Abstract
Exogenously administered nerve growth factor (NGF) repairs injured axons, but it does not cross the blood-brain barrier. Thus, agents that could potentiate the neuritogenic ability of endogenous NGF would be of great utility in treating neurological injuries. Using the PC12 cell model, here we show that unfractionated green tea polyphenols (GTPP) at low concentrations (0.1 μg/ml) potentiate the ability of low concentrations of NGF (2 ng/ml) to induce neuritogenesis at a level comparable to that induced by optimally high concentrations of NGF (50 ng/ml) alone. In our experiments, GTPP by itself did not induce neuritogenesis or increase immunofluorescent staining for β-tubulin III; however, it increased expression of mRNA and proteins for the neuronal markers neurofilament-L and GAP-43. Among the polyphenols present in GTPP, epigallocatechin-3-gallate (EGCG) alone appreciably potentiated NGF-induced neurite outgrowth. Although other polyphenols present in GTPP, particularly epigallocatechin and epicatechin, lack this activity, they synergistically promoted this action of EGCG. GTPP also induced an activation of extracellular signal-regulated kinases (ERKs). PD98059, an inhibitor of the ERK pathway, blocked the expression of GAP-43. K252a, an inhibitor of TrkA-associated tyrosine kinase, partially blocked the expression of these genes and ERK activation. Antioxidants, catalase (cell-permeable form) and N-acetylcysteine (both L and D-forms) inhibited these events and abolished GTPP potentiation of NGF-induced neuritogenesis. Taken together, these results show for the first time that GTPP potentiates NGF-induced neuritogenesis likely through the involvement of sublethal levels of reactive oxygen species and suggest that unfractionated GTPP is more effective in this respect than its fractionated polyphenols.
Keywords: green tea, epigallocatechin gallate, extracellular signal-regulated kinase, neurofilament-L, GAP-43
INTRODUCTION
Nerve growth factor (NGF) is a member of the neurotrophin family known to stimulate growth and differentiation of neurons during development (Bothwell 1995). NGF and its receptors also have been reported to be elevated in activated neurons, microglia, and astrocytes after brain injury raising the possibility that NGF may serve to protect injured neurons from neurotoxic damage and effect growth of damaged axons (Sofroniew et al., 2001). Indeed, the exogenous delivery of NGF induces growth of injured axons (Oudega and Hagg 1996; Thuret et al., 2006). However, a practical drawback to the clinical use of NGF supplementation as a therapeutic intervention is the fact that NGF does not readily cross the blood-brain barrier making its use dependent on invasive neurological procedures that require direct infusion of the trophin into the cerebroventricular system (Brinton and Yamazaki 1998). In addition, because of the diversity of cell types that respond to NGF both peripherally and centrally, NGF supplementation has led to a number of undesirable side effects in both animals and patients limiting the effectiveness of its use as a therapeutic intervention to treat brain injury (Hao et al., 2000; Jonhagen 2000; Winkler et al., 2000).
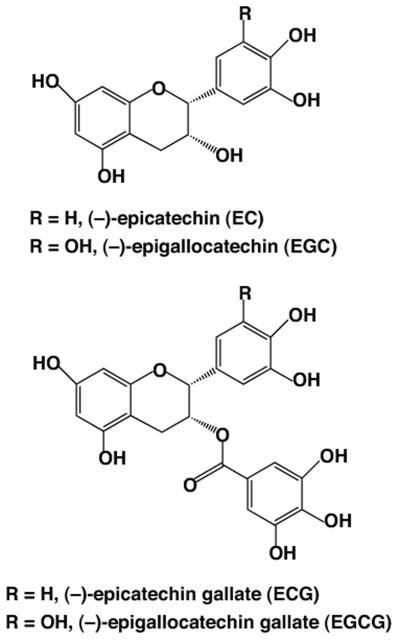
An alternative to the above approach is the enhancement of the neuritogenic ability of endogenous NGF via administration of exogenous potentiators. This approach would be without the systemic effects of exogenous NGF administration. Fortunately, there are a variety of natural products that are known to directly induce neurite outgrowth or potentiate the action of NGF which may be suited for the treatment of neuronal injury and are without the logistical drawbacks and high costs of synthetic drug development (Guo et al., 2006; Kano et al., 2008; Li et al., 2003; Sagara et al., 2004; Shibata et al., 2008; Tohda et al., 2005). One such potential compound noteworthy for study is green tea (Camelia sinensis), one of the most popular and widely consumed beverages in the world. Epidemiological and experimental data support the health benefits of green tea in neoplastic, cardiovascular, and neurological diseases (Gupta et al., 2001; Mukhtar and Ahmad 2000; Park et al., 2009; Yang et al., 2002; Zaveri 2006; Zhang et al., 2006). A typical cup of green tea contains approximately 150 mg of water-soluble green tea polyphenols (GTPP), commonly known as catechins. These include galloylated polyphenols (−)-epigallocatechin-3-gallate (EGCG) and (−)-epicatechin-3-gallate (ECG) as well as nongalloylated polyphenols (−)-epigallocatechin (EGC) and (−) epicatechin (EC) (Fig. 1). The pioneering work of Mandel, Youdim, and their associates has shown that EGCG, the major constituent of GTPP, possesses antioxidant, neuroprotective, and neurorescuing properties (Mandel et al., 2005). However, the detailed mechanisms by which these agents work to induce diverse cellular effects are not completely understood.
Fig. 1.
Structures of major polyphenols present in green tea.
Our hypothesis is that GTPP can interact with NGF in a convergent manner to enhance neurite outgrowth by up-regulating common pathways linked to the induction of NGF signaling. Possible points of convergence include (1) activating transmembrane receptor-associated tyrosine kinase, TrkA (Huang and Reichardt 2003; Vaudry et al., 2002) which leads to a sustained activation of extracellular signal-regulated kinases (ERKs) necessary for neurite outgrowth (Pang et al., 1995) and (2) induction of sublethal levels of oxidative stress that lead to the activation of ERK (Gopalakrishna et al., 2008).
We have tested this hypothesis through a series of experiments using the rat pheochromocytoma cell line PC12 (Greene and Tischler 1976) which has been used previously for the screening of several natural compounds for their ability to induce neuritogenesis. We show for the first time that both GTPP and EGCG potentiate the action of NGF in inducing neuritogenesis likely through sublethal levels of reactive oxygen species (ROS). Additionally, we demonstrate the superiority of unfractionated GTPP over fractionated polyphenols in this process.
