Abstract
Prenatal cocaine exposure induces cytoarchitectural changes in the embryonic neocortex; however, the biological mechanisms and type of cortical neurons involved in these changes are not known. Previously we found that neural progenitor proliferation in the neocortical ventricular zone (VZ) is inhibited by cocaine; here we examine the changes in cortical neurogenesis and migration of glutamate and GABA neurons induced by prenatal cocaine exposure. Pregnant rats received 20 mg/kg of cocaine intraperitoneally twice at an interval of 12 hr during three periods of neocortical neurogenesis. Neocortical area and distribution of developing neurons were examined by counting Tuj1+, glutamate+ or GABA+ cells in different areas of the cerebral cortex. Cocaine decreased neocortical area by reducing the size of the Tuj1+ layer, but only when administered during early periods of neocortical neurogenesis. The number of glutamatergic neurons was increased in the VZ, but was decreased in the outer cortical laminae. Although the number of GABA+ neurons in the VZ of both the neocortex and ganglionic eminences was unchanged, GABA+ cells decreased in all other neocortical laminae. Tangential migration of GABA+ cells was also disrupted by cocaine. These findings suggest that in utero cocaine exposure disturbs radial migration of neocortical neurons, possibly due to decreased radial glia guiding support through enhanced differentiation of neocortical VZ progenitors. Cocaine interrupts radial migration of both glutamatergic and GABAergic neurons within the neocortex, in addition to the tangential migration of GABAergic neurons from the subcortical telecephalon. This may result in abnormal neocortical cytoarchitecture and concomitant adverse functional effects.
Keywords: cocaine, GABAergic neurons, glutamate neurons, neocortical development, neural progenitor cells, cell migration
INTRODUCTION
Thousands of infants are born every year following exposure to cocaine, resulting in a spectrum of adverse developmental consequences (Chiriboga, 2003). Although confounding variables such as timing and dose of cocaine, prenatal health, and poly-drug use have led to differing reports on the presence and degree of abnormalities (Frank et al., 1998; Thompson et al., 2009), studies involving higher levels of cocaine exposure in utero have demonstrated consistent neurological and behavioral changes (Chiriboga et al., 1999; Chiriboga et al., 2007; Delaney-Black et al., 1996; Mirochnick et al., 1995; Sallee et al., 1995; Tronick et al., 1996).
It has been suggested that cocaine exerts its effect on neurobehavioral development through impaired fetal brain growth (Behnke et al., 2006; Singer et al., 2002; Singer et al., 2008). Fetal cocaine exposure causes abnormal morphology of brain structures, including the neocortex (Bellini et al., 2000). In animals, interference with neocortical development causes cognitive and motor defects (Berger-Sweeney and Hohmann, 1997). Cocaine exposure in utero has been shown to have persistent developmental effects in humans, such as cognitive impairment (Alessandri et al., 1998; Singer et al., 2002; Singer at al., 2008), language delays (Bandstra et al., 2004; Lewis et al., 2004; Morrow et al., 2004), and delays in motor development (Arendt et al., 1999; Miller-Loncar et al., 2005; Thyssen Van Beveren et al., 2000), all of which are related to neocortical function. Studying the changes in neocortical development induced by cocaine exposure may result in a better understanding of the neurobehavioral and developmental deficits resulting from in utero cocaine exposure.
In primates, prenatal cocaine exposure causes multiple neocortical cytoarchitectural abnormalities, including neuronal displacement in the cerebral wall, decreased numbers of cortical neurons, and decreased neocortical volume (Lidow, 1995; Lidow and Song, 2001). These defects are only observed when cocaine is administered during the second trimester, when neural progenitor proliferation, differentiation, and migration are most active (Lidow et al., 2001). In humans, cocaine use in the second trimester is also associated with microcephaly and neurobehavioral changes (Bellini et al., 2000; Gaultney et al., 2005; Richardson et al., 1999; Richardson et al., 2008). The specific actions of cocaine in the second trimester and the decrease in neurons in the neocortex suggest that cocaine may affect important developmental functions of neural progenitor cells.
