Human plasminogen (Plg) is a 791 amino acid single-chain protein zymogen, which is activated to the two-chain serine protease, plasmin (Plm), by mammalian activators, e.g., tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA), as well as bacterial activators, e.g., streptokinase (SK), staphylokinase (Sak) [1], and a surface protease (Pla) from Yersinia pestis [2]. Activation occurs as a result of cleavage of a single peptide bond at Arg561-Val562, yielding a heavy-chain linked by two disulfide bonds to a light-chain. The former region possesses 5 kringle domains that are responsible for binding of Plg/Plm to effector molecules, and the latter polypeptide chain contains the serine protease catalytic triad, His603, Asp646, and Ser741 (human Plg numbering). Plm is the primary fibrinolytic enzyme, functioning in maintaining vascular patency through degradation of fibrin-rich thrombi, and in clearing extravascular fibrin, e.g., as occurs in ligneous conjunctivitis, in humans and in mice [3, 4]. While Plm directly degrades fibrin and matrix proteins, it also catalyzes activation of other zymogens, e.g., pro-matrix metalloproteases [5, 6], latent forms of growth factors [7, 8], and complement proenzymes [9], thus functioning as a major pathophysiological extracellular protease.
A number of receptors for Plg/Plm are present on mammalian normal and tumor cells [10, 11], as well as on bacterial cells [12, 13]. Several candidate receptors have been identified to which Plg/Plm binds utilizing the lysine binding sites (LBS) present in Plg/Plm kringles 1, 2, 4, and 5. Many Plg/Plm receptors, e.g., α-enolase [14] and annexin II [15], thus possess COOH-terminal lysine residues. Plg/Plm interactions with receptors have also been shown to occur via arrangements of internal side chains that are isosteric with lysine, an example of which is the binding of human Plg to the group A streptococcal virulence protein, PAM [16, 17]. Plg/Plm-cellular receptor interactions function to facilitate the assembly of the fibrinolytic machinery on cell surfaces. This allows for efficient generation of cell-associated Plm activity, which can regulate cell migration through degrading protein barriers, a function important for a number of pathophysiological processes, such as angiogenesis associated with tumor growth and dissemination [18, 19].
Mice with a targeted total deficiency of Plg have been generated in two laboratories [20, 21]. These mice are viable and survive to adulthood, but some of the spontaneous phenotypes that arise include diminished growth rates, fibrin deposition in a number of organs, gastrointestinal ulcerations, and rectal prolapse [21]. Since the generation of these mice, a number of challenge models have been developed to determine the importance of Plg in regulating normal and pathological physiologies, and studies have been published investigating a Plg deficiency in models of vascular injury and repair, arthritis, glomerulonephritis, pulmonary fibrosis, inflammatory cell recruitment, cancer, wound healing, and neurological-related processes (reviewed in [22]). Most in vivo investigations attempting to distinguish between the fibrinolytic activity of Plm and its other potential proteolytic and nonproteolytic functions have relied on double deletions of Plg and fibrinogen [23]. While a very valuable approach, it nonetheless complicates the pathophysiology of the animal because of the lack of two proteins with other functions. In an attempt to circumvent some of these issues, and to allow definition of plasminogen-related functions due exclusively to Plm protease activity, we generated mice in which the murine Plm active site residue, Ser743 (identical to Ser741 in human Plg), has been mutated to Ala. In Plg/fibrinogen pleiotropy studies, this mouse line allows the Plg and fibrinogen genes to remain, while at the same time, eliminating any possible proteolytic activity of Plm. Initial phenotypes of this new mouse line are reported in this communication.
For the generation of these mice, a plasmid (pPE7neoW-F2LF), that contains a single loxP site, two flippase recombination target (FRT) sites, and a neomycin resistance (NEO) cassette (from Dr. K. Yusa, Osaka University, Osaka, Japan), was modified to provide a targeting vector (TV) backbone (pPE7neoW-F2L2) in which the NEO cassette was flanked by two loxP sites and two FRT sites.