MATERIALS AND METHODS
Materials
Purified GTPP constituents (EGCG, ECG, EGC, and EC), catalase-polyethylene glycol, and N-acetyl-L-cysteine (L-NAC) were from Sigma (St. Louis, MO). N-Acetyl-D-cysteine (D-NAC) was from Research Organics (Cleveland, OH). Mouse NGF was from Upstate (Lakeplacid, NY). K252a and PD98059 were from Alexis Biochemicals (San Diego, CA). Anti-MAP kinase ERK1/2 antibodies and anti-phospho-MAP kinase ERK1/2 (pThr202/Tyr204) antibodies were from Cell Signaling (Denvers, MA). Mouse anti-GAP-43 monoclonal antibodies and goat polyclonal antibodies raised against neurofilament-L (NF-L) were from Santa Cruz Biotechnology (Santa Cruz, CA). β-tubulin III mouse monoclonal antibodies were from Covance (Emeryville, CA). Decaffeinated extract of GTPP, which was standardized to contain 97% polyphenols and nearly 70% catechins, was obtained from Pharmanex (Provo, UT). The typical preparation contained the following polyphenols expressed as percentage of original weight of GTPP preparation: EGCG (36%), ECG (15%), EC (7%), and EGC (3%) (Morre et al., 2003). Similar composition was also reported with another batch of GTPP suggesting a high degree of consistency in the composition of this GTPP preparation (Lu et al., 2005).
Cell Culture and Treatments
PC12 cells, originally obtained from Dr. Christine Pike (University of Southern California), were grown in MEM supplemented with 10% heat-inactivated horse serum, 5% fetal calf serum, 50 units/ml penicillin, and 0.05 mg/ml streptomycin. GTPP and constituent polyphenols were dissolved in dimethylsulfoxide and the aliquots of samples were stored at −80°C which minimizes autooxidation of polyphenols. The samples were diluted with water on the day of use. When agents were dissolved in organic solvents, appropriate solvent controls were used.
Quantitation of Neurite Outgrowth
PC12 cells were plated in polylysine-coated 6-well plates in a growth medium at a density of 1 × 104 cells/ml. The medium was changed to MEM with 2% heat-inactivated calf serum and 1% fetal calf serum. After overnight growth, cells were treated with NGF, GTPP, or other agents either alone or in combination for three days. The cells were then scored for the presence of neurites (Katoh et al., 1997). For each treatment, 200 cells in each of three separate fields were scored. Cells with outgrowths longer than two diameters of the cell body were scored positive for neurites and were expressed as a percentage of the total cell number.
Quantitative RT-PCR for Analyzing the Expression of Neuronal Marker Genes
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). Then it was cleaned using an RNase-free DNase set (Qiagen, Valencia, CA) to eliminate genomic DNA contamination. RNA was used for quantitative real-time PCR (qRT-PCR) analysis of the mRNA for neurofilament-L and GAP-43. qRT-PCR analysis was performed with the MyiQ Single-Color Real-Time PCR detection System (Bio-Rad, Hercules, CA) using SYBR Green PCR Master Mix (Bio-Rad) according to manufacturer's protocol. The amplification protocol consisted of one cycle at 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The dissociation curve protocol was performed at the end of the amplification to confirm a single peak near the calculated melting temperature of each amplicon. All amplifications were run in triplicate. A standard curve of cycle thresholds using serial dilutions of cDNA samples was used to calculate the relative abundance. The difference in the initial amount of total RNA between the samples was normalized in every assay using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression as an internal standard. The sequences of forward and reverse primers for GAPDH, NF-L, and GAP-43 were previously given (Gopalakrishna et al., 2008).
Immunofluorescence Staining of β-tubulin III
PC12 cells were seeded at a low density on polylysine-coated culture slides (BD Biosciences, Bedford, MA) and were grown for 24 h. The cells were then treated with NGF, GTPP or a combination of both. Cells were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 min, then washed three times in phosphate-buffered saline and subsequently permeabilized with 0.1% Triton X-100 for 30 min. Cells were blocked in 1% bovine serum albumin before being incubated with β-tubulin III mouse monoclonal antibody (1:200 dilution) at 37°C for 1 hr. The cells were washed and incubated with Alexa Fluor 488-conjugated goat antimouse IgG secondary antibody (Invitrogen, Carlsbad, CA) for 1 h at room temperature. Then, the washed cells were mounted with Vectashield (Vector, Laboratories, Burlingame, CA) containing 4′-6-diamidino-2-phenylindole (DAPI) for nuclear staining and viewed on an LSM 510 laser-scanning microscope (Carl Zeiss, Thornwood, NY). No fluorescence was detected when the primary antibody was omitted.
Western Blotting Analysis of Proteins
Cell extracts were prepared and subjected to SDS-polyacrylamide gel electrophoresis as described previously (Gopalakrishna et al., 2008). Electrophoretically separated proteins were transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% dry milk. For quantitation of NF-L, the membranes were incubated with goat polyclonal antibodies to NF-L followed by donkey anti-goat secondary antibodies conjugated with horseradish peroxidase. For quantitation of GAP-43, we used mouse anti-GAP-43 monoclonal primary antibodies and goat anti-mouse secondary antibodies. The immuno-reactive bands were visualized by an enhanced chemiluminescence Western blot detection kit (Thermo Scientific, Rockford, IL). These bands were analyzed by densitometric scanning using the Omega 12 IC Molecular Imaging System and UltraQuant software. Actin staining served to confirm equal loading of protein in all electrophoretic lanes.
Measuring Activation of MAP Kinases ERK1/2
Activation of MAP kinase was determined by Western immunoblotting using anti-phospho-MAP kinase ERK1/2 (pThr202/Tyr204) rabbit antibodies. Total ERK1/2 (unphosphorylated and phosphorylated forms) was determined using anti-MAP kinase ERK1/2 antibodies, which served to confirm equal loading of protein in all electrophoretic lanes.
Statistical Analysis
Statistical analyses were performed in StatView software. Data are expressed as the mean ± SE and were compared for statistical significance by paired t-test analyses. P<0.05 was considered significant.
RESULTS
Unless otherwise mentioned, NGF at 2 ng/ml was used as the low concentration, and NGF at 50 ng/ml was used as the high concentration. 50 ng/ml was previously determined to be the optimal concentration of NGF to induce neurite outgrowth. GTPP was used at a concentration of 0.1 μg/ml. EGCG was used at a concentration of 1 μM. Due to the instability of green tea polyphenols in the culture medium (Hong et al., 2002), they were added once daily to the medium.
GTPP Potentiates NGF-induced Neuritogenesis
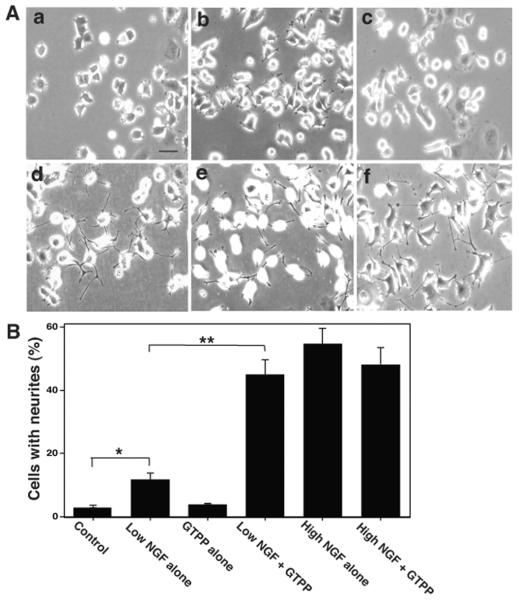
At low concentration, NGF induced only a limited degree of neurite outgrowth in PC12 cells (Fig. 2A and B). GTPP alone did not induce neurite outgrowth to an appreciable extent even at high concentrations (1 –5 μg/ml) or when the treatment was extended for an additional five days. However, in the presence of GTPP, a low concentration of NGF induced neurite outgrowth to an extent equivalent to that achieved with the high concentration of NGF alone (Fig. 2A and B). A combination of GTPP and NGF, besides increasing the number of neurites, increased the length of the neurites and the degree of branching (Figure 2d). In this case, the neurites grew to a length of nearly 2- to 4-fold longer than cell body diameter. GTPP at concentrations as low as 0.025 μg/ml potentiated neuritogenesis induced by a low concentration of NGF (data not shown). However, addition of GTPP to a high concentration of NGF did not further enhance neurite outgrowth. Interestingly, at very high concentrations (1-5 μg/ml), GTPP failed to potentiate neuritogenesis induced by a low concentration of NGF. In fact, very high concentrations of GTPP (1-5 μg/ml) decreased the neurite outgrowth directly induced by high concentration NGF (50 ng/ml). This decrease in neuritogenesis may be due to lethal effects of GTPP at high concentrations. GTPP did not inhibit proliferation of PC12 cells at low concentrations (<0.5 μg/ml). However, GTPP did inhibit cell growth at high concentrations (>5 μg/ml).