Neural progenitor cells develop in the neuroepithelium and subsequently differentiate into neurons to form the cortical layers. Alterations in their cell cycle have been linked to changes in the number of neurons in the cortical layers and abnormalities in cerebrocortical size (Dehay and Kennedy, 2007; Sheen et al., 2004). Previously, we demonstrated that oxidative ER stress induced by cocaine causes down-regulation of cyclin A, a key regulator of G1-to-S phase cell cycle transition, thus promoting inhibition of neural progenitor cell proliferation in the developing neocortex (Lee et al., 2008). Because cell cycle duration is regulated mainly via G1 phase duration and/or the G1-to-S transition during embryonic neurogenesis (Dehay et al., 2001; Miyama et al., 1997), cocaine-induced G1/S cell cycle arrest may have a substantial impact on proliferation of progenitor cells, as well as on ensuing differentiation and migration, both of which contribute to determining numbers of neurons and brain cytoarchitecture.
Our previous findings, along with other studies on cocaine-induced neocortical abnormalities, led us to examine the biological mechanisms involved in these cocaine-induced cytoarchitectural changes and the cortical neuron populations that are affected during neocortical development. The neocortex is composed of two principal populations of neurons: glutamatergic projection neurons and GABAergic interneurons. Neurotransmitters have recently been shown to have much broader actions than their role in transmission at established synapses, and emerging evidence points to their importance in brain development and maintenance of homeostasis (Luján et al., 2005). Neurotransmitters are important components of the chemical environment for developing neurons, and both glutamate and GABA modulate proliferation of cortical progenitor cells, neurogenesis, and migration of young neurons during fetal brain development (Behar et al., 1999; Behar et al., 2000; Cuzon et al., 2006; Haydar et al., 2000; Heng et al., 2007; LoTurco et al., 1995). Effects of cocaine on tangential migration of GABAergic neurons have previously been reported (Crandall et al. 2004). Clarifying the actions of cocaine on differentiation and migration of the two types of neocortical neurons may therefore provide significant insights into the mechanisms underlying cytoarchitectural modifications of the neocortex and developmental deficits due to fetal cocaine exposure.
MATERIAL AND METHODS
Animals and experimental design
Timed-pregnant Sprague-Dawley rats (Charles River Laboratories, Wilmington, DE) received cocaine at early (E13 and E14), middle (E15 and E16), or late periods (E17 and E18) of neocortical neurogenesis (Fig. 1A). Rats received 20 mg/kg cocaine IP twice, at an interval of 12 hr (9 PM on the first day, 9 AM on the second). Treatment of 20 mg/kg cocaine twice daily is frequently used for in vivo studies to determine cocaine-induced neurological changes (Crandall et al., 2004; Ren et al., 2004), and achieves a concentration of cocaine in the fetal rat brain comparable to human conditions (Lee et al., 2008). Because our goal was to study cocaine-induced cytoarchitectural alterations during specific stages of neocortical neurogenesis, our treatment paradigm was limited to two cocaine treatments in 24 hours. We employed a dose of 20 mg/kg cocaine, which has been shown to inhibit neural progenitor cell proliferation in vivo (Lee et al., 2008), and to produce prominent effects on an acute treatment schedule. Bromodeoxyuridine (BrdU), when used, was administered as a single 50 mg/kg dose 24 hr after the second injection of cocaine. Rats were euthanized by CO2 inhalation 2 hr after BrdU, or 24 hr after the second cocaine treatment if BrdU was not given. Control animals received physiological saline. All animal procedures were performed according to the “Guide for the Care and Use of Laboratory Animals,” according to an animal protocol approved by the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program.
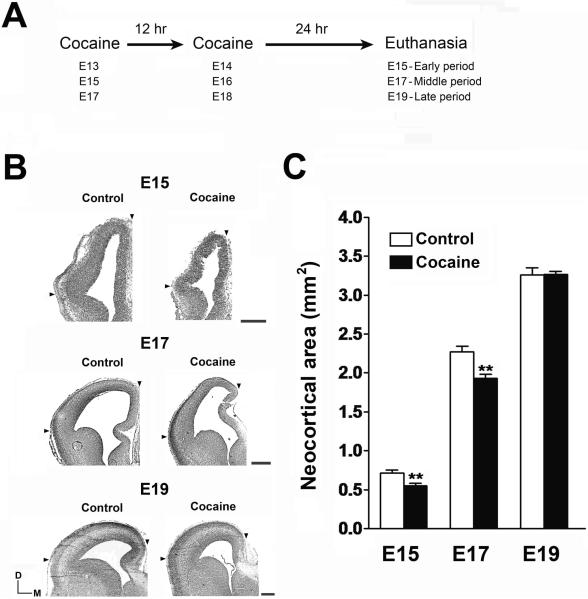
Fig. 1.