A C57Bl/6 mouse BAC clone containing the Plg locus (RPRI 23-218G19; BACPAC Resources Center, Children’s Hospital Oakland Research Institute) was purified and electroporated into a modified E . coli strain (SW106) that is capable of temperature-inducible homologous recombination and arabinose-inducible expression of Cre recombinase [24]. The PlgS743A mutation was then introduced in Plg exon 19 of BAC Plg in SW106, using overlapping PCR along with a selection-counterselection strategy with plasmid pRpsL-Neo (GeneBridges, Heidelberg, Germany) to select correctly targeted cells containing PlgS743A (218G19-PlgS743A). Following this, the loxP-FRT-NEO-FRT-loxP cassette from pPE7neoW-F2L2 was inserted between the 3’ end of Plg exon 19 and the 3’ end of Scl22a3 exon 11 in the BAC DNA from the cells containing 218G19-PlgS743A. Correctly targeted cells containing 218G19-PlgS743A-NEO (RPR-218G19-PlgS743A-NEO) were then selected. For the final TV, the modified Plg was retrieved from RPR-218G19-PlgS743A-NEO and used to construct the plasmid pMCS-DTA-Plg, containing Plg exon 17 and Scl22a3 exon 10 flanking a Spe1 site. This latter restriction site was used to insert the region from exon 18 of Plg to exon 10 of Scl22a3 of 218G19-PlgS743A-NEO into pMCS-DTA-Plg, yielding the final TV for PlgS743A, which contained the negative autotoxic Diphtheria toxin A (DTA) gene [25] upstream of Plg exon 17 (Figure 1a). This plasmid was then electroporated into C57Bl/6 embryonic stem (ES) cells. Those ES cells surviving the negative selection were positively selected by kanamycin and ampicillin and screened by PCR for proper recombination (Fig. 1b). Final selection was made after sequencing of the lox and FRT 5’ and 3’ flanks. Next, injections of the recombined ES cells into Balb/c blastocysts were performed. Chimeric males were identified and mated with female C57Bl/6 mice. The resulting F1 offspring were tested for proper germline transmission by PCR and sequence analyses.
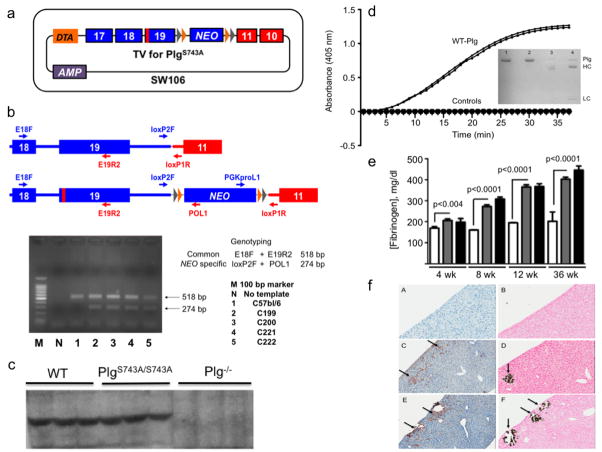
Figure 1.