Fig. 2.
GTPP potentiates NGF-induced neurite outgrowth. A: morphological changes in PC12 cells treated with the following: a, control; b, low concentration of NGF (2 ng/ml) alone; c, GTPP (0.1 μg/ml) alone; d, low concentration of NGF and GTPP; e, high concentration of NGF (50 ng/ml) alone; f, high concentration of NGF and GTPP. PC12 cells were seeded at a low density (1 × 104 cells/ml) on polylysine-coated 6-well plates in MEM medium supplemented with 2% heat-inactivated horse serum and 1% fetal calf serum. Cells were treated with the indicated agents and morphological changes were photographed after three days. Scale bar = 50 μm. B: Cells with neurites were counted andexpressed as the percentage of the total cell number. Each value is the mean ± SE obtained from three experiments. The values obtained with “low NGF alone” and “low NGF and GTPP” were compared with their respective controls as indicated in the figure by the paired t test (*, P<0.05; **, P<0.01).
GTPP Induces Expression of Neuronal Marker mRNA and Proteins
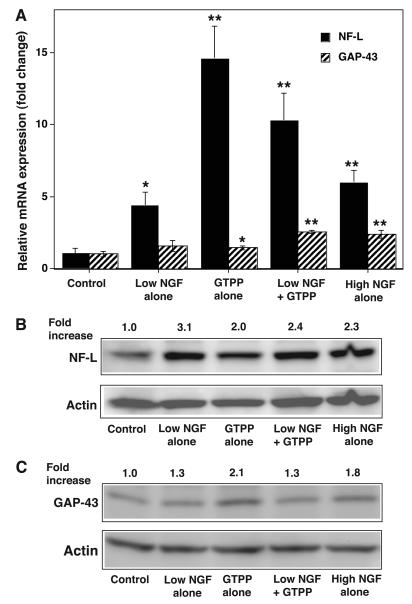
The expression of neuronal marker genes NF-L and GAP-43 were analyzed by qRT-PCR in PC12 cells treated with GTPP or a low concentration of NGF to determine whether the expression of these genes correlates with the observed synergistic interaction between GTPP and NGF. At a low concentration, NGF alone induced an increase in expression of both genes (Fig. 3A). GTPP induced a nearly 15-fold increase in the expression of NF-L which was higher than that expressed in cells treated with a low concentration of NGF. GTPP also moderately induced the expression of GAP-43 to a level similar to that induced by a low concentration of NGF. A combination of GTPP and a low concentration of NGF induced the expression of NF-L, but this was neither synergistic nor additive. Conversely, GAP-43 expression was higher when GTPP and a low concentration of NGF were combined than with either agent independently. NGF at a high concentration induced the expression of both neuronal marker genes to a degree comparable to that induced by a combination of GTPP and a low concentration of NGF.
Fig. 3.
GTPP induces expression of mRNA and proteins for NF-L and GAP-43. A: expression of mRNA for NF-L and GAP-43 by GTPP. PC12 cells were grown in polylysine-coated 100-mm Petri dishes, changed to serum-free medium, and treated with indicated agents for 24 h. Total RNA was extracted from control and the treated cells and used for qRT-PCR analysis of the mRNAs for NF-L and GAP-43. The qRT-PCR reading for each sample was normalized to that of glyceraldehyde-3-phosphate dehydrogenase. The results are shown as the mean ± SE for three estimations. Values obtained with the various agents were compared with the control by the paired t test (*P<0.05; **, P<0.01). B: expression of NF-L protein by Western immunoblot analysis. C: expression of GAP-43 protein by Western immunoblot analysis. β-actin was used as the loading control. The band density of NF-L and GAP-43 was quantitated by densitometry and normalized to the band density of actin present in the same lane and expressed as a relative-fold increase compared with that of the control.
Since GTPP induced an increase in expression of neuronal marker genes NF-L and GAP-43 but not a morphological neuronal differentiation, we quantitated NF-L and GAP-43 proteins by Western immunoblotting. There was an increase in the levels of both proteins in PC12 cells treated with GTPP that was comparable to that seen with the high concentration of NGF (Fig. 3B and C). NF-L expression is affected not only by events at the transcriptional level but also by post-transcriptional regulation and translational efficiency (Thyagarajan et al., 2007). Factors associated with the latter two processes could account for the discrepancy between the 15-fold elevation of NF-L mRNA and the only 2-fold increase in its protein after GTPP treatment.
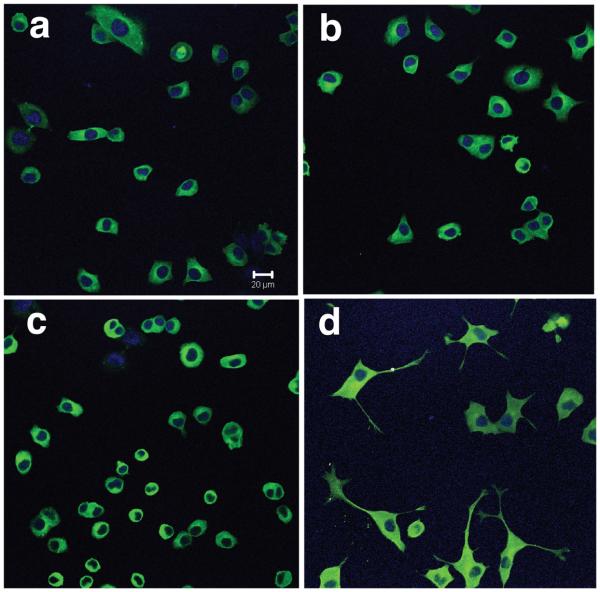
We also carried out immunofluorescence staining for neuron-specific β-tubulin III in the PC12 cells treated with GTPP and a low concentration of NGF. This experiment has two purposes; one to confirm that the threadlike processes induced by a combination of GTPP and a low concentration of NGF were indeed neurites, and the other was to determine whether there is an increase in this protein in PC12 cells treated with these agents. Treatment of PC12 cells with either GTPP or a low concentration of NGF for two days did not result in an appreciable increase in the fluorescence intensity of β-tubulin III (Fig. 4). However, a combination of both agents increased the fluorescence staining intensity of β-tubulin III in the cell bodies as well as the threadlike processes induced by these agents. This provides strong evidence that the morphological changes observed with the combined treatment of GTPP and low concentration of NGF were indeed due to neuronal differentiation. In cells treated with a combination of GTPP and NGF, β-tubulin-stained cells with neurites were approximately 36% of the total. There were virtually no β-tubulin-stained neurites in the control cells, NGF-treated, or GTPP-treated cells. These results further suggest that while GTPP alone increases NF-L and GAP-43 protein and mRNA, it does not increase the expression of β-tubulin III protein which is strongly associated with neuronal differentiation.
