an important role in the storage and activation of latent TGF-β to its active form. Since TGF-β has been associated with numerous ocular diseases, it would also be of interest to examine the relationship of TGM2 and TGF-β in other ocular tissues. Since TGM2 inhibition ameliorates experimental diabetic nephropathy, glomerular scarring and fibrosis, TGM2 inhibitors should be examined as potential treatments for ocular fibrotic diseases. The pro- and/or anti-apoptotic effect of TGM2 has been virtually unexplored in ocular tissues. Finally, the membrane-bound form of TGM2 binds GTP and may function as a G protein. This action has not been studied in ocular tissues.
Acknowledgments
The authors acknowledge grant support (EY-012783) to R.J. W from the National Eye Institute at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tara Tovar-Vidales, Email: tara.tovar-vidales@unthsc. edu.
Abbot F. Clark, Email: abe.clark@unthsc.edu.
Robert J. Wordinger, Email: robert.wordinger.@unthsc.edu.
References
- Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2008;49:622–628. doi: 10.1167/iovs.07-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XH, Lee EH, Koh HJ, Song J, Kim EK, Kim CY, Lee JB, Kim SY, Yao K, Lee JH. Enhanced expression of transglutaminases 2 in anterior polar cataracts and its induction by TGF-β in vitro. Br J Ophthalmol. 2002;86:1293–1298. doi: 10.1136/bjo.86.11.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]


