Abstract
The externalizing dimension is viewed as a broad dispositional factor underlying risk for numerous disinhibitory disorders. Prior work has documented deficits in event-related brain potential (ERP) responses in individuals prone to externalizing problems. Here, we constructed a direct physiological index of externalizing vulnerability from three ERP indicators and evaluated its validity in relation to criterion measures in two distinct domains: psychometric and physiological. The index was derived from three ERP measures that covaried in their relations with externalizing proneness the error-related negativity and two variants of the P3. Scores on this ERP composite predicted psychometric criterion variables and accounted for externalizing-related variance in P3 response from a separate task. These findings illustrate how a diagnostic construct can be operationalized as a composite (multivariate) psychophysiological variable (phenotype).
Keywords: externalizing, disinhibition, feedback-related negativity, P300, event-related potential
Experts in the mental health field have called for systematic efforts to integrate neurobiological concepts and findings into systems for diagnosing mental disorders (Hyman, 2007), toward the aim of enhancing the effectiveness of assessment, prevention, and treatment of such disorders (Insel & Scolnick, 2006). One effort in this direction entails developing reliable neurobiological indicators (biomarkers) of psychopathology constructs. Most research of this kind has focused on identifying individual indicators of specific disorders. However, little work has been done to evaluate patterns of relations among varying physiological indicators of differing disorders. Should separate physiological (e.g., event-related potential) indicators demonstrate convergence indicative of a common neural substrate, their joint consideration may be important for identifying individuals at risk prior to the emergence of active pathology and for elucidating the neurobehavioral mechanisms underlying such disorders.
With this prospect in mind, the current study examined convergence among multiple psychophysiologic indicators of general proneness to externalizing disorders a spectrum of psychopathology marked by deficient impulse control (Krueger et al., 2002). Specifically, a common factor was extracted reflecting the shared variance among differing brain response indicators of externalizing proneness, and the validity of this physiologically-based composite for predicting external criterion measures of interest was evaluated. A secondary aim was to illustrate a general research strategy for developing stable neurobiological indices of individual difference constructs relevant to psychopathology.
The Externalizing Construct
The construct of externalizing has been proposed as a common dispositional factor underlying the spectrum of disorders marked by deficient impulse control (aka “disinihibition”; Gorenstein & Newman, 1980; Sher & Trull, 1994) including child and adult antisocial deviance and substance-related disorders. Evidence for the existence of this broad factor emerged out of structural analyses of diagnostic data in adult epidemiologic samples. For example, Krueger (1999) reported that the covariance among various DSM-defined disorders could be accounted for by two broad factors: internalizing, encompassing mood and anxiety disorders, and externalizing, encompassing antisocial personality disorder and alcohol and drug dependence. These broad factors can be viewed as reflecting general dispositional vulnerabilities to disorders of each type (Krueger et al., 2002; Mineka, Watson, & Clark, 1998). Consistent with this perspective, available data indicate that scores on the general externalizing factor are highly (> 80%) heritable (Kendler, Prescott, Myers, & Neale, 2003; Krueger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000).
Personality traits in the domains of impulsivity, aggression, and sensation seeking have also been identified as indicators of the broad externalizing factor (Krueger, McGue, & Iacono, 2001; Krueger, Markon, Patrick, Benning, & Kramer, 2007). The implication is that the externalizing construct encompasses normal-range personality traits as well as pathological behavioral tendencies along a common vulnerability continuum. This conceptualization inspired the development of the Externalizing Spectrum Inventory (ESI; Krueger et al., 2007), a 415-item self-report questionnaire that indexes externalizing vulnerability comprehensively in terms of scores on 23 unidimensional subscales. The ESI was developed using factor analysis and item-response theory techniques to optimize the psychometric properties and structural coherence of its subscales. The subscales of the ESI index a range of distinctive but interrelated trait-dispositional and behavioral constructs in domains of impulsiveness, sensation seeking, irresponsibility, blame externalization, dishonesty, aggression, and substance abuse.
Psychophysiological Indicators of Externalizing Proneness
As noted, scores on the broad externalizing factor appear highly heritable more heritable in fact than individual disorders with which it is associated (Krueger et al., 2002) making it a compelling target for studies aimed at identifying neurobiological mechanisms of impulse control problems. The most extensively documented neurobiological indicator of externalizing proneness is the P300/P3, a positive-going event-related potential (ERP), maximal at parietal scalp sites, that occurs following the presentation of attended stimuli. Reductions in P3 amplitude have been documented in relation to disorders including alcohol dependence, drug dependence, conduct disorder, adult antisocial personality, and attention-deficit hyperactivity disorder (e.g., Bauer & Hesselbrock, 1999; Biggins, MacKay, Clark, & Fein, 1997; Costa et al., 2000; Kim, Kim, & Kwon, 2001; Porjesz, Begleiter, & Garozzo, 1980), and recent studies have linked the P3 to the broad externalizing factor that these disorders share (Patrick et al., 2006; Venables et al., 2005). Subsequent work demonstrating that the relationship between the externalizing dimension and diminished P3 is primarily attributable to genetic influence (Hicks et al., 2007) lends supports to the idea that P3 is a biomarker of externalizing proneness.