Fig. 4.
Immunofluorescence staining of β-tubulin III in PC12 cells treated with NGF and GTPP. PC12 cells were grown on polylysine-coated culture slides and treatedwith a low concentration of NGF, GTPP, or a combination of both for two days. Cells were fixed, permeabilized, and incubated with β-tubulin III monoclonal antibody followed by Alexa Fluor 488-conjugated secondary antibody (green). Cells were viewed on a confocal microscope. The nuclei were stained blue by 4′,6′-diamidino-2-phenyl-indole. a, control untreated cells; b, low concentration NGF alone; c, GTPP alone; d, combination of GTPP and NGF. Scale bar = 20 μm.
Potentiation of Neuritogenesis by Individual Polyphenols Present in GTPP
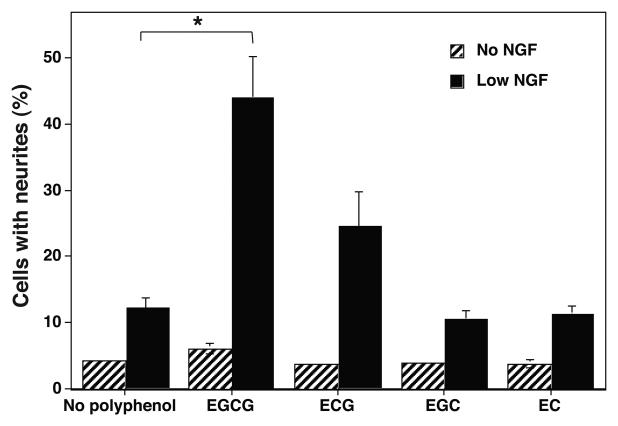
Four major polyphenolic compounds present in GTPP extract were tested in pure form for their ability to potentiate NGF-induced neurite outgrowth. None of these polyphenolic compounds induced a significant increase in neurite outgrowth in the absence of NGF (Fig. 5). Galloylated EGCG appreciably potentiated the action of NGF to induce neurite outgrowth, followed by ECG. On the other hand, nongalloylated polyphenols EC and EGC did not potentiate NGF-induced neurite outgrowth. This suggests that the galloyl group in the polyphenolic compounds tested may be required for the potentiation of NGF to induce neurite outgrowth.
Fig. 5.
Potentiation of NGF-induced neurite outgrowth by individual polyphenols present in GTPP. PC12 cells were treated with 1 μM EGCG, ECG, EGC, or EC in the presence or absence of a low concentration of NGF for three days. Cells with neurites were counted and expressed as the percentage of the total cell number. Each value is the mean ± SE obtained from three experiments. The values obtained with low NGF and polyphenols were compared with values obtained with low NGF but no polyphenol by the paired t test. *, significantly different values (P<0.05).
Synergistic Interaction Among Polyphenols Present in GTPP
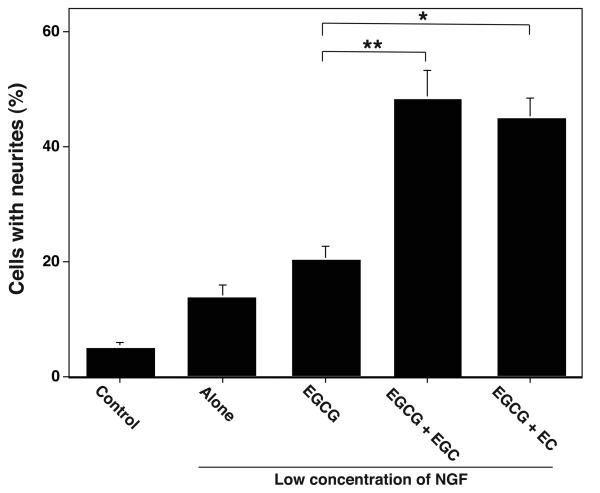
The amount of isolated EGCG (1 μM or 0.458 μg/ml) required for the optimal potentiation of NGF-induced neurite outgrowth was nearly 12-fold higher than the EGCG (0.036 μg/ml) present in the amount of GTPP extract (0.1 μg/ml) that is needed for the optimal potentiation of NGF-induced neuritogenesis. Therefore, we determined whether the polyphenols EC and EGC present in GTPP, which lack the ability to potentiate NGF-induced neurite outgrowth on their own, can synergistically promote this action of EGCG. As shown in Fig. 6, when EGCG was used at a suboptimal concentration (0.1 μM), EC and EGC enhanced the ability of EGCG to potentiate NGF-induced neuritogenesis. Thus, it is possible that the mixture of polyphenolic agents present in the unfractionated GTPP may work better than the isolated individual components due to their synergistic interactions. Therefore, we carried out the majority of the studies with GTPP extract instead of pure compounds. However, since EGCG is the major polyphenol responsible for the action of GTPP, we also carried out limited studies with it to determine whether this polyphenolic compound can elicit effects similar to that of the GTPP mixture.
Fig. 6.
Synergistic interaction among polyphenols present in GTPP in potentiating NGF-induced neurite outgrowth. PC12 cells were treated with a suboptimal concentration of EGCG (0.1 μM) either alone or in combination with 1 μM EGC or EC for three days. Neurite outgrowth was determined as indicated in Materials and Methods. The results are shown as the mean ± SE for three experiments. The values obtained were compared as indicated in the figure by the paired t test (*, P<0.05; **, P<0.01).
