Abstract
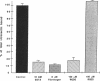
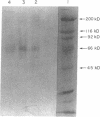
Vitronectin (Vn) is a multifunctional 75-kD glycoprotein that is present in plasma and the extracellular matrix. Vn functions as a complement regulatory protein in plasma, and promotes the growth and attachment of cells in tissue culture. Recent cDNA cloning reveals that like other adhesive proteins, Vn contains the sequence Arg-Gly-Asp and binds to some members of the integrin class of adhesive membrane receptors. In liposomes, the platelet membrane glycoprotein complex IIb/IIIa binds Vn, as well as fibrinogen, von Willebrand factor, and fibronectin. We examined the binding of purified Vn to resting and stimulated human platelets. Vn bound to thrombin-stimulated platelets in a calcium-dependent, specific, and saturable manner with a Kd of 320 nM and 8,000 sites per platelet. Epinephrine or ADP stimulation led to specific binding with KdS of 93 and 116 nM, respectively. Binding was inhibited by the tetrapeptide Arg-Gly-Asp-Ser and by monoclonal and polyclonal antibodies to GPIIb/IIIa. Endogenous platelet Vn stores were identified in immunoblots of gel-filtered platelets and the surface expression of endogenous platelet Vn was thrombin inducible. Monoclonal as well as polyclonal antibodies to Vn inhibited platelet aggregation, suggesting that Vn plays a role in the formation of stable platelet aggregates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetscher M., Pumplin D. W., Bloch R. J. Vitronectin at sites of cell-substrate contact in cultures of rat myotubes. J Cell Biol. 1986 Aug;103(2):369–378. doi: 10.1083/jcb.103.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. W., Silnutzer J. Isolation of human serum spreading factor. J Biol Chem. 1983 Oct 25;258(20):12548–12552. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Podack E. R. Characterization of human S protein, an inhibitor of the membrane attack complex of complement. Demonstration of a free reactive thiol group. Biochemistry. 1985 Apr 23;24(9):2368–2374. doi: 10.1021/bi00330a036. [DOI] [PubMed] [Google Scholar]
- Dahlbäck K., Löfberg H., Dahlbäck B. Localization of vitronectin (S-protein of complement) in normal human skin. Acta Derm Venereol. 1986;66(6):461–467. [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K. M., Hennessy S. W., Grant G. A., Santoro S. A., Frazier W. A. A monoclonal antibody against human thrombospondin inhibits platelet aggregation. Proc Natl Acad Sci U S A. 1985 May;82(10):3472–3476. doi: 10.1073/pnas.82.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K., Hennessy S. W., Broekelmann T. J., McDonald J. A., Grant G. A., Santoro S. A., Frazier W. A. Inhibition of platelet aggregation by a monoclonal antibody against human fibronectin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3844–3848. doi: 10.1073/pnas.82.11.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K., Hennessy S. W., Broekelmann T. J., McDonald J. A., Grant G. A., Santoro S. A., Frazier W. A. Inhibition of platelet aggregation by a monoclonal antibody against human fibronectin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3844–3848. doi: 10.1073/pnas.82.11.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman R. D., Lubowsky J., Charo I., Zabinski M. P. The lumi-aggregometer: a new instrument for simultaneous measurement of secretion and aggregation by platelets. J Lab Clin Med. 1977 Jul;90(1):125–129. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Ohara S., Hawiger J. Thrombin-induced exposure and prostacyclin inhibition of the receptor for factor VIII/von Willebrand factor on human platelets. J Clin Invest. 1982 Jun;69(6):1212–1222. doi: 10.1172/JCI110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. M., Hynes R. O. Interaction of fibronectin with its receptor on platelets. Cell. 1985 Sep;42(2):439–448. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Walz D. A., Aiken M., Starr-Spires L., Ogilvie M. L. Antibodies against a 23Kd heparin binding fragment of thrombospondin inhibit platelet aggregation. Biochem Biophys Res Commun. 1984 Oct 15;124(1):290–295. doi: 10.1016/0006-291x(84)90950-1. [DOI] [PubMed] [Google Scholar]