Although multiple variants of the P3 exist, the most extensively studied has been the P3 response to target stimuli in frequent-infrequent (“oddball”) tasks, commonly termed the “P300” or “P3b.” Another is the novel P3 (“P3a”), a P3 response to unexpected novel events that exhibits a somewhat earlier latency and a more anterior scalp distribution. Other variants of the P3 occur in tasks in which the familiarity or meaningfulness of stimuli is varied. We use the term “P3” in the current paper to refer to this broad family of ERP components, which includes the P3a and P3b. Available data indicate that differing variants of the P3 overlap in terms of their underlying neural generators, with structures including the inferior parietal lobe, temporoparietal junction, anterior cingulate cortex, and prefrontal cortex (PFC) playing some role in each (see Linden, 2005). However, the relative contribution of particular brain regions to the P3 can differ as a function of stimulus and task parameters. For example, the topography of the novel-stimulus P3 (P3a) tends to be more frontocentral than that of the oddball-target P3 (P3b) and is thought to engage frontal brain regions such as lateral PFC more so than the P3b.
In addition to variants of the P3, externalizing and other constructs involving disinhibition have been linked to the response-locked ERN, a negative-going brain potential, maximal at frontocentral electrode sites, that follows performance errors in speeded response tasks (Falkenstein, Hohnsbein, & Hoormann, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). In terms of underlying neural sources, substantial evidence points to the anterior cingulate cortex (ACC; Dehaene, Posner, & Tucker, 1994), as well as the supplementary motor area, as primary sources of the ERN, with other structures including the PFC (Gehring & Knight, 2000) playing a supporting role. Reduced ERN amplitude has been documented for individuals scoring low on socialization (reflecting rebelliousness, impulsivity, and aggression; Dikman & Allen, 2000) and conscientiousness (a Big Five personality dimension reflecting tendencies toward responsibility, reliability, and dutifulness; Pailing & Segalowitz, 2004), as well as for individuals scoring highly on disinhibitory traits such as impulsiveness (Pailing, Segalowitz, Dywan, & Davies, 2002; Potts, George, Martin, & Barratt, 2006) and psychoticism (Santesso, Segalowitz, & Schmidt, 2005). Hall, Bernat, & Patrick (2007) extended this prior work by testing the hypothesis that the ERN would be related to the general externalizing factor that, as mentioned previously, reflects proneness to problems of impulse control and affiliated traits (e.g., impulsivity, aggression, and irresponsibility). Consistent with prediction, Hall et al. found that individuals high in externalizing proneness (as measured by an abbreviated version of the ESI) showed reduced amplitude of the ERN over frontocentral scalp locations where the ERN tends to be maximal.
An important question that has yet to be addressed is whether these differing ERP measures (P3, ERN) represent overlapping or unrelated indicators of externalizing proneness, and whether they index some neural process in common that accounts for their individual relations with the externalizing construct. Despite differing scalp topographies, some indirect evidence exists to link P3 and ERN responses as indicators of externalizing tendencies. As noted earlier, frontal brain regions including ACC and PFC are known to be involved in the generation of each (Dehaene, Posner, & Tucker, 1994; Dien, Spencer, & Donchin, 2003; Miltner et al., 1997; Nieuwenhuis, Aston-Jones, & Cohen, 2005), and frontal brain dysfunction has also been implicated in differing forms of disinhibitory psychopathology (Morgan & Lilienfeld, 2000; Peterson & Pihl, 1990). Based on these lines of evidence, we hypothesized, as described below, that some overlap would be evident in the bivariate relations of P3 and ERN responses with the externalizing construct.
Present Study Aims and Hypotheses
A primary aim of the current study was to evaluate the relationships among differing psychophysiological indicators of externalizing proneness using data from a preexisting sample (for prior reports of findings from this sample, see Bernat, Nelson, Steele, Gehring, & Patrick, 2009; Hall et al., 2007; Venables et al., 2005). As detailed above, externalizing proneness has been related to various ERP components in past work. However, it has not been clear whether these observed relations reflect deviations in distinctive cognitive processes associated with each component (e.g., in the case of P3, deficits in context-updating; in the case of ERN, deficits in performance monitoring), or whether amplitude reductions in these differing components reflect some more basic process (or set of processes) that spans tasks.
We addressed this question in the current study by directly examining relations between P3 and ERN responses in the current sample and evaluating the extent to which these brain response components overlap in their relations with externalizing proneness. Specifically, we evaluated whether differing ERP indicators would evidence a sufficient degree of convergence to permit a common factor to be derived reflecting their covariance. In addition, we evaluated whether the observed covariance among indicators reflected externalizing-proneness or not by examining the association of the common ERP factor with an omnibus index of externalizing (i.e., the ESI) that had evidenced associations with each individual ERP indicator. We also evaluated the validity of this shared ERP-based factor in relation to separate criterion measures of externalizing proneness from two distinct measurement domains: psychometric (self-report) assessment and physiological (ERP) measurement. We included physiological criterion variables along with more traditional diagnostic variables because we were interested in comparing predictive relations for criteria in the same domain versus a different measurement domain.
Data were available for three tasks: a flanker discrimination task, a gambling feedback task, and a visual oddball task. The following three measures from the flanker and gambling tasks were utilized as primary indicators in analyses aimed at delineating a common neurophysiological factor: P3 response to target stimuli in the flanker task, P3 response to feedback stimuli in the gambling task, and ERN response following performance errors in the flanker task. P3 responses to stimuli (target, novel) in the oddball task were reserved as criterion measures in follow-up validation analyses. Oddball P3 responses were utilized as criterion variables because extensive research documents diminished oddball task P3 as an indicator of externalizing proneness, and because these responses were measured in a separate task from the primary ERP indicators.