Effects of cocaine on neocortical growth. (A) The experimental paradigm consisted of two cocaine injections (20 mg/kg for each, IP) administered at 12 h intervals to pregnant rats at three different gestational periods. (B) Coronal cortex sections stained with cresyl violet were prepared from control and cocaine-exposed fetuses at three different periods of neocortical neurogenesis. Arrowheads show the boundaries of the regions used for the measurements of the size of neocortex. Scale bar = 400 μm. (C) Reductions in neocortical area in cocaine-exposed fetal brains were seen only at E15 and E17, when cocaine was administered during early and middle periods of neocortical neurogenesis. **p<0.01 compared to control, n = 5–7 per group from three individual pregnant dams.
Measurements of neocortical area
The fetal brains were immersed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) overnight and cryoprotected in 20% sucrose in PBS. Cryostat sections (10 μm) were thaw-mounted on gelatin-subbed slides. Total neocortical area was determined using coronal brain sections stained with cresyl violet, and was measured for both hemispheres using three sections for each animal, corresponding to defined marks [superior sagittal sinus (SSS), junction between dorsal pallium and lateral pallium, arrowheads on Fig. 1B]. Tuj1-positive and Tuj1-negative neocortical areas were measured along the same area of the cortex, defined by the distinct boundary which was evident after staining with Tuj1 (red arrows on Fig. 2A and 2B). A stronger contrast, accomplished by Adobe Photoshop, was used in Figure 2A and 2B to distinguish a clear boundary between Tuj1-positive and Tuj1-negative laminae of the neocortex. Tuj1 is also known as beta-III tubulin and is a neuron-specific cytoskeletal protein. Both the total neocortical area and Tuj1-positive/negative neocortical areas were calculated using both hemispheres, and were averaged over three sections for each brain, for a final of five to seven brains obtained from three individual pregnant dams. The identity of the cortical sections (control or cocaine-exposed) was unknown to the investigator performing the neocortical area measurements. Neocortical area measurements, including Tuj1-positive and Tuj1-negative areas, were performed with a Zeiss Axiovert microscope and the software used was UTHSCSA (University of Texas Health Science Center at San Antonio) Image tool (http://ddsdx.uthscsa.edu/dig/itdesc.html).
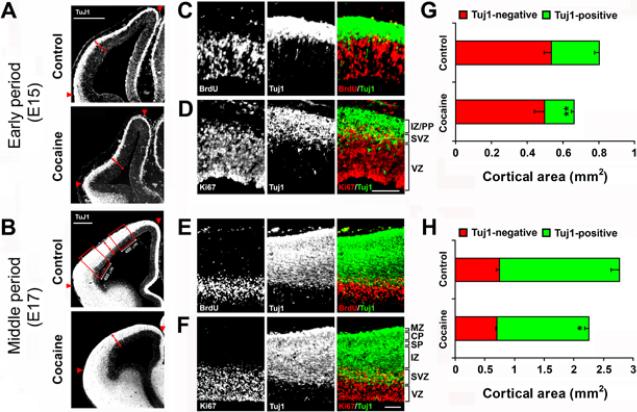
Fig. 2.
Effects of cocaine on the composition of the developing neocortex. (A, B) Immunostaining for Tuj1 in the fetal cortex of control and cocaine-exposed embryos at E15 (early period of neocortical neurogenesis) and E17 (middle period of neocortical neurogenesis) shows boundaries marking Tuj1-positive and Tuj1-negative areas (red arrows). The neocortical area between the red arrowheads was used for quantification. The boxed areas (400 μm in width) at E17 of the control group (B) were used for quantification of the densities of Tuj1-, glutamate-, and GABA-positive cells in the neocortex. Scale bar is 400 μm. (C, E) Co-immunostaining of BrdU (red) and Tuj1 (green) in the control neocortex at E15 (C) and E17 (E) show that the Tuj1-negative area essentially corresponds to the VZ. (D, F) Co-immunostaining for Ki67 (red) and Tuj1 (green) in the control neocortex at E15 (D) and E17 (F) shows that the area of overlap of Ki67 and Tuj1 staining corresponds to the SVZ, and the Tuj1-positive area is composed of the SVZ, IZ and other outer laminae of the neocortex (PP at E15 and SP/CP/MZ at E17). Scale bar = 100 μm for C-F. VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; PP, preplate; SP, subplate; CP, cortical plate; MZ, marginal zone. (G, H) The Tuj1-negative neocortical area (red) was not changed by cocaine exposure, but the Tuj1-positive area (green) was significantly decreased, so that the change in the Tuj1-positive area accounted for the overall reduction in total neocortical area (red and green together) seen in cocaine-exposed fetuses, for both the early and middle periods of neocortical neurogenesis. *p<0.05, **p<0.01 compared to control, n = 6–7 per group from three individual pregnant dams.