Generation and characterization of mice expressing an inactivating Plm active site mutation. (a). The final targeting vector for the PlgS743A mutation. Plg exons are in blue and Slc22a3 in red. The red vertical bar in Plg exon 19 is the location of the S743A mutation. The DTA autotoxic gene is present upstream of exon 17 such that undesired recombinants that possessed DTA would not survive. Lox (gray) and FRT (orange) sites flank the NEO gene in the 3’ UTR region of Plg. Positive selection of ES cells containing the NEO gene was made via kanamycin/ampicillin resistance. (b). PCR- and sequence-based identification of ES cells carrying the mutated allele. A common forward primer in Plg exon 18 (E18F) and a reverse primer in Plg exon 19 (E19R2) yields an amplicon of 518 bp for both WT Plg and PlgS743A. This amplified region was sequenced to confirm the S743A mutation. The primers loxP2F in the 3’ region of Plg and POL1 in the NEO gene provide a 274 bp amplicon only in the case of PlgS743A, and was sequenced to confirm the integrity of the 5’ lox and FRT sites. The amplicon generated by primers PGKproL1 and loxP1R was sequenced to confirm the identity of the 3’ FRT and lox sites. (c). Western blot analysis of plasmas from WT, PlgS743A/S743A, and Plg−/− mice using anti-mouse Plg chemiluminescence detection. (d). Activation of plasma Plg by uPA. Purified WT and PlgS743A/S743A (20 μg/ml) were incubated with 0.25 mM S2251 at 37°C. The activation of Plg was accelerated with 100 IU (relative to the WHO International Standard of high-molecular weight human uPA)/ml of recombinant high-molecular weight mouse uPA. Plm generation was continually monitored at 405 nm through hydrolysis of S2251 with liberation of p-nitroanilide. Control reactions, which are indistinguishable from each other in the Figure panel, consisted of WT Plg and PlgS743A/S743A without uPA, uPA without Plg, and S2251 without proteins. Plm activity was only detected in the duplicate samples of WT Plg with uPA. Insets are reduced SDS gels of purified plasma WT Plg (1) and PlgS743A/S743A (2), and WT Plg activated with uPA(3) and PlgS743A/S743A activated with uPA (4). Plm heavy (HC) and light (LC) chains are noted upon uPA treatment of WT Plg and PlgS743A/S743A in lanes 3 and 4. However, since active Plm is formed upon WT Plg activation, a doublet heavy chain consisting of Glu1-HC and Lys78-HC of Plm is seen. Active Plm is not formed upon activation of PlgS743A/S743A and only the Glu1-HC of Plm is observed. (e). Fibrinogen levels in resting WT and PlgS743A/S743A mice between 4–36 wk of age. The white, gray, and black bars represent WT Pg, PlgS743A/S743A, and Plm−/−mice, respectively at the various ages. N = 6–10 mice or each genotype at each age. (f). Histological stains of liver slices (4 μm) from WT (A, B). Plg−/− (C, D), and PlgS743A/S743A (E, F) mice for anti-fibrin(ogen) (A, C, E) immunostaining and von Kossa silver staining for calcium (B, D, F), scanned at 20X. The arrows indicate examples of the positive areas of the slides.
F1 mice were then bred with mice expressing flippase, Tg-CAG_FLPe37, in order to remove the NEO cassette. Genomic DNA was obtained from tail snips of WT and PlgS743A mice, and exon 19 from both lines (N = 4 for each) was amplified with specific 5’ and 3’ primers and sequenced. The results confirmed that Ser743 (AGT) was mutated to Ala743 (GCC) in the genomic DNA. Mice with a double allele of this mutation expressed similar levels of Plg in plasma as WT mice (Fig. 1c). Plg was purified from the pooled plasmas of 12–15 WT and PlgS743A/S743A mice by affinity chromatography on Sepharose-Lysine. Approximately 1 mg of Plg was obtained from ~8 ml of plasma from each line, further confirming the nearly equal expression levels of WT Plg and PlgS743A/S743A. The Plg samples were activated by uPA. Whereas WT Plg provided two-chain Plm with amidolytic activity toward the Plm chromogenic substrate, S2251, PlgS743A/S743A displayed no activity toward S2251, despite formation of two-chain Plm (Figure 1d). This latter result is consistent with previous in vitro studies, wherein the active site Ser of recombinant human Plg was mutated to Ala [26] or Cys [27], yielding an inactive two-chain Plm with all Plg activators examined. These results demonstrate that the genomic DNA was successfully modified to express PlgS743A, that mice with the double allele for this mutation expressed PlgS743A/S743A equal to Plg expression in WT mice, that the PlgS743A/S743A was activated to its two-chain equivalent Plm, and that the PlmS743A/S743A was inactive.