Our primary study hypothesis, based on extensive prior research examining P3 response to oddball task stimuli, was that P3 responses to flanker and feedback stimuli would, along with ERN response as previously reported by Hall et al. (2007), evidence significant negative relations with externalizing proneness as indexed by the ESI. Findings in line with this prediction would indicate that the P3-externalizing relationship generalizes across differing stimuli and experimental conditions. Our additional hypotheses, pertaining to coherence among ERP indices of externalizing proneness, were predicated on this primary hypothesis and thus were somewhat more tentative. First, in view of data indicating a role for anterior brain structures in the generation of both ERN and P3, we postulated some degree of overlap in the psychophysiological process(es) tapped by each individual ERP indicator. Specifically, we hypothesized that scores on the three primary ERP indicators (gambling feedback-P3, flanker target-P3, flanker response-ERN) would correlate with one another, as a function of overlap in associated processes. We posited further that variance in common among these differing electrocortical indicators would reflect, at least in part, psychophysiological processes related to externalizing proneness. Based on this presumption, we hypothesized that scores on a common ERP factor, reflecting the overlap among primary P3 and ERN indicators, would significantly predict scores on the ESI as well as scores on separate criterion measures of externalizing proneness representing psychometric and physiological assessment domains namely, scores on self-report measures of disinhibitory problems/traits, and reductions in amplitude of P3 responses measured within an oddball task. Regarding relations of the ERP-based factor with criterion measures, we expected that correlations for oddball P3 measures would exceed correlations for self-report measures as a function of same versus differing assessment domains (cf. Campbell & Fiske, 1959).
Method
Participants
Participants were undergraduates pre-selected from a larger sample of students (N = 1,637) based on their scores on the ESI. Individuals were selected to represent the full range of scores, with participants scoring in the upper and lower quartiles of the distribution of ESI scores oversampled to ensure strong representation of high and low scoring individuals. Data for the three study tasks (gambling, flanker, oddball; Bernat et al., 2009; Hall et al., 2007; Venables et al., 2005) were available for the 92 participants included in the Hall et al. (2007) ERN study. Two of these participants were dropped from the current analyses due to excessive ERP signal artifact in the oddball task, and two others were dropped due to excessive artifact in the gambling task, yielding a final N of 88 (55 female; M age = 20.47 years, SD = 2.57).
Questionnaire Measures
Externalizing Spectrum Inventory (ESI)
Participants completed an abbreviated (100-item) version of the ESI (Krueger et al., 2007), a self-report measure developed to assess a range of behavioral and personality characteristics associated with externalizing spectrum psychopathology. Higher ESI scores indicate greater externalizing tendencies. Internal consistency reliability (Cronbach’s α) for the ESI in the current sample was .95.
Participants also completed other self-report questionnaires that served as separate criterion measures of externalizing tendencies; descriptions of these measures, with α coefficients for the current sample noted in parentheses after scale abbreviations, are as follows:
Alcohol Dependence Scale (ADS; α = .88)
The ADS (Skinner & Allen, 1982) is a 29-item measure with questions related to alcohol use, abuse, and dependence. The ADS yields a total score such that higher scores indicate more extreme alcohol-related problems.
Short Drug Abuse Screening Test (SDAST; α = .77)
The SDAST (Skinner, 1982) is a 20-item questionnaire that indexes problems involving drug use, including drug abuse and dependence. High SDAST total scores indicate more severe drug-related problems.
Behavior Report on Rule-Breaking (BHR; α = .92)
The BHR is a questionnaire of adolescent and adult antisocial behaviors composed of items from several other published measures (Clark & Tifft, 1966; Nye & Short, 1957; Hindelang, Hirschi, & Weis, 1981). The measure includes 33 items about unlawful or inappropriate behavior, and each item requests a rating for both adolescence (before age 18) and adulthood (age 18 and up) behavior.
Socialization Scale (So; α = .84)
The So Scale (Gough, 1960) is a 52-item self-report measure that indexes socialization, a construct with similarities to the externalizing construct. High scores indicated higher levels of rebelliousness, aggression, and impulsivity.
Procedure
Experimental stimuli were presented centrally on a 21-in Dell high-definition CRT color monitor, using E-Prime version 1.1 software (Psychology Software Tools, Inc.). Behavioral responses were made using the PST Serial Response Box from the same company. During a single physiologic recording session, participants completed the following three tasks sequentially:
Flanker discrimination task
This task, consisting of six 100-trial blocks, was a variant of the Eriksen flanker task (Eriksen & Eriksen, 1974). As described in Hall et al. (2007), participants viewed target letter arrays (HHHHH, SSSSS, HHSHH, and SSHSS; 86% of trials) and pressed a button (left or right) to indicate the central letter (“H” or “S”) in the array. The task also included non-target stimuli (XXXXX, SSXSS, HHXHH; 14% of trails) to which no response was made. Each stimulus was presented for 150 ms, followed by a 1000 ms response window and a 1500–2500 ms (M = 2000 ms) fixation point prior to the onset of the next trial. To enhance task difficulty and increase performance errors, hand-letter assignment was reversed prior to the start of each new block of trials.
Gambling feedback task
This task, consisting of twelve 32-trial blocks, was modified from the procedure of Gehring and Willoughby (2002). On each trial, participants selected between two numeric options (5–5, 25–25, 5–25, 25–5) and then received feedback indicating whether their choice resulted in a gain or a loss of money. Outcomes were signaled by changes in the color of boxes enclosing the two numeric options: The box around the chosen option turned red or green to indicate either a win or loss, and the box enclosing the unchosen box turned red or green to indicate what the outcome would have been had the participant made the other choice. Color-outcome mapping was counterbalanced across participants. The choice stimulus remained on the screen until a selection was made, after which a blank screen appeared for 100 ms. The feedback stimulus appeared for 1000 ms, followed by a blank screen for 1500 ms preceding the onset of the next trial.