Immunohistochemistry
Coronal tissue sections stained with an anti-BrdU antibody were pretreated with 2N HCl for 30 min at 37 °C. Sections were blocked with 10% normal goat serum in PBS with 0.01% Triton X-100 and processed for immunohistochemistry using the following antibodies: mouse anti-Tuj1 (1:1000, Promega, Madison, USA), rabbit anti-Tuj1 (1:1000, Covance, Emeryville, USA), mouse anti-BrdU (1:200, BD Biosciences, San Jose, CA), rabbit anti-Ki67 (1:200, Vector Laboratories, Burlingame, USA), rabbit anti-glutamate (1:2000, Sigma, Saint Louis, MO), rabbit anti-GABA (1:1000, Sigma), and rabbit anti-tyrosine hydroxylase (TH) (1:500, Pel-Freez, Rogers, Arkansas). Sections were developed using the secondary antibodies Alexa Fluor 594 and Alexa Fluor 488 (Invitrogen, Carlsbad, CA) diluted 1:500 in PBS, and nuclei were labeled with 4'-6-Diamidino-2-phenylindole (DAPI) (Invitrogen).
Quantification of Tuj1-, glutamate-, and GABA-positive cells
Manual counts of cells positive for various markers in cortical regions were determined using a Zeiss Axiovert microscope (Oberkochen, Germany) and employed methods similar to those described previously by Crandall et al. (2004) and Cuzon et al. (2008). A 10× objective lens was first used to determine the borders of the different cortical regions and a 40× objective lens was then used while adjusting the focus through the depth of the section to clearly distinguish the presence of overlapping cells and to count individual cells, as shown in Supplementary Fig. 1A and 1B. To establish a consistent guideline for counting individual cells, only cell densities larger than 5 μm were counted. Signals smaller than 5 μm were excluded to avoid counting neurites, nerve terminals, and false signals. In Figures 3 and 4, contrast was enhanced using Adobe Photoshop to allow for a clear distinction between immunopositive cell densities of the control and cocaine-treated groups. Densities [number of cells/ area (mm2)] of Tuj1-, glutamate-, and GABA-positive cells in the various laminae were calculated in regions of 400 μm width (red rectangles in Fig. 2B) centered at one-third and two-thirds the length of the neocortex, as defined by arrowheads at the SSS and the junction between dorsal pallium and lateral pallium in Figure 2B. Four regions per section (two for each brain hemisphere) were averaged from three sections using eight different brains obtained from three individual pregnant dams. Counts of Tuj1- and GABA-positive cells from the medial and lateral ganglionic eminences (MGE and LGE) were determined within regions 400 μm in width, measured along the ventricular surface and extending through the VZ proliferative zone (see Fig. 4A). GABA cell counts along the migratory stream crossing the corticostriatal boundary and next to developing pool of GABA-positive cells were each evaluated within a 150 μm/side square. The tangential stream (Fig. 4A and 4C, Box A) was placed at the top of the corticostriatal junction with one side aligned diagonally along the Tuj1 border and containing the SVZ / lower intermediate zone. Box B, representing cells that stay in the subcortical region of the ventral telecephalon below the corticostriatal junction, was positioned horizontally with its upper right corner below Box A and shifted laterally until outside the pool of developing GABAergic neurons (Fig. 4A and 4C, Box B). All counts of cells in the MGE, LGE, Box A, and Box B were comprised of the average between both hemispheres from three brain sections across eight brains obtained from three individual pregnant dams. The identity of cortical sections (control or cocaine-treated) were unknown to the investigator performing the cell counting.