GTPP Induces the Activation of ERK1/2
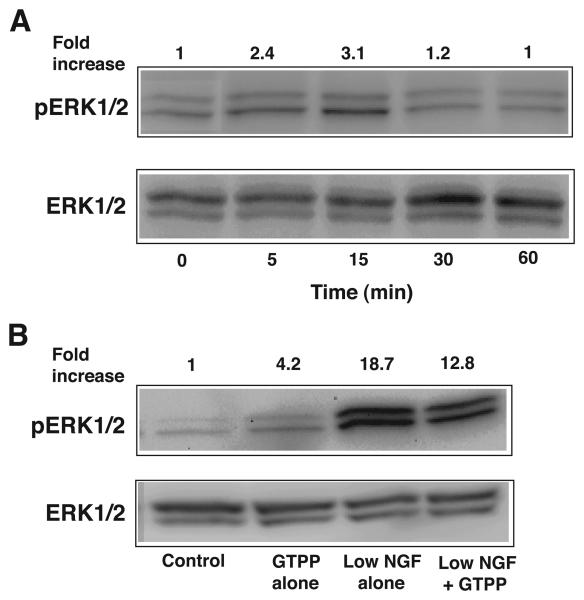
Since a sustained activation of ERK is required for the induction of neuritogenesis (Pang et al., 1995), we predicted that GTPP might increase the intensity and/or the duration of ERK activation in cells treated with a low concentration of NGF. Therefore, we determined the ERK1/2 activity by Western immunoblotting PC12 cells treated with GTPP alone, with NGF alone, and with a combination of both. As shown in Fig. 7A, although GTPP alone induced activation of ERK1/2, this was less intense and short duration. Contrary, a low concentration of NGF induced an intense and sustained activation of ERK (data not shown). Unexpectedly, when combined, GTPP did not increase NGF-induced ERK1/2 activation, but rather slightly decreased the intensity of NGF-induced ERK1/2 activation (Fig. 7B). Similar results were obtained when experiments were carried out with EGCG (data not shown). Based on these results, GTPP-induced activation of ERK likely does not additionally contribute to the already high activity of ERK induced by a low concentration of NGF.
Fig. 7.
A: Time course of induction of ERK activity by GTPP. At confluence, PC12 cells were serum starved for 24 h and then treated with GTPP (0.1 μg/ml) for the indicated time periods. Cell lysates were subjected to Western immunoblotting to determine phosphorylation of ERK1/2. B: Lack of additive nature of ERK activity induced by GTPP and NGF. Serum-starved cells were treated with GTPP alone, low concentration NGF alone, or a combination of both for 10 min. Phosphorylation of ERK was then determined. The band density of the phosphorylated ERK1/2 was quantitated by densitometry and normalized to the band density of the total ERK1/2 present in the same lane and expressed as a relative-fold increase compared with that of the untreated control.
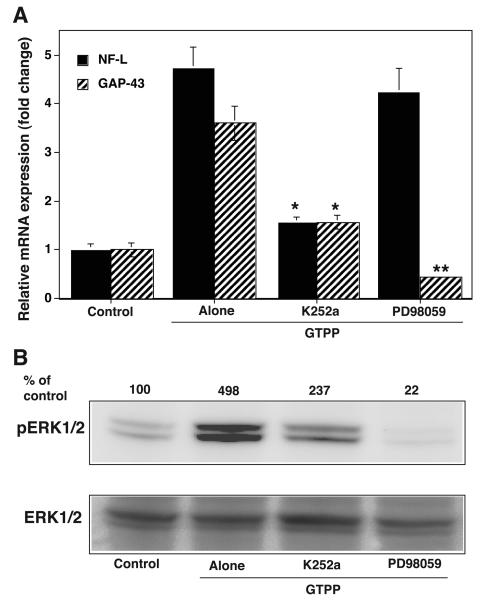
Since the ERK pathway plays a key role in the induction of gene expression caused by NGF-induced neuritogenesis, we determined whether the ERK activation induced by GTPP eventually plays a role in the expression of these genes. PD98059, a mitogen-activated protein kinase kinase (MEK) inhibitor which inhibits the ERK pathway, abolished the GTPP-induced increase in the expression of mRNA for GAP-43 but not for NF-L (Fig. 8A). In addition, PD98059 inhibited GTPP potentiated NGF-induced neurite outgrowth, but it did not induce toxicity when treated alone as a control (data not shown).
Fig. 8.
A: Effect of K252a and PD98059 on GTPP-induced expression of NF-L and GAP-43. Cells were grown in polylysine-coated 100-mm Petri dishes, changed to serum-free medium, and treated with GTPP (0.1 μg/ml) either alone or in combination with K252a (200 nM) or PD98059 (50 μM) for 24 hr. qRT-PCR analyses of mRNA for NF-L and GAP-43 were carried out as described in Materials and Methods. The results are shown as the mean ± SE for three estimations. Values obtained with GTPP in combination with K252a and PD98059 were compared with those for GTPP alone by the paired t test (*P<0.05; **, P<0.01). B: Effect of K252a and PD98059 on GTPP-induced increase in activation of ERK. Confluent cells were serum starved for 24 h and treated with GTPP in the presence or absence of K252a or PD98059 for 10 min. ERK activation was determined by analyzing phosphorylated ERK1/2 by Western immunoblotting. The band density of the phosphorylated ERK1/2 was quantitated by densitometry and normalized to the band density of the total ERK1/2 present in the same lane andexpressed as a percentage of the control not treated with GTPP.
Possible Role of TrkA-associated Tyrosine Kinase in GTPP Effects
Since the action of NGF is mediated by binding to and activating its transmembrane receptor TrkA (Huang and Reichardt 2003; Vaudry et al., 2002), which eventually leads to the expression of proteins that are necessary for neuritogenesis, we determined whether GTPP-induced expression of the NF-L and GAP-43 genes is inhibited by K252a, a TrkA tyrosine kinase inhibitor. K252a (200 nM) substantially blocked the GTPP-induced mRNA expression levels for NF-L and GAP-43 suggesting that this action of GTPP may be directly or indirectly mediated by TrkA-associated tyrosine kinase activity (Fig. 8A). Since TrkA activation triggers the activation of the ERK pathway, we determined whether the blocking of TrkA-associated tyrosine kinase activity could inhibit GTPP-induced ERK activation. K252a inhibited nearly 50% of the GTPP-induced activation of ERK1/2 whereas PD98059, a MEK inhibitor, completely blocked the GTPP-induced activation of ERK1/2 (Fig. 8B). K252a inhibited GTPP potentiated NGF-induced neurite outgrowth, but it did not induce toxicity when treated alone as a control (data not shown).
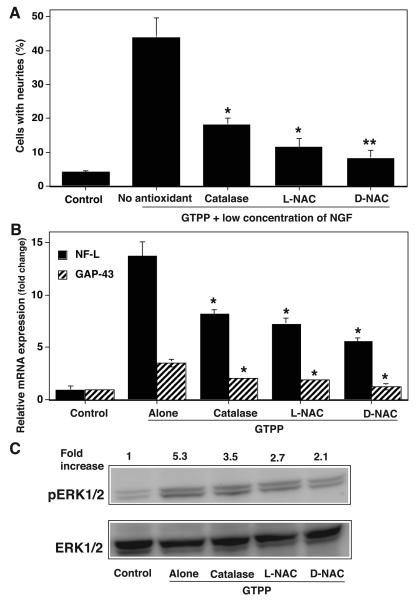
Antioxidants Inhibit the GTPP Potentiation of NGF-induced Neuritogenesis
Having established that both GTPP and EGCG potentiate NGF-induced neurite outgrowth, we determined whether this action is mediated by ROS generated in response to treatment with these agents. Cell-permeable polyethylene glycol (PEG)-catalase inhibited GTPP potentiation of NGF action while, its negative control, heat-inactivated PEG-catalase, did not (Fig. 9A). This suggests the possible role of hydrogen peroxide in the potentiating action of GTPP. Similarly, L-NAC (2.5 mM) inhibited GTPP-potentiated NGF-induced neuritogenesis (Fig. 9A). However, it did not show toxicity when treated alone as a control. Since L-NAC can serve as a precursor for the synthesis of cellular glutathione, we used D-NAC which is not a precursor of glutathione synthesis. D-NAC (2.5 mM) also inhibited GTPP-potentiated NGF-induced neuritogenesis. This suggests a possible involvement of ROS in this process. All three antioxidants inhibited the GTPP-induced expression of neuronal markers NF-L and GAP-43 (Fig. 9B). Similarly, these antioxidants decreased GTPP-induced activation of ERK1/2 (Fig. 9C). This suggests that sublethal levels of ROS may play a role in the GTPP-mediated induction of NF-L and GAP-43 as well as in its activation of ERK.