- George J. N., Nurden A. T., Phillips D. R. Molecular defects in interactions of platelets with the vessel wall. N Engl J Med. 1984 Oct 25;311(17):1084–1098. doi: 10.1056/NEJM198410253111705. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J., Ryckwaert J. J., Pierschbacher M., Pytela R., Ruoslahti E., Plow E. F. Immunochemical and amino-terminal sequence comparison of two cytoadhesins indicates they contain similar or identical beta subunits and distinct alpha subunits. J Biol Chem. 1987 Apr 25;262(12):5437–5440. [PubMed] [Google Scholar]
- Ginsberg M. H., Wencel J. D., White J. G., Plow E. F. Binding of fibronectin to alpha-granule-deficient platelets. J Cell Biol. 1983 Aug;97(2):571–573. doi: 10.1083/jcb.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., Coller B. S. Fibrinogen competes with von Willebrand factor for binding to the glycoprotein IIb/IIIa complex when platelets are stimulated with thrombin. Blood. 1984 Oct;64(4):797–800. [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ohgren Y., Ruoslahti E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ill C. R., Ruoslahti E. Association of thrombin-antithrombin III complex with vitronectin in serum. J Biol Chem. 1985 Dec 15;260(29):15610–15615. [PubMed] [Google Scholar]
- Jenne D., Hugo F., Bhakdi S. Interaction of complement S-protein with thrombin-antithrombin complexes: a role for the S-protein in haemostasis. Thromb Res. 1985 May 15;38(4):401–412. doi: 10.1016/0049-3848(85)90138-0. [DOI] [PubMed] [Google Scholar]
- Jenne D., Stanley K. K. Molecular cloning of S-protein, a link between complement, coagulation and cell-substrate adhesion. EMBO J. 1985 Dec 1;4(12):3153–3157. doi: 10.1002/j.1460-2075.1985.tb04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. C., Plow E. F., D'Souza S. E., Cheresh D. A., Frelinger A. L., 3rd, Ginsberg M. H. Isolation and characterization of a platelet membrane protein related to the vitronectin receptor. J Biol Chem. 1989 Mar 5;264(7):3742–3749. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989 Nov 1;74(6):2022–2027. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L. Role of thrombospondin in platelet aggregation. J Clin Invest. 1984 Nov;74(5):1764–1772. doi: 10.1172/JCI111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B. Fibrinogen receptor exposure and aggregation of human blood platelets produced by ADP and chilling. Blood. 1981 Apr;57(4):663–670. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Podack E. R., Dahlbäck B., Griffin J. H. Interaction of S-protein of complement with thrombin and antithrombin III during coagulation. Protection of thrombin by S-protein from antithrombin III inactivation. J Biol Chem. 1986 Jun 5;261(16):7387–7392. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977 Dec;119(6):2024–2029. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Rao G. H., Johnson G. J., White J. G. Influence of epinephrine on the aggregation response of aspirin-treated platelets. Prostaglandins Med. 1980 Jul;5(1):45–58. doi: 10.1016/0161-4630(80)90090-7. [DOI] [PubMed] [Google Scholar]
- Silver M. J., Smith J. B., Ingerman C., Kocsis J. J. Arachidonic acid-induced human platelet aggregation and prostaglandin formation. Prostaglandins. 1973 Dec;4(6):863–875. doi: 10.1016/0090-6980(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Thiagarajan P., Kelly K. L. Exposure of binding sites for vitronectin on platelets following stimulation. J Biol Chem. 1988 Feb 25;263(6):3035–3038. [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus P. W. Inhibition of human platelet aggregation by monovalent antifibrinogen antibody fragments. J Clin Invest. 1975 Jun;55(6):1259–1268. doi: 10.1172/JCI108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer T., Sims P. J. Effect of complement proteins C5b-9 on blood platelets. Evidence for reversible depolarization of membrane potential. J Biol Chem. 1985 Jul 5;260(13):8014–8019. [PubMed] [Google Scholar]