Oddball task
This task, consisting of 240 trials, was a 3-stimulus variant of the “rotated-heads” visual oddball task (Begleiter, Porjesz, Bihari, & Kissin, 1984). Task stimuli included nontarget ovals (70% of trials), target “heads,” (15% of trials) containing a nose and one ear, and (3) novel nontarget stimuli (15% of trials) consisting of pleasant, neutral, and unpleasant pictures from the International Affective Picture System (Center for Study of Emotion and Attention, 1999). Participants responded to target heads with a right or left button press to indicate the side on which the ear appeared. Stimuli appeared for 100 ms each and were separated by ITIs (with central fixation) of 4000 to 5000 ms.
Psychophysiological Data Acquisition and Reduction
EEG activity was recorded using a 64-channel Neuroscan Synamps2 amplifier system. EEG electrodes (sintered Ag-AgCl) were positioned in accordance with the International 10–20 system (Jasper, 1958) using a Quick-Cap electrode array. Impedances at all sites were below 10 kΩ. Ocular activity was recorded from above and below the left eye. EEG signals were referenced online to electrode site CPz and digitized at 1000 Hz, and then re-referenced off-line to linked mastoids and re-sampled to 128 Hz. The response-locked ERN was epoched from 1000 ms before to 1000 ms after response onset; all stimulus-locked P3 measures were epoched from 1000 ms before to 2000 ms after stimulus onset. Trial-level EEG data were corrected for ocular and movement artifacts using an algorithm developed by Semlitsch, Anderer, Schuster, and Presslich (1986), as implemented in the Neuroscan EDIT software (version 4.3). For the response-locked ERN, a 1-Hz high-pass filter was also applied to reduce the effect of slow-wave motor potentials that can contaminate response-locked signals. The stimulus- and response-locked ERPs from the flanker task (P3 and ERN, respectively) were averaged across all target stimulus trials on which a response occurred. The feedback-locked P3 (gambling task) was averaged across stimulus trials involving gain and loss outcomes.
Because the ERP components were measured from varying tasks with differing procedural parameters, measurement windows for each ERP variable were defined according to task-specific waveforms (Picton et al., 2000), resulting in variations in the time windows employed across tasks. The flanker ERN was defined as the maximum negative-voltage peak, relative to a −250 to −50 ms pre-response baseline, occurring within a window beginning with the onset of an incorrect button-press response and terminating at 125 ms post-response. (To facilitate comparisons with the P3 response variables, raw ERN scores were inverted such that higher positive values reflected larger ERN amplitudes.) P3 components were computed as maximum voltage peaks relative to a pre-stimulus baseline within designated time windows as follows: flanker P3, peak 320.31 to 500 ms post-stimulus relative to −148.44 to −7.81 ms pre-stimulus baseline; feedback P3, 296.88 to 500 ms relative to −101.56 to −7.81 ms baseline;1 and oddball target and novel P3, 250 to 562.5 ms relative to −148.44 to −7.81 ms baseline. (Note: Window onset and offset times contain decimals as they represent bins of 128 Hz re-sampled data.)
For each ERP measure, data from the frontocentral (FCz) electrode location were used in the analyses reported here in order to facilitate comparisons and because associations with externalizing scores tended to be maximal at this scalp location. In this regard, Hall et al. (2007) reported that the ERN/externalizing association was distributed frontocentrally on the scalp and focused their analyses of the ERN on electrode site FCz, where the magnitude of the correlation with externalizing scores was r = .29. Mirroring Hall et al., we operationalized the ERN in terms of (inverted) amplitude for error trials at FCz. For the P3 measures, we evaluated associations for each with externalizing at representative frontal, central, and parietal electrode sites. Consistent with the idea that externalizing tendencies entail deficits in anterior brain function (and consistent with the topography of ERN effects), we found that externalizing-related amplitude reductions for each P3 measure were more pronounced at frontocentral as compared to parietal sites. For example, the correlation between ESI-100 externalizing scores and flanker-stimulus P3 was −.37 at FCz but only −.25 at Pz; similarly, the association of feedback-stimulus P3 with externalizing was −.24 at FCz compared with −.17 at Pz. For P3 responses to target and novel stimuli in the oddball task, rs at electrode site FCz were −.31 and −.32, respectively, compared with −.11 and −.13 at Pz. Notably, these patterns contrasted with the topography of P3 response across participants in the sample as a whole, where amplitudes tended to be maximal at parietal locations as is typical of the P3 (e.g., Katayama & Polich, 1999).
Data Analyses
The analyses are described in several parts below. First, we present the three ERP-based indicators of externalizing and show correlational and exploratory factor analyses demonstrating their coherence. Second, we describe follow-up analyses demonstrating that the coherence among these ERP variables reflects a common externalizing-related process rather than some other common brain process unrelated to externalizing. Finally, we present correlations between a composite variable, derived from the three ERP measures using principal axis factor analysis,2 and an array of criterion measures (representing self-report diagnostic and physiological response domains) to examine the validity of this ERP composite in relation to other known indicators of externalizing proneness. In addition, for the physiological (oddball task P3) criterion measures, hierarchical regression analyses are presented to directly evaluate the extent to which scores on the ERP-based composite account for externalizing-related variance in these measures.
Supplemental analyses were performed to test for possible moderating effects of age and gender. Neither variable showed any evidence of a moderating effect on the relationship between externalizing proneness (indexed by scores on the ESI) and any of the physiological (ERP) measures included in the analyses. Thus, results are presented without inclusion of these demographic variables in the analysis.