Fig. 9.
A: Effect of antioxidants on GTPP potentiation of NGF-induced neurite outgrowth. GTPP potentiated NGF-induced neurite outgrowth was measured in the presence and absence of antioxidants, cell-permeable PEG-catalase (125 units/ml), L-NAC (2.5 mM), and D-NAC (2.5 mM). The results are shown as the mean ± SE for three experiments. The values obtained for GTPP-potentiated neurite outgrowth in the presence of antioxidants were compared with those obtained for GTPP-potentiated neurite outgrowth in the absence of antioxidants by the paired t test (*P<0.05; **, P<0.01). B: Effect of antioxidants on GTPP-induced expression of NF-L and GAP-43. PC12 cells were pretreated with PEG-catalase for 24 h or L-NAC or D-NAC for 3 h to ensure the entry of the molecules into the cells. Then the cells were treated with GTPP (0.1 μg/ml) for 24 hr. qRT-PCR analyses of mRNA for NF-L and GAP-43 were carried out. The results are shown as the mean ± SE for three estimations. Values obtained with GTPP in combination with antioxidants were compared with those obtained with GTPP without antioxidants by the paired t test (*P<0.05). C: Effect of antioxidants on GTPP-induced activation of ERK. Initially, cells were serum starved for 24 h and pretreated with antioxidants. Then cells were treated with GTPP for 10 min and phosphorylation of ERK was determined by Western immunoblotting. The band density of the phosphorylated ERK1/2 was quantitated by densitometry and normalized to the band density of the total ERK1/2 present in the same lane and expressed as a relative-fold increase compared with that of the control not treated with GTPP.
DISCUSSION
This is the first report showing a potentiation of NGF-induced neuritogenesis by GTPP or EGCG. Although it has been shown that EGCG and other polyphenolic compounds undergo autooxidation in cell culture medium (Hong et al., 2002; Sang et al., 2007), it is possible that limited amounts of polyphenols remaining after autooxidation may be sufficient to induce this potentiation effect. It is also possible that the initial effects of unoxidized GTPP and EGCG may be sufficient to trigger their later effects. The following key points emerge from this study.
First, the concentrations of GTPP required for potentiation of NGF-induced neurite outgrowth are extremely low (0.025 to 0.1 μg/ml). In our study GTPP or EGCG did not induce neuritogenesis in the absence of NGF. In this respect, these studies are clearly different from the previously published studies where EGCG alone or green tea extract induced neurite outgrowth (Reznichenko et al., 2005; Shurygin et al., 2004). However, we do not exclude the possibility that these agents may directly induce neurite outgrowth in some clones of PC12 cells as elegantly shown by others (Reznichenko et al., 2005). Consistent with this possibility, we observed that GTPP by itself increased the expression of mRNA and protein for neuronal markers NF-L and GAP-43. Nevertheless, GTPP did not increase β-tubulin III, another neuronal marker protein. Although an increase in GAP-43 protein is associated with neuritogenesis in NGF-treated PC12 cells, a dissociation between GAP-43 and neuritogenesis has been previously reported in some cases (Burry and Perrone-Bizzozero 1993). Conceivably, GTPP may be inducing some signaling for expression of NF-L and GAP-43, but this signaling may not be sufficient for the direct induction of neuritogenesis.
It is also possible that at low concentrations NGF may be activating some pathways but not all of those needed for neuritogenesis. GTPP might activate these missing pathways and thereby complement the action of NGF so that NGF even at a low concentration can induce neuritogenesis. This is particularly important because, at sites of neuronal injury, NGF is suboptimal for the induction of neuritogenesis. Delivery of optimal concentrations of NGF just to sites of neuronal injury is a daunting task. Thus, the NGF potentiating activity of GTPP and EGCG may be a highly useful tool in the treatment of neuronal injuries.
Second, this study reveals that the mixture of polyphenols present in unfractionated GTPP is more effective than its isolated individual polyphenols in potentiating NGF-induced neurite outgrowth. EGCG is the active polyphenolic agent responsible for the potentiation of NGF-induced neuritogenesis and activation of the ERK pathway, but other polyphenols present in GTPP may synergistically enhance this action of EGCG. This is particularly important considering the fact that the individual polyphenols present in GTPP are poorly absorbed (Lee et al., 2002). For example, EGCG concentration is low in the plasma after ingestion of EGCG (Lee et al., 2002), but its cellular uptake has been shown to be enhanced by EC (Suganuma et al., 1999; Suganuma et al., 1998). In this study, we used concentrations of EGCG roughly equivalent to plasma concentrations achieved after consumption of two to three cups of green tea. Although we have seen toxicity of GTPP and EGCG at very high (1-5 μg/ml) concentrations, such high concentrations are unlikely to be reached in the plasma after consumption few cups of green tea due to the limited absorption of these polyphenols in the intestinal tract. These observations have implications for future preclinical or clinical studies aimed at enhancing recovery from neuronal injury. In such studies, whole extract of GTPP, but not the isolated polyphenols, should be used, and only in limited doses.
Third, we demonstrated a mild and transient activation of ERK1/2 by GTPP. However, neither GTPP nor EGCG increased or prolonged the ERK activation induced by a low concentration of NGF. This suggests that the observed potentiation of NGF by GTPP and EGCG was unlikely caused by the additional contribution to ERK activity. Previous studies have shown an induction of ERK activation by some naturally occurring compounds correlating with their ability to induce neurite outgrowth, and that the blocking of this pathway results in inhibition of neuritogenesis induced by these agents (Hur et al., 2004; Ina et al., 2007; Kano et al., 2008; Sagara et al., 2004). Some agents which potentiate NGF-induced neuritogenesis lack their own ability to induce ERK activity and yet prolong ERK activation induced by a suboptimal concentration of NGF (Qi et al., 2006). Other agents associated with potentiation of NGF induced neuritogenesis neither induce ERK activation nor enhance NGF-induced ERK activation (Li et al., 2003). GTPP and EGCG are different from all these agents; they induce a rapid but mild activation of ERK but do not enhance or prolong the ERK activation induced by a low concentration of NGF. Although GTPP induced ERK activation only to a limited extent, this is still significant because PD98059, an ERK pathway inhibitor, partially inhibited the GTPP-induced expression of GAP-43 (Fig. 10).
Fig. 10.