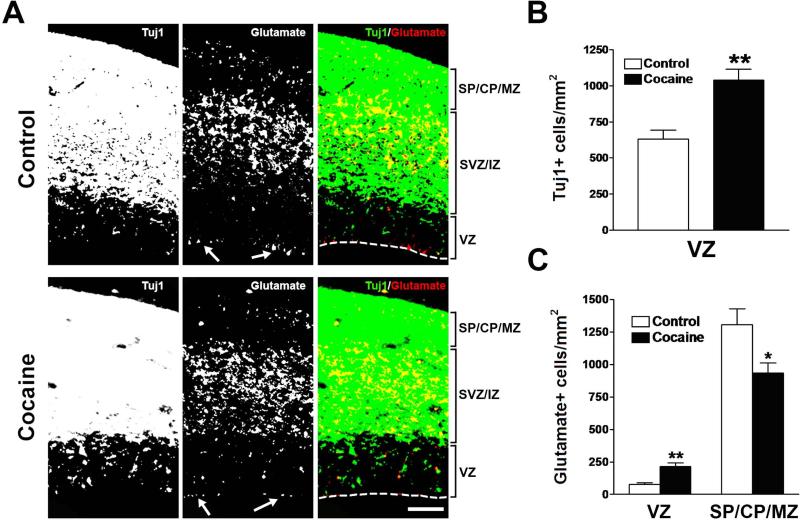
Fig. 3.
Effects of cocaine on the densities of Tuj1- and glutamate-positive cells in the neocortex during the middle period of neocortical neurogenesis. (A) Immunostaining for Tuj1 (green) and glutamate (red) in the neocortex of control and cocaine-exposed fetuses. Glutamate-like staining at the ventricular surface, which is often seen at the edges of the sections, was excluded from quantification (white arrows show examples). Scale bar = 100 μm. VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; SP, subplate; CP, cortical plate; MZ, marginal zone. (B) Tuj1-positive cells in the VZ were increased in cocaine-exposed fetuses. **p<0.01 compared to control, n = 8 per group from three individual pregnant dams. (C) Cocaine exposure caused an increase in the density of glutamate-positive cells in the neocortical VZ, but a decrease in the SP/CP/MZ. *p<0.05, **p<0.01 compared to control, n = 8 per group from three individual pregnant dams.
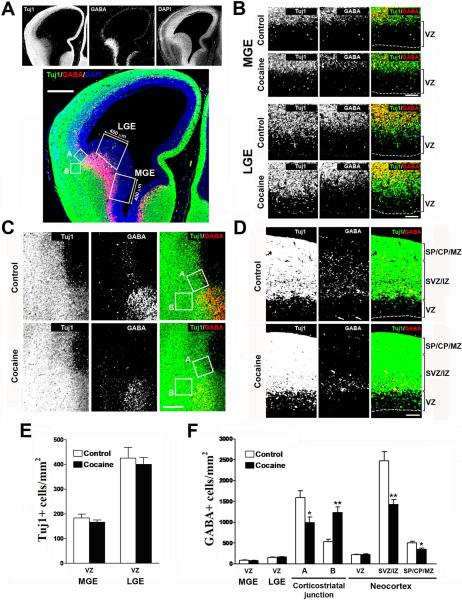
Fig. 4.
Effects of cocaine on the density of GABA-positive cells in the fetal cortex during the middle period of neocortical neurogenesis. (A) Immunostaining for Tuj1 (green), GABA (red), and DAPI (blue) in the fetal cortex. The boxed areas (400 μm in width) mark the medial and lateral ganglionic eminences (MGE and LGE), and were used for quantification of the densities of Tuj1- and GABA-positive cells. The square boxes (A and B, 150×150 μm) were used for quantification of the GABA-positive cells either migrating tangentially to the neocortex (Box A) or accumulating in the ventral telencephalon below the corticostriatal junction (Box B). Scale bar = 400 μm. (B) Immunostaining of Tuj1 (green) and GABA (red) in the MGE and LGE of control and cocaine-exposed fetuses. Scale bar = 100 μm. VZ, ventricular zone. (C) Immunostaining of Tuj1 (green) and GABA (red) in the corticostriatal junction of control and cocaine-exposed fetuses. Scale bar = 200 μm. (D) Immunostaining of Tuj1 (green) and GABA (red) in the neocortex of control and cocaine-exposed fetuses. GABA-like staining at the ventricular surface that is often seen at the edge of the section was excluded from quantification (white arrows show examples). Scale bar = 100 μm. VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; SP, subplate; CP, cortical plate; MZ, marginal zone. (E) Densities of Tuj1-positive cells in the VZ of the MGE and LGE were unaltered by cocaine exposure. N = 8 per group from three individual pregnant dams. (F) Densities of GABA-positive cells were unchanged in the VZ of the MGE and LGE in cocaine-exposed fetuses. Cocaine exposure caused the densities of GABA-positive cells to decrease in the neocortical SVZ / lower IZ (Box A), but concomitantly increase in the subcortical region (Box B). Quantification of GABA-positive cells showed no differences in the neocortical VZ as compared to control, but a decrease in both the SVZ/IZ and SP/CP/MZ. *p<0.05, **p<0.01 compared to control, n=8 per group from three individual pregnant dams.