Results
Constructing a Multivariate ERP-Based Index of Externalizing Proneness
Bivariate relations of individual ERP variables with externalizing tendencies
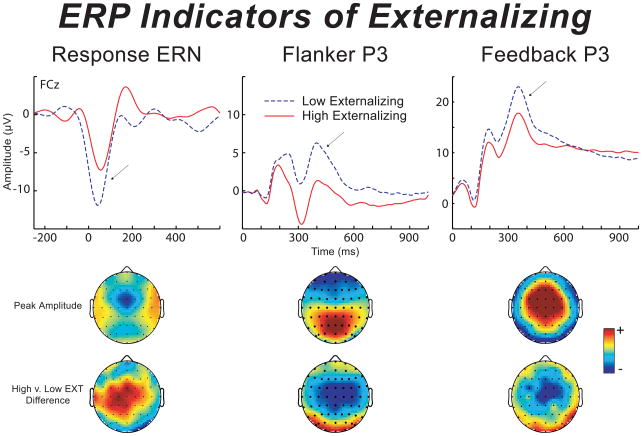
To illustrate the primary ERP response variables on which our analyses focused (flanker response ERN, flanker stimulus P3, feedback stimulus P3), Figure 1 presents waveforms for these three variables at electrode site FCz for participants high (top quartile) versus low (bottom quartile) on the ESI. As indicated in Table 1, the correlation between continuous ESI externalizing scores and (inverted) ERN amplitude in the sample as a whole (N = 88) was −.29. Mirroring findings from prior studies using conventional oddball task P3s, the flanker and feedback P3 responses also evidenced significant negative associations with externalizing scores, rs = −.37 and −.24.
Figure 1.
Average ERN (on error trials), flanker P3, and feedback P3 response waveforms for subgroups low and high in externalizing tendencies (top and bottom 25% of scorers on the ESI) at electrode site FCz. Color topographic maps below the waveform plots depict (1) the overall peak amplitude of each ERP measure (upper row of topographic plots), and (2) the relative magnitude and directionality of group differences (low minus high externalizing) across scalp sites for each ERP response measure (bottom row).
Table 1.
Correlations Among Physiological and Questionnaire Indicators of Externalizing Proneness
| Response ERN | Flanker P3 | Feedback P3 | |
|---|---|---|---|
| Flanker P3 | .27* | ||
| Feedback P3 | .24* | .26* | |
| ESI Questionnaire | −.29** | −.37** | −.24* |
p < .05;
p < .01;
p < .001
Correlations among ERP indicators and derivation of a multivariate ERP composite
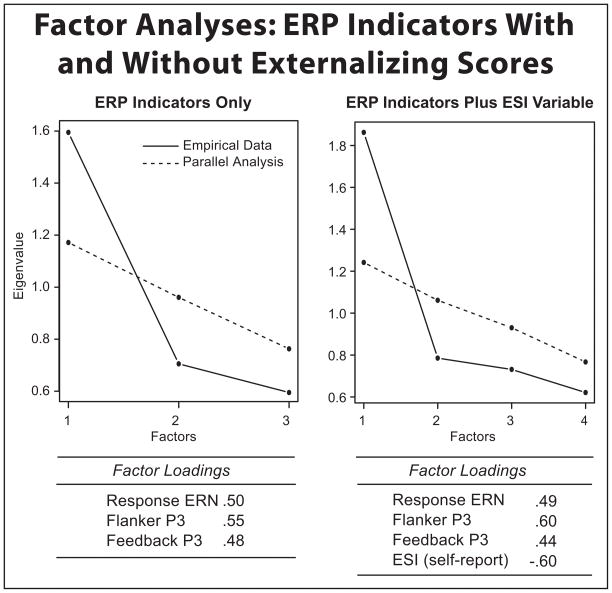
As shown in Table 1, the three primary ERP variables correlated significantly with one another (rs = −.24 to −.27). To evaluate the possibility that these measures index some process or processes in common, a principal axis exploratory factor analysis of these measures was performed. This analysis yielded evidence of a single dominant factor accounting for covariance among the three ERP indicators (Figure 2, left plot). This one-factor solution was evident both by visual inspection of the scree plot and by parallel analysis, a technique for determining the number of factors to retain by comparing the eigenvalues of the sample data with those of randomly generated data (Horn, 1965).3 Each ERP indicator loaded appreciably and to a comparable degree on the shared factor (range = 0.48 to 0.55), indicating that the three ERP variables index something in common and that each contributes similarly to the shared factor.
Figure 2.
Scree plots and variable loadings for two factor analyses. On the left side is an analysis incorporating the three primary ERP indicators of externalizing vulnerability (response ERN, flanker P3, and feedback P3). On the right is an analysis incorporating the three ERP indicators along with the self-report externalizing (ESI) variable. In each plot, actual eigenvalues (solid line) are accompanied by eigenvalues estimated from a parallel analysis (dashed line) based on 100 random samples.
Although the factor analysis indicated that these differing ERP variables index something in common, further analysis was required to determine whether this covariance reflected an externalizing-related brain process, as opposed to overlap in brain activity unrelated to externalizing proneness. To this end, a second factor analysis was performed in which scores on the self-report ESI measure were included together with scores on the three ERP indicators. The rationale was that if the ERP variables covaried due to externalizing-related variance, then a factor analysis of these variables along with scores on the ESI should yield a solution in which all four variables load appreciably on a common factor. This is indeed what was found (see Figure 2, right plot). Thus, despite the fact that one of the variables included in this analysis was from a different measurement domain (self-report) than the others (physiological), the variables appear to index something in common that relates to externalizing proneness.
Predictive Validity of the Multivariate ERP Composite
To evaluate the predictive validity of the ERP factor in relation to its individual brain response indicators, scores on the common factor derived from the three ERP measures (Figure 2, left plot) were computed using the regression method and examined as predictors of criterion measures known to be related to externalizing proneness.