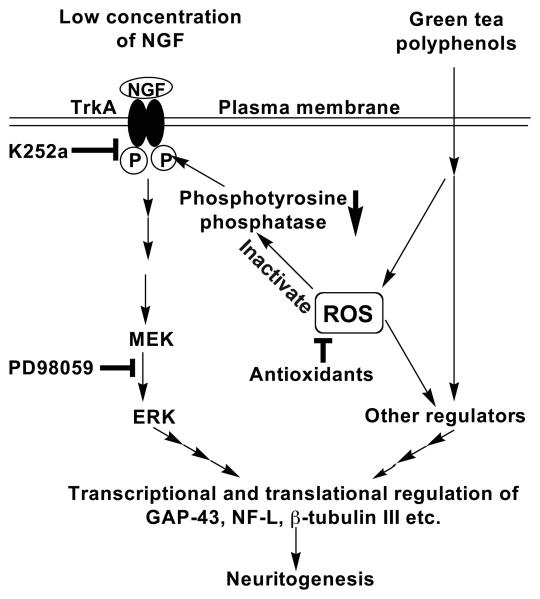
Schematic presentation of a possible mechanism by which GTPP potentiates NGF-induced neurite outgrowth. In this study, we have demonstrated therole of TrkA, ERK and ROS in the GTPP potentiation of NGF action by using appropriate blocking agents. Our hypothesis is that under basal conditions TrkA is phosphorylated at its tyrosine residues, but a phosphotyrosine protein phosphatase deposphorylates TrkA and inhibits its activation and subsequent downstream signaling. GTPP, via ROS generation, inactivates this phosphatase and increases the phosphorylation of TrkA and subsequent activation of ERK. A lack of further increase in NGF-induced ERK activation by GTPP suggests that additional pathways, regulated either by GTPP directly or by ROS generated by GTPP, may complement the ERK pathway to increase the expression of neuronal marker genes and potentiate NGF-induced neuritogenesis.
In this study, PD98059 inhibited the GTPP potentiated NGF-induced neuritogenesis. Nevertheless, this does not imply that the ERK pathway is responsible for the effects of GTPP on NGF since neurite outgrowth induced by high concentrations of NGF alone also involves the activation of ERK (Pang et al., 1995; Perron and Bixby 1999). Therefore, PD98059-induced inhibition of neuritogenesis may be due to the blocking of GTPP and/or NGF action.
K252a-induced partial inhibition of ERK activation and induction of NF-L and GAP-43 suggests the role of TrkA tyrosine kinase activity in mediating these GTPP effects. Autophosphorylation of TrkA at its tyrosine residues leads to activation of ERK via the Ras pathway (Huang and Reichardt 2003). It is intriguing that GTPP activates this pathway. Previous studies have shown high affinity binding of EGCG to certain cellular proteins (Bode and Dong 2009). However, there are no reports of EGCG binding to TrkA. K252a was originally characterized as an inhibitor of protein kinase C and cAMP-dependent protein kinase. However, at nanomolar concentrations, it has been shown to be a specific inhibitor of NGF-induced biochemical effects and neurite outgrowth in PC12 cells (Berg et al., 1992). It cannot be excluded that K252a, in addition to its effect on TrkA tyrosine kinase activity, may also directly affect one or more downstream kinases. Therefore, the role of TrkA in mediating GTPP or EGCG action is cautiously interpreted. TrkA inhibition did not completely abolish GTPP-induced ERK activation, whereas MEK inhibition by PD98059 did. This suggests that other kinases such as protein kinase C or phosphoinositide 3-kinase may also be contributing to ERK activation by acting upstream to MEK but independently from TrkA.
Finally, we show here that at low concentrations both GTPP and EGCG potentiate NGF action via induction of sublethal levels of ROS as evidenced by the blocking of these effects by antioxidants. Previous studies have shown an induction of apoptosis in tumor cells by GTPP and EGCG at high concentrations mediated by ROS generated by autooxidation of these agents (Yang et al., 1998). Therefore, it is possible that low concentrations of GTPP might be inducing the production of sublethal level of ROS which then act as a second messenger. We have recently shown that sublethal levels of ROS induce neuritogenesis (Gopalakrishna et al., 2008). While the precise mechanism remains to be elucidated, our results strongly suggest a causal relationship between oxidant-induced cell signaling and GTPP potentiation of NGF-induced neuritogenesis. Previous elegant studies have demonstrated the antioxidant properties of EGCG (Mandel et al., 2005). Our suggestion of a role for reactive oxygen species in the action of GTPP and EGCG does not contradict with these previous studies as several antioxidants are also prooxidants (Halliwell 2008; Yang et al., 2004). Furthermore, sublethal oxidative stress is known to induce an antioxidant cellular response (Crawford and Davies 1994).
It has been previously reported that oxidants inactivate phosphotyrosine protein phosphatase 1B and thereby activate TrkA-associated tyrosine kinase and trigger downstream activation of ERK (Shibata et al., 2008). It is intriguing that this is in agreement with our current observations that ERK activation and gene expression are inhibited by both antioxidants and K252a.
A variety of natural products have been identified that can potentiate the action of NGF to induce neurite outgrowth in cell culture. However, pharmacokinetic data for many of these compounds has not been established. Moreover, whether these agents can cross the blood-brain barrier is not known, and the safety of these agents to humans has not been determined. Meanwhile, pharmacokinetic data on GTPP constituents are well established in humans (Lee et al., 2002), and green tea constituents such as EGCG have been shown to cross the blood-brain barrier (Suganuma et al., 1998). In addition, green tea and EGCG have been shown to be safe for humans, and clinical trials are underway examining the use of these agents in the prevention of cancer. As such, GTPP can be easily and safely evaluated in humans for promotion of recovery after neuronal injuries as well as for the reversal of age-related loss of neuronal plasticity.
ACKNOWLEDGMENTS
We thank Ms. Kelley Mowatt, Mr. Harry Mai, and Mr. Javid Pour-Ghasemi for their excellent technical assistance.
Contract grant sponsor: National Institutes of Health Grant; Contract grant number: NS 046538 (to T. H. M.)