Statistical analysis
All values were expressed as means ± standard error of the mean (SEM). For each comparison, mean values were composed of all samples collected from three independent pregnant rats and analyzed using the two-tailed Student's t-test. Significance was defined as p<0.05.
RESULTS
Cocaine inhibits growth of the neocortex only when administered during early neocortical neurogenesis
The relative neocortical sizes were reduced by 23% and 15% when cocaine was injected during the early (E13–E15) and middle (E15–E17) periods of neurogenesis, respectively (Fig. 1B and 1C). Neocortical size was not, however, changed by injections during the late (E17–E19) period of neurogenesis, and further experiments therefore omitted this treatment period (Fig. 1A–C). This result is consistent with our previous conclusion that cocaine perturbs the expansion of neocortex only during earlier periods of neocortical neurogenesis, that involve active proliferation of neural progenitor cells in the ventricular zone (VZ) (Lee et al., 2008).
Cocaine inhibits the expansion of the Tuj1-positive lamina of the neocortex, without affecting the size of the VZ
In coronal sections, a marked boundary between Tuj1-positive and Tuj1-negative laminae of the fetal cortex was evident (Fig 2A and 2B). The innermost part of the subventricular zone (SVZ) was defined as the boundary between the outer half of the VZ (the half farthest from the ventricle) containing a layer of closely-packed cells positive for BrdU S-phase incorporation, and the bottom of the SVZ layer with a relatively light scattering of BrdU-positive cells (Fig. 2C and 2E). The Tuj1-positive lamina extended from the pial surface of the cortex to the inner boundary of the SVZ. Ki67 immunocytochemistry labels the total fraction of VZ and SVZ progenitor cells that are active in the cell cycle. Thus, the lamina of the neocortical wall where Ki67- and Tuj1-positive staining overlaps represents the SVZ (Fig. 2D and 2F). These data show that the Tuj1-negative area essentially corresponds to the VZ, while the Tuj1-positive area represents the SVZ, intermediate zone (IZ) and the rest of the outer cortical laminae [the preplate (PP) in early neocortical neurogenesis, which then splits into the subplate (SP), cortical plate (CP), and marginal zone (MZ) during middle neocortical neurogenesis] (Fig. 2D and 2F). Notably, the cocaine-induced reduction in total neocortical area seen in the early and middle periods of neocortical neurogenesis was entirely due to reductions in the Tuj1-positive area, of 39% and 24%, respectively (Fig. 2A, 2B, 2G, and 2H).
Because we have shown previously that cocaine blocks cell cycle progression of VZ progenitor cells (Lee et al., 2008), we expected to see the shrinking of the VZ progenitor pool and fewer immature neurons that migrated to the cortex. The Tuj1-negative area (i.e. VZ) was not, however, affected by cocaine treatment during either period (Fig. 2A, 2B, 2G, and 2H). The size of the embryonic neocortex expands during corticogenesis via proliferation of neural progenitor cells in the VZ and migration of postmitotic cells into the overlying cortex. Therefore, the lack of change in the size of the VZ suggests that cocaine causes deficits in the migration of neurons outward into the developing neocortex.
Cocaine causes Tuj1-positive cells to accumulate in the VZ
We have previously found that cocaine inhibits the proliferation of neural progenitor cells in the VZ, without affecting cell survival (Lee et al., 2008). We therefore asked whether an increase in the number of postmitotic neurons in the VZ counteracts cocaine-induced deficits in the proliferation of VZ progenitors, thus resulting in the size of the VZ remaining unchanged. We examined postmitotic neurons by determining the fraction of cells labeled with Tuj1 in the VZ during the middle period of neocortical neurogenesis, when neuronal production and total VZ area are at their peak levels, and there is more mature development and stratification of neocortical laminae compared to the early period of neurogenesis (Dehay and Kennedy, 2007; Takahashi et al., 1995). Quantification of the density of Tuj1-positive cells in the VZ revealed a 1.6-fold increase in the cocaine-exposed fetuses (Fig. 3A and 3B). This result suggests that prenatal exposure to cocaine causes postmitotic neurons to accumulate in the VZ.