Criterion measures of substance problems, antisocial behavior, and disinhibitory tendencies
The upper part of Table 2 presents correlations for the ERP-based factor and its individual indicators with scores on available self-report measures of alcohol dependence, drug abuse, antisocial behavior, and disinhibitory tendencies. Scores on the ERP-based factor were correlated with each of the self-report criterion variables in the predicted direction, with 5 of the 7 correlations achieving significance. Also notable is the fact that correlations of the ERP factor with criterion measures tended to be higher than correlations for the individual ERP indicators.
Table 2.
Individual ERP Indicators and Multivariate ERP Composite: Correlations with Externalizing-Related Criterion Variables
| Criterion Variable | N | Response ERN | Flanker P3 | Feedback P3 | Composite |
|---|---|---|---|---|---|
| Psychometric | |||||
| ESI | 88 | −.29** | −.37*** | −.24* | −.43*** |
| ADS | 87 | −.31** | −.29** | −.24* | −.40*** |
| SDAST | 86 | −.03 | −.07 | −.14 | −.11 |
| BHR | |||||
| Total | 87 | −.28** | −.29** | −.24* | −.38*** |
| Adult | 87 | −.25* | −.23* | −.24* | −.33** |
| Adolescent | 87 | −.26* | −.31** | −.19 | −.36** |
| Socialization Scale | 87 | .16 | .06 | .09 | .15 |
| Physiologic | |||||
| Oddball-Target P3 | 88 | .42*** | .58*** | .43*** | .68*** |
| Oddball-Novel P3 | 88 | .48*** | .53*** | .46*** | .69*** |
Note:
p < .05;
p < .01;
p < .001.
ESI = Externalizing Spectrum Inventory; ADS = Alcohol Dependence Scale; SDAST = Short Drug Abuse Screening Test; BHR = Behavior Report on Rule-Breaking.
Prediction of oddball task P3 amplitude
We also evaluated the ability of ERP factor scores to predict separate brain-based indices of externalizing proneness namely, P3 responses to target and novel stimuli from the oddball task measured at electrode site FCz. Oddball-target and oddball-novel P3 responses were utilized as criterion measures because they came from a separate task than the response variables that contributed to the ERP-based composite; further, oddball task P3 is well-established status as an indicator of externalizing proneness. Across participants in the current sample, oddball-target P3 and oddball-novel P3 responses were highly correlated with one another (r = .76) and showed correlations of −.31 and −.32, respectively, with ESI scores. As shown in Table 2 (lower part), scores on the common factor reflecting the overlap among primary ERP indicators (from flanker and gambling tasks) strongly predicted P3 responses to both target and novel stimuli in the oddball task. Data in the lower part of the Table also show that correlations with both oddball P3 responses tended to be stronger for the ERP composite variable than for the individual ERP indicators that went into the composite.
To quantitatively evaluate the extent to which the composite outperformed individual ERP indicators in predicting ESI externalizing scores, hierarchical regression analyses were performed in which scores on the ERP factor were entered as a predictor in step 2, following entry of one or the other oddball P3 variable in step 1. For both oddball-target and oddball-novel P3, the addition of the ERP-based factor as a predictor in the second step (following entry of oddball P3 in the first step) of the model (1) reduced the predictive (beta) coefficient for the oddball P3 variable to nonsignificance (betas in steps 1 and 2 were, respectively, oddball-target P3, Bs = −.31 and −.03, ps = .004 and .847; oddball-novel P3, Bs = −.32 and −.05, ps = .002 and .717); and (2) produced a significant increase in R2 for the overall model (for oddball-target P3, R2 increased from .31 in step 1, F(1,86) = 8.86, p = .004, to .43 in step 2, F(2,85) = 9.70, p < .001, R2 change F(1,85) = 9.65, p = .003; for oddball-novel P3, R2 increased from .32 in step 1, F(1,86) = 10.01, p = .002, to .43 in step 2, F(2,85) = 9.76, p < .001, R2 change F(1,85) = 8.63, p = .004). Thus, the ERP-based composite variable significantly outperformed individual comparison ERP variables in predicting externalizing proneness.
Discussion
Prior work has documented relations between differing indices of physiological response and externalizing proneness, a broad dispositional factor encompassing tendencies toward impulsivity, antisocial behavior, and alcohol and drug problems that has been conceptualized as reflecting a general vulnerability to problems of impulse control. In particular, amplitude reductions in oddball task P3 and response-ERN have been found in relation to externalizing tendencies. The current study provided an initial demonstration of associations with externalizing proneness for two other variants of the P3. One of these consisted of P3 response to target flanker stimuli in a procedure in which the typical phenomenon of interest is the ERN response that follows performance errors. In this procedure, the target stimulus was an array of letters, and the task involved discriminating the central letter from flanking letters to determine whether to make a left or right button response. The second variant consisted of P3 response to gain and loss feedback in a simulated gambling task in which individuals selected one of two monetary options and then processed feedback as to whether their choice resulted in a gain or a loss of money. Deficits in the amplitude of P3 response to task-relevant stimuli have been interpreted as reflecting impairment of some kind in post-perceptual processing of stimulus input across differing tasks. That these non-oddball variants of the P3 demonstrated associations with externalizing tendencies implies that the P3-externalizing relationship may generalize across a wide range of stimuli and task conditions.
Another key finding involved the topography of the externalizing-related reduction in P3 amplitude relative to the topography of the P3 component itself. As is typical of the P3 component, peak amplitude in the current study tended to be maximal at parietal electrode sites, yet the P3 amplitude reduction associated with externalizing proneness was largest at frontocentral sites. This dissociation is consistent with the notion that the externalizing-related cognitive processing deficit indexed by P3 amplitude reduction involves anterior brain structures, despite the role of more posterior structures in the generation of the P3 response overall. Given evidence (noted earlier) that the P3 reflects activity in a range of underlying brain regions including anterior as well as posterior regions, the current findings suggest that the basis of the reduction in P3 amplitude associated with externalizing proneness may lie more in anterior brain structures (e.g., ACC, PFC) that contribute to P3.