Abbreviations used
- NGF
nerve growth factor
- GTPP
green tea polyphenols
- EGCG
(−)-epigallocatechin gallate
- ECG
(−)-epicatechin-3-gallate
- EGC
(−)-epigallocatechin
- EC
(−)-epicatechin
- ERK
extracellular signal regulated kinase
- MEK
mitogen-activated protein kinase kinase
- NF-L
neurofilament-L
- L-NAC
N-acetyl-L-cysteine
- D-NAC
N-acetyl-D-cysteine
- ROS
reactive oxygen species
- PEG
polyethylene glycol
REFERENCES
- Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev Res. 2009;2:514–517. doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Yamazaki RS. Advances and challenges in the prevention and treatment of Alzheimer's disease. Pharm Res. 1998;15:386–398. doi: 10.1023/a:1011963929012. [DOI] [PubMed] [Google Scholar]
- Burry RW, Perrone-Bizzozero NI. Nerve growth factor stimulates GAP-43 expression in PC12 cell clones independently of neurite outgrowth. J Neurosci Res. 1993;36:241–251. doi: 10.1002/jnr.490360302. [DOI] [PubMed] [Google Scholar]
- Crawford DR, Davies KJ. Adaptive response and oxidative stress. Environ Health Perspect. 1994;10:25–28. doi: 10.1289/ehp.94102s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R, Gundimeda U, Schiffman JE, McNeill TH. A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J Biol Chem. 2008;283:14430–14444. doi: 10.1074/jbc.M801519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xu J, Li Y, Watanabe R, Oshima Y, Yamakuni T, Ohizumi Y. Iridoids and sesquiterpenoids with NGF-potentiating activity from the rhizomes and roots of Valeriana fauriei. Chem Pharm Bull (Tokyo) 2006;54:123–125. doi: 10.1248/cpb.54.123. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476:107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Hao J, Ebendal T, Xu X, Wiesenfeld-Hallin Z, Eriksdotter Jonhagen M. Intracerebroventricular infusion of nerve growth factor induces pain-like response in rats. Neurosci Lett. 2000;286:208–212. doi: 10.1016/s0304-3940(00)01107-1. [DOI] [PubMed] [Google Scholar]
- Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. TRK recepors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Hur JY, Lee P, Kim H, Kang I, Lee KR, Kim SY. (−)-3,5-Dicaffeoyl-muco-quinic acid isolated from Aster scaber contributes to the differentiation of PC12 cells: through tyrosine kinase cascade signaling. Biochem Biophys Res Commun. 2004;313:948–953. doi: 10.1016/j.bbrc.2003.11.178. [DOI] [PubMed] [Google Scholar]
- Ina A, Hayashi K-I, Nozaki H, Kamei Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int J Dev Neurosci. 2007;25:63–68. doi: 10.1016/j.ijdevneu.2006.09.323. [DOI] [PubMed] [Google Scholar]
- Jonhagen ME. Nerve growth factor treatment in dementia. Alzheimer Dis Assoc Disord. 2000;14:S31–S38. doi: 10.1097/00002093-200000001-00006. [DOI] [PubMed] [Google Scholar]
- Kano Y, Horie N, Doi S, Aramaki F, Maeda H, Hiragami F, Kawamura K, Motoda H, Koike Y, Akiyama J, Eguchi S, Hashimoto K. Artepillin C derived from propolis induces neurite outgrowth in PC12m3 cells via ERK and p38 MAPK pathways. Neurochem Res. 2008;33:1795–1803. doi: 10.1007/s11064-008-9633-9. [DOI] [PubMed] [Google Scholar]
- Katoh S, Mitsui Y, Kitani K, Suzuki T. Hyperoxia induces the differentiated neuronal phenotype of PC12 cells by producing reactive oxygen species. Biochem Biophys Res Commun. 1997;241:347–351. doi: 10.1006/bbrc.1997.7514. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Li P, Yamakuni T, Matsunaga K, Kondo S, Ohizumi Y. Nardosinone enhances nerve growth factor-induced neurite outgrowth in a mitogen-activated protein kinase- and protein kinase C-dependent manner in PC12D cells. J Pharmacol Sci. 2003;93:122–125. doi: 10.1254/jphs.93.122. [DOI] [PubMed] [Google Scholar]
- Lu Q-Y, Jin Y-S, Pantuck A, Zhang Z-F, Heber D, Belldegrun A, Brooks M, Figlin R, Rao J. Green tea extract modulates actin remodeling via Rho activity in an in vitro multistep carcinogenic model. Clin Cancer Res. 2005;11:1675–1683. doi: 10.1158/1078-0432.CCR-04-1608. [DOI] [PubMed] [Google Scholar]
- Mandel SA, Avramovich-Tirosh Y, Reznichenko L, Zheng H, Weinreb O, Amit T, Youdim MBH. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. NeuroSignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- Morre DJ, Morre DM, Sun H, Cooper R, Chang J, Janle EM. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX) Pharmacol Toxicol. 2003;92:234–241. doi: 10.1034/j.1600-0773.2003.920506.x. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Park JW, Jang YH, Kim JM, Lee H, Park WK, Lim MB, Chu YK, Lo EH, Lee SR. Green tea polyphenol (−)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J Neurosci Res. 2009;87:567–575. doi: 10.1002/jnr.21847. [DOI] [PubMed] [Google Scholar]
- Perron JC, Bixby JL. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol Cell Neurosci. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- Qi J, Han C, Sasayama Y, Nakahara H, Shibata T, Uchida K, Ojika M. Granulatoside A, a starfish steroid glycoside, enhances PC12 cell neuritogenesis induced by nerve growth factor through an activation of MAP kinase. ChemMedChem. 2006;1:1351–1354. doi: 10.1002/cmdc.200600190. [DOI] [PubMed] [Google Scholar]
- Reznichenko L, Amit T, Youdim MBH, Mandel S. Green tea polyphenol (−)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J Neurochem. 2005;93:1157–1167. doi: 10.1111/j.1471-4159.2005.03085.x. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Vanhnasy J, Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem. 2004;90:1144–1155. doi: 10.1111/j.1471-4159.2004.02563.x. [DOI] [PubMed] [Google Scholar]
- Sang S, Yang I, Buckley B, Ho C-T, Yang CS. Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (−)-epigallocatechin-3-gallate: studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radical Biol Med. 2007;43:362–371. doi: 10.1016/j.freeradbiomed.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Nakahara H, Kita N, Matsubara Y, Han C, Morimitsu Y, Iwamoto N, Kumagai Y, Nishida M, Kurose H, Aoki N, Ojika M, Uchida K. A food-derived synergist of NGF signaling: identification of protein tyrosine phosphatase 1B as a key regulator of NGF receptor-initiated signal transduction. J Neurochem. 2008;107:1248–1260. doi: 10.1111/j.1471-4159.2008.05686.x. [DOI] [PubMed] [Google Scholar]
- Shurygin AY, Viktorov IV, Ignatova EA, Skorokhod NS, Abramova NO, Malysh OS. Stimulatory effect of green tea extract on the growth of neurites in the rat spinal ganglion culture. Bull Exptl Biol Med. 2004;138:262–263. doi: 10.1007/s10517-005-0016-9. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (--)-epigallocatechin gallate with (--)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nature Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Thyagarajan A, Strong MJ, Szaro BG. Post-transcriptional control of neurofilaments in development and disease. Exp Cell Res. 2007;313:2088–2097. doi: 10.1016/j.yexcr.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Tohda C, Kuboyama T, Komatsu K. Search for natural products related to regeneration of the neuronal network. NeuroSignals. 2005;14:34–45. doi: 10.1159/000085384. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Winkler J, Ramirez GA, Thal LJ, Waite JJ. Nerve growth factor (NGF) augments cortical and hippocampal cholinergic functioning after p75NGF receptor-mediated deafferentation but impairs inhibitory avoidance and induces fear-related behaviors. J Neurosci. 2000;20:834–844. doi: 10.1523/JNEUROSCI.20-02-00834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Hong J, Hou Z, Sang S. Green tea polyphenols: antioxidative and prooxidative effects. J Nutr. 2004;134:3181S. doi: 10.1093/jn/134.11.3181S. [DOI] [PubMed] [Google Scholar]
- Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5:1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]