Cocaine changes the distribution of glutamate-positive cells in the neocortex
The neocortex is composed of two major types of neurons: glutamatergic projection neurons, which originate in the neocortical VZ and migrate radially towards the pial surface, and GABAergic interneurons, which originate in the subcortical ganglionic eminence and migrate tangentially to the neocortex (Gupta et al., 2002; Marín and Rubenstein, 2001). Once GABAergic neurons enter the cortex, some migrate towards the cortical plate, but many initially migrate towards the VZ to obtain positional information, and then subsequently move radially towards the cortical plate (Kriegstein and Noctor, 2004).
To determine which population contributes to the neuronal accretion in the neocortical VZ caused by cocaine, we quantified the density of glutamate-positive cells in the VZ during the middle period of neocortical neurogenesis. There was a 2.8-fold increase in glutamate-positive cells in the VZ of cocaine-exposed fetuses as compared to controls, suggesting that some postmitotic cortical projection neurons failed to reach their positions in the developing neocortex, as shown in Figure 3A and C.
We then examined the density of glutamate-positive cells in the outer cortical laminae and found a 29% decrease in the number of glutamate-positive cells in the SP/CP/MZ of cocaine-exposed fetuses (Fig. 3A and 3C). Evaluation of glutamate-positive cells in the middle laminae (SVZ/IZ) was not possible because most glutamate-positive cells aggregated together, as confirmed by confocal microscopic observations with a 100× objective, thus hampering precise counting of cells. These data indicate that cocaine causes glutamatergic neurons to accumulate in the VZ, and thus fail to assume their normal positions in the developing laminar neocortex.
Cocaine changes the distribution of GABA-positive cells in the fetal cortex
We next examined the effects of cocaine on the developing GABAergic neurons. GABAergic interneurons are born in the VZ of the medial and lateral ganglionic eminences (MGE and LGE) (Anderson et al., 2001). No differences in the number of Tuj1- or GABA-positive cells in the VZ of the MGE or LGE during the middle period of neocortical neurogenesis were found (Fig. 4B, 4E, and 4F). These results indicate that cocaine did not affect GABAergic neuron production or migration in the proliferative zones of the subcortical ganglionic eminences.
GABAergic neurons derived from ganglionic eminences subsequently migrate tangentially towards the dorsal neocortex, crossing the corticostriatal boundary, and populating primarily the SVZ and lower IZ as they enter into the developing neocortex (Marín and Rubenstein, 2001). Two areas, shown as boxes A and B in Figure 4A and 4C, were chosen to represent the tangential migration of GABAergic neurons from the ganglionic eminence (Box A) and GABAergic neurons that do not migrate to the cortex, but accumulate below the corticostriatal junction (Box B). Examination of these two corticostriatal areas showed a decrease in the number of GABA-positive cells in the tangential migratory pathway of the neocortical SVZ and lower IZ (Box A in Fig. 4C and 4F), but an increase in the number of GABA-positive cells in a subcortical area located laterally outside the pool of developing GABAergic neurons (Box B in Fig. 4C and 4F).
These results show that cocaine interrupts the tangential migration of GABAergic interneurons from the ganglionic eminences to the neocortex, which may ultimately cause an accumulation of these neurons in the subcortical telencephalon. Cell counts of GABA-positive cells in the different laminae of the neocortex revealed no change in the number of cells in the VZ, but decreases in the SVZ/IZ and the SP/CP/MZ (Fig. 4D and 4F), which suggests that the radial migration of GABAergic neurons towards the cortical plate within the neocortex was affected by cocaine.
TH-Positive axons penetrate the rat neocortex until the late period of neocortical neurogenesis
Whether dopamine is involved in cocaine-induced disturbances in neocortical development is not clear. It has been reported that a significant number of TH-positive axons can extend into the neuroepithelial area of the mouse cerebral neocortex at E15 and settle in close proximity to proliferating precursor cells, leading to a suggestion that dopamine may influence cortical neurogenesis after exposure to cocaine (Bhide, 2009). We did not observe TH-positive staining in the neocortex of the developing rat at E15 or E17, and only a few TH-positive axons were observed in the upper cortical lamina at E19 (Fig. 5).
Fig. 5.