The PTs (10.9 ± 0.3 sec, n=6, for Plg−/− mice; 10.6 ± 0.8 sec, n=3, for PlgS743A/S743A mice; and 11.6 ± 0.7, n=4, for WT mice), and aPTTs (35 ± 4 sec, n=6, for Plg−/− mice; 42 ± 3 sec, n=5, for PlgS743A/S743A mice; and 34 ± 1 sec, n=4, for WT mice), were similar for the three mouse lines. However, resting plasma fibrinogen levels were higher in Plg743A/743A mice at all ages between 4–36 wk, and the differences in plasma fibrinogen concentrations between the WT and Plg743A/743A mice became larger as the mice aged. This effect closely followed that of Pg−/− mice (Fig. 1e) and likely is an acute phase response consistent with increased spontaneous disease progression in the absence of Plm, along with the inability of Plg−/− and Plg743A/743A mice to clear fibrin. In this regard, in both the PlgS743A/S743A and Plg−/− mice, histologically-evident (N = 4) focal areas of fibrin deposits and microcalcifications are observed in the liver (Fig. 1f). Also noted in both mutated lines is focal ulceration, inflammation, and fibrin deposition at the ano-rectal junction (not shown), as well as several enlarged lymph nodes proximal to the pancreas. The WT mice do not present these pathologies, and appear normal microscopically.
As to additional spontaneous abnormalities, at 6 wk, PlgS743A/S743A mice were growth retarded (18.8 ± 0.5 g, n=4), relative to WT mice (20.8 ± 0.5 g, n=6, p=0.03) and developed rectal prolapse during a time frame very similar to that experienced by Plg−/−mice.
The results from this study demonstrate that an inactivating mutation of the latent active site serine of Plm in mice recapitulates many of the spontaneous phenotypes observed in Plg−/− mice, suggesting that plasminolytic activity is critical for normal maintenance of hemostasis in mice. This newly developed PlgS743A/S743A mouse line is important to studies aimed at selectively eliminating protease activity of Plm, while maintaining binding of effector molecules to Plg and Plm via their intact kringle domains.
Acknowledgments
The authors wish to thank Ms. Mayra Sandoval-Cooper for assistance with histology and Ms. Deborah L. Donahue for performing animal surgeries. This study was supported by grants HL013423 and HL073750 from the NIH (to FJC).
References
- 1.Castellino FJ, Ploplis VA. Plasminogen Structure, activation, and regulation. Kluwer Academic/Plenum Publishers; 2003. Human plasminogen: structure, activation, and function; pp. 3–17. [Google Scholar]
- 2.Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, Goguen JD. A surface protease and the invasive character of plague. Science. 1992;258:1004–7. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 3.Schott D, Dempfle CE, Beck P, Liermann A, Mohr-Pennert A, Goldner M, Mehlem P, Azuma H, Schuster V, Mingers AM, Schwarz HP, Kramer MD. Therapy with a purified plasminogen concentrate in an infant with ligneous conjunctivitis and homozygous plasminogen deficiency. N Engl J Med. 1998;339:1679–86. doi: 10.1056/NEJM199812033392305. [DOI] [PubMed] [Google Scholar]
- 4.Drew AF, Kaufman AH, Kombrinck KW, Danton MJS, Daugherty CC, Degen JL, Bugge TH. Ligneous conjunctivitis in plasminogen-deficient mice. Blood. 1998;91:1616–24. [PubMed] [Google Scholar]
- 5.Atkinson SJ, Ward RV, Reynolds JJ, Murphy G. Cell-mediated degradation of type IV collagen and gelatin films is dependent on the activation of matrix metalloproteinases. Biochem J. 1992;288 ( Pt 2):605–11. doi: 10.1042/bj2880605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy G, Atkinson S, Ward R, Gavrilovic J, Reynolds JJ. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann NY Acad Sci. 1992;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PG, Andress DL. Plasmin degradation of insulin-like growth factor-binding protein-5 (IGFBP-5): regulation by IGFBP-5-(201–218) Am J Physiol. 1997;273:E996–1004. doi: 10.1152/ajpendo.1997.273.5.E996. [DOI] [PubMed] [Google Scholar]