Notably, the P3 is not the only ERP component that has evidenced relations with externalizing tendencies. As demonstrated by Hall et al. (2007), the ERN response is also negatively associated with tendencies toward externalization. Unique to the current study, however, is the finding that the externalizing-related processing impairments indexed by P3 and ERN appear to be overlapping, despite theorized differences in the mechanisms that underlie ERN and P3. Specifically, ERN response correlated significantly with both feedback P3 and flanker P3 and loaded to a comparable degree with these two variants of P3 on a common factor that in turn predicted criterion measures relevant to externalizing psychopathology. The implication is that reductions in ostensibly distinct brain measures assessed in differing tasks contexts may reflect common or intersecting deficits associated with externalizing proneness.
A challenge in future research will be to identify exactly what externalizing-related processing deficit may be tapped by these ERP indicators. The topography of externalizing-related effects for these differing indicators supports the idea that it is a frontally driven process, but the extent to which the process in question is one commonly presumed to be indexed by ERN or P3 (e.g., recognition of errors or other performance-related outcomes; incorporation of perceptual input into a mental model of an ongoing task) remains unclear. This question can be addressed in future research by developing hybrid P3/ERN procedures and manipulating task parameters to test alternative hypotheses regarding processes underlying convergence of differing ERP indicators with externalizing measures. Regarding specific brain mechanisms, a plausible hypothesis is that overlapping externalizing-related impairments in these differing ERP indicators reflect dysfunction in anterior brain circuitry including ACC and/or PFC structures known to contribute to the ERN as well as the P3. Studies using other neuroimaging methods in conjunction with EEG/ERP measurement will be valuable in addressing this hypothesis.
Given that ERN and differing variants of P3 in the current study overlapped in terms of their relations with ESI externalizing scores, we sought to create an aggregate physiological index of externalizing proneness from these measures. Specifically, we extracted a common factor reflecting the covariance among the three primary ERP components and evaluated the predictive validity of this factor in relation to self-report and physiological criterion measures. In this regard, some limitations of the current study warrant mention. Although it could be argued that the sample size was acceptable in terms of number of subjects per indicator variable (i.e., > 25), the sample size was relatively modest for a factor analytic investigation. Similarly, the number of indicators available was too limited to provide for a compelling evaluation of the underlying factor structure of externalizing-related brain measures. Future studies of this type would benefit from larger samples, a wider array of brain response measures, and use of confirmatory factor analytic methods to evaluate alternative models of structure. In particular, it would be desirable to include other ERP components (like the ERN) that have been localized to particular neuroanatomic locations. Furthermore, utilization of data from clinical samples would extend the generalizability of the current findings to populations with more severe psychopathology.
Another important issue involves the specificity of ERP measures such as P3 and ERN as indicators of externalizing proneness, in view of findings indicating relations with other common disorders outside the externalizing spectrum. For example, anxiety disorder symptoms have been associated with enhancements in both the ERN and P3 (Bruder et al., 2002; Gehring, Himle, & Nisenson, 2000; Hajcak, Franklin, Foa, & Simons, 2008), and depression has been associated with reductions in the amplitude of the P3 response (Bruder et al., 1995; Yanai, Fujikawa, Osada, Yamawaki, & Touhouda, 1997). Findings for ERN in relation to depression have been more mixed (Chiu & Deldin, 2007; Ruchsow, Herrnberger, Beschoner, Grön, Spitzer, & Kiefer, 2006). Nonetheless, it will be important in follow-up studies to concurrently assess for symptoms of other disorders (in particular, commonly-occurring conditions such as mood- and anxiety-related disorders) in order to establish the specificity of composite ERP variables as biomarkers for externalizing proneness.4
Notwithstanding these limitations, our factor analysis of ERP indicators yielded a number of intriguing findings. Consistent with prediction, scores on the ERP factor composite related in predictable ways to differing self-report indices of disinhibitory tendencies. Correlations with measures of adolescent and adult antisocial deviance and alcohol dependence were most robust. Correlations for measures of socialization and drug abuse, although in predicted directions (negative and positive, respectively), were nonsignificant. The implication is that these specific psychometric indices of externalizing proneness were less reflective of neural processing deviations than other psychometric indices within the current sample. In part, this may reflect unreliability of measurement for narrow manifest indicators (cf. Vaidyanathan, Patrick, & Bernat, 2009); in line with this, it is notable that the highest observed validity coefficient was for prediction of broad ESI scores using the ERP composite. Another factor that may have contributed to weaker associations for some criteria in the current study is limitations associated with self-report measurement. To address this point, it will be valuable in future studies to include interview-based criterion variables along with measures of disinhibitory behaviors and traits derived from self-report.
Notably, the ERP common factor estimate generally outperformed constituent ERP indicators (ERN, flanker P3, feedback P3) in the prediction of externalizing-related criterion measures. Further, the ERP composite outperformed individual P3 indicators from a separate task (oddball-target and oddball-novel P3) in the prediction of ESI externalizing scores, such that ERP composite scores contributed significantly to prediction over and above these alternative psychophysiological indicators. This makes sense from psychometric perspective, insofar as aggregation across indicators enhances reliability of measurement and proportion of “true score” variance available for prediction. From this standpoint, scores on the common ERP factor represented a purer index of externalizing tendencies than scores on any individual ERP indicator, presumably because factor scores more purely reflected the externalizing-related process tapped by each individual indicator.