Immunohistochemical staining of BrdU and TH in the neocortex of control rat embryos at E15, E17, and E19. (A) Representative BrdU- and TH-immunoreactive cortical sections are shown for different stages of development. TH immunoreactivity (yellow arrows) was found in the neostriatum at each development stage. The anti-TH antibody used consistently detected blood vessels distributed throughout the cerebral cortex. The blood vessels found in the cerebral cortex have two possible spatial orientations: blood vessels oriented horizontally relative to the plane of dissection are shown as yellow arrowheads and blood vessels oriented vertically relative to the plane of dissection are shown as green arrowheads in Fig. 5A, 5B, and 5C. Scale bar = 100 μm. Fig. 5B and 5C show enlargements of the neocortex and neostriatum from Fig. 5A, respectively. (B) A few TH-positive axons were observed in the neocortex of E19 embryos. An example of TH-positive axon is shown in the enlarged box area. Scale bar = 50 μm. (C) In the neostriatum, some apparently TH+ cells were found at E15, but only cross-sections of TH-positive axons were be found at E17 and E19. Scale bar = 50 μm.
DISCUSSION
The present data suggest that cytoarchitectural abnormalities observed in the neocortex due to in utero cocaine exposure during neocortical neurogenesis result from disturbances in the development and migration of glutamatergic and GABAergic neuronal populations.
Cocaine exposure in utero disturbs radial migration of neurons in the developing neocortex
During neocortical neurogenesis, the VZ develops first, followed by the SVZ. After the middle period of neurogenesis, the VZ begins to decrease, and in the late period cortical germinal zones are comprised of only the SVZ (Takahashi et al., 1995). Cocaine perturbs the expansion of the neocortex only during earlier periods of neocortical neurogenesis, and thus the VZ is the specific target of cocaine in the developing neocortex.
Once progenitor cells differentiate into neurons in the VZ, they start to migrate to the outer cortical laminae. Following cocaine exposure, we observed an increase in the number of postmitotic neurons in the VZ, but a decrease in the Tuj1-positive area of the neocortex. This suggests that cocaine disturbs the radial migration of neurons within the neocortex.
We have shown previously that cocaine inhibits proliferation of neocortical VZ progenitor cells by inducing G1/S cell cycle arrest through oxidative ER stress-mediated down-regulation of cyclin A (Lee et al., 2008). It has been shown that the length of the G1 phase in the cell cycle of cortical progenitor cells is involved in determining the timing of the shift from proliferation to differentiation: a short G1 phase leads to self-renewal of progenitors, whereas a long G1 phase encourages progenitors to exit the cell cycle to differentiate into neurons (Calegari and Huttner, 2003; Hodge et al., 2004; Lukaszewicz et al., 2002). Longer G1 phases promote differentiation, possibly by increasing the window of time during which cells are sensitive to differentiation signals (Dehay and Kennedy, 2007). Cocaine (1 to 100 μM, 7 days) was shown in an in vitro study to induce both G1/S phase arrest and neuronal differentiation of human fetal brain-derived neural precursor cells (Hu et al., 2006). Enhanced neurogenesis and inhibition of proliferation of cortical progenitor cells has also been observed when the G1/S phase transition is arrested by other means (Kim et al., 2006). Cocaine self-administration in rats was shown to both enhance adult neurogenesis and inhibit neural progenitor proliferation in the subgranular zone of the hippocampus, as indicated by a decrease in progenitor proliferation after 3 weeks, and an increase in the number of immature neurons after 4 additional weeks of cocaine self-administration (Noonan et al., 2008). Although cocaine seems to increase differentiation of neural progenitor cells, this alone is not sufficient to explain our results. If only differentiation was affected, newborn neurons would simply be transported to the cortical plate and increase neocortical area. It seems probable that decreased migration resulting from cocaine exposure would also be necessary to explain the accumulation of neurons in the VZ and decreased neocortical area.
In fact, radial glia comprise the vast majority of neural precursor cells in the developing neocortical VZ, and are responsible for both generation of new neurons and guidance of those neurons to their final cortical positions (Anthony et al., 2004; Noctor et al., 2002). Exposure to cocaine causes decreased proliferation of neural progenitor cells in the VZ (Lee et al., 2008), probably resulting in enhanced differentiation of VZ progenitors, decreasing numbers of radial glia, and thus decreasing glial scaffolding available for radial migration of neurons to the cortical plate. This may cause an accumulation of neurons in the VZ because they would not be able to migrate to the developing outer cortical