- 8.Rifkin DB, Mazzieri R, Munger JS, Noguera I, Sung J. Proteolytic control of growth factor availability. APMIS. 1999;107:80–5. doi: 10.1111/j.1699-0463.1999.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 9.Heinz HP, Loos M. Activation of the first component of complement, C1: comparison of the effect of sixteen different enzymes on serum C1. Immunobiology. 1983;165:175–85. doi: 10.1016/S0171-2985(83)80058-8. [DOI] [PubMed] [Google Scholar]
- 10.Miles LA, Hawley SB, Baik N, Andronicos NM, Castellino FJ, Parmer RJ. Plasminogen receptors: the sine qua non of cell surface plasminogen activation. Front Biosci. 2005;10:1754–62. doi: 10.2741/1658. [DOI] [PubMed] [Google Scholar]
- 11.Ranson M, Andronicos NM, O'Mullane MJ, Baker MS. Increased plasminogen binding is associated with metastatic breast cancer cells: differential expression of plasminogen binding proteins. Br J Cancer. 1998;77:1586–97. doi: 10.1038/bjc.1998.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullberg M, Kronvall G, Wiman B. New receptor for human plasminogen on gram positive cocci. APMIS. 1989;97:996–1002. doi: 10.1111/j.1699-0463.1989.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 13.Ullberg M, Kronvall G, Karlsson I, Wiman B. Receptors for human plasminogen on gram-negative bacteria. Infect Immun. 1990;58:21–5. doi: 10.1128/iai.58.1.21-25.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: Identification of α-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–91. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 15.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen tissue plasminogen activator. 1. Identity with annexin II. J Biol Chem. 1994;269:21191–7. [PubMed] [Google Scholar]
- 16.Wistedt AC, Kotarsky H, Marti D, Ringdahl U, Castellino FJ, Schaller J, Sjobring U. Kringle 2 mediates high affinity binding of plasminogen to an internal sequence in streptococcal surface protein PAM. J Biol Chem. 1998;273:24420–4. doi: 10.1074/jbc.273.38.24420. [DOI] [PubMed] [Google Scholar]
- 17.Rios-Steiner JL, Schenone M, Mochalkin I, Tulinsky A, Castellino FJ. Structure and binding determinants of the recombinant kringle-2 domain of human plasminogen to an internal peptide from a group A Streptococcal surface protein. J Mol Biol. 2001;308:705–19. doi: 10.1006/jmbi.2001.4646. [DOI] [PubMed] [Google Scholar]
- 18.Bugge TH, Lund LR, Kombrinck KK, Nielsen BS, Holmback K, Drew AF, Flick MJ, Witte DP, Dano K, Degen JL. Reduced metastasis of Polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene. 1998;16:3097–104. doi: 10.1038/sj.onc.1201869. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 20.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Gene Develop. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 21.Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–93. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 22.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–54. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 23.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–19. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 24.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagawa Y, Kobayashi T, Ohnishi M, Kobayashi T, Tamura S, Tsuzuki T, Sanbo M, Yagi T, Tashiro F, Miyazaki J. Enrichment and efficient screening of ES cells containing a targeted mutation: the use of DT-A gene with the polyadenylation signal as a negative selection maker. Transgenic Res. 1999;8:215–21. doi: 10.1023/a:1008914020843. [DOI] [PubMed] [Google Scholar]
- 26.Lijnen HR, Van Hoef B, De Coek F, Okada K, ueshima S, Matsuo O, Collen D. On the mechanism of fibrin-specific plasminogen activation by staphylokinase. J Biol Chem. 1991;266:11826–32. [PubMed] [Google Scholar]
- 27.Horrevoets AJG, Pannekoek H, Nesheim ME. Production and characterization of recombinant human plasminogen(S741C-Fluorescein). A novel approach to study zymogen activation without generation of active protease. J Biol Chem. 1997;272:2176–82. doi: 10.1074/jbc.272.4.2176. [DOI] [PubMed] [Google Scholar]