The broader implication is that multivariate psychometric techniques such as factor analysis, which have long been utilized in the self-report domain to refine measurement of psychological constructs, might similarly be applied to physiological response measures to develop reliable physiologically-based protocols for assessing dispositional constructs relevant to mental disorders. Just as questionnaire items are evaluated in terms of their psychometric properties, so too might ERP (or other physiological) response indicators be evaluated quantitatively in terms of their utility in the assessment of individual difference constructs. Following this approach, it should be possible to develop physiologically-based measures of individual difference constructs relevant to psychopathology that possess sound psychometric properties (e.g., high internal consistency, high test-retest reliability, stable convergent and discriminant validity). Assessment measures of this type would be of substantial value both for neurobiological research studies and clinical prevention and treatment efforts that emphasize underlying neurobiological mechanisms.
Acknowledgments
This manuscript is based on work completed by the first author in partial fulfillment of the requirements for the degree of Master of Arts at the University of Minnesota, under the supervision of the second author. The work was supported by grants MH65137, MH17069, MH072850, MH080239, MH089727, and AA12164 from the National Institutes of Health.
Footnotes
In prior work using this task and dataset (Bernat et al., 2009), the feedback P3 was operationalized as a time-frequency component to separate it from a somewhat overlapping, negative-polarity component of higher frequency. In the current study, to simplify presentation and facilitate comparisons with other ERP variables, we scored the feedback P3 using the more common time-domain peak approach. Peak scores correlated very highly with scores based on the time-frequency approach, r = .92.
Principal axis factor analysis was used rather than principal components analysis because the focus of our interest was on evaluating the coherence among indicators and extracting a composite reflecting this coherence (i.e., we explicitly wanted to capture the shared variance attributable to a common factor underlying the differing indicators and exclude variance unique to each indicator).
Here, eigenvalues were computed from the similated data and compared to those of the empirical data. In the current study, eigenvalues for 100 random data samples were computed and averaged. Similar results were found using the 95th percentile of the random data eigenvalues.
Although we did not systematically assess for symptoms of mood and anxiety disorders in the current study, global self-report measures of depression and trait anxiousness consisting of the Self-Rating Depression Scale (SDS; Zung, 1965) and the State-Trait Anxiety Inventory (STAI; Spielberger, 1985) were collected as a supplement to criterion measures of externalizing proneness. No significant correlations were evident for either the SDS or the STAI with any of the available ERP measures (rs = .00 to −.19, ns). Further, predictive relations for each ERP measure with ESI externalizing proneness remained significant after controlling for SDS and STAI scores.
References
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: Effects on P300 during the Stroop test. Neuropsychopharmacology. 1999;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and brain responses to gain/loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. 2009 doi: 10.1037/a0022124. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins CA, MacKay S, Clark W, Fein G. Event-related potential evidence for frontal cortex effects of chronic cocaine dependence. Biological Psychiatry. 1997;42:472–485. doi: 10.1016/S0006-3223(96)00425-8. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, et al. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, Quitkin FM. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clinical Electroencephalography. 2002;33:119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin. 1959;56:81–105. [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention [CSEA-NIMH] The international affective picture system: Digitized photographs. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Clark JP, Tifft LL. Polygraph and interview validation of self-reported deviant behavior. American Sociological Review. 1966;31:516–523. [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, et al. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Cognitive Brain Research. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJB. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review. 1980;87:301–15. [PubMed] [Google Scholar]
- Gough HG. Theory and measurement of socialization. Journal of Consulting Psychology. 1960;24:23–30. [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindelang MJ, Hirschi T, Weis JG. Measuring delinquency. Beverly Hills, CA: Sage; 1981. [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Hyman SM. Can neuroscience be integrated into the DSM-V? Nature Reviews: Neuroscience. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: Raising the bar for mental health research. Molecular Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Katayama J, Polich J. Auditory and visual P300 topography from a 3 stimulus paradigm. Clinical Neurophysiology. 1999;110:463–468. doi: 10.1016/s1388-2457(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Kendler K, Prescott C, Myers J, Neale M. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry and Human Development. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning S, Kramer M. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, McGue M, Iacono WG. The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences. 2001;30:1245–1259. [Google Scholar]
- Linden DEJ. The P300: Where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measure of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nye FI, Short JF., Jr Scaling delinquent behavior. American Sociological Review. 1957;22:326–331. [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO. Information processing, neuropsychological function, and the inherited predisposition to alcoholism. Neuropsychology Review. 1990;1:343–369. doi: 10.1007/BF01109029. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Garozzo R. Visual evoked potential correlates of information processing deficits in chronic alcoholics. In: Begleiter H, editor. Biological effects of alcohol. New York: Plenum; 1980. pp. 603–623. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MRM, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters. 2006;397:130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Beschoner P, Grön G, Spitzer M, Kiefer M. Error processing in major depressive disorder: Evidence from event-related potentials. Journal of Psychiatric Research. 2006;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10 year-olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull T. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner A. The Drug Abuse Screening Test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Assessment of state and trait anxiety: Conceptual and methodological issues. The Southern Psychologist. 1985;2:6–16. [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Bernat EM, Hall JR, Steffen BV, Cadwallader M, Krueger RF, et al. Neurophysiological correlates of behavioral disinhibition: Separable contributions of distinct personality traits. Psychophysiology. 2005;42:S126. [Google Scholar]
- Yanai I, Fujikawa T, Osada M, Yamawaki S, Touhouda Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. Journal of Affective Disorders. 1997;46:264–271. doi: 10.1016/s0165-0327(97)00100-6. [DOI] [PubMed] [Google Scholar]
- Young S, Stallings M, Corley R, Krauter K, Hewitt J. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:684–695. [PubMed] [Google Scholar]
- Zung WWK. A self-rating depression scale. Archives of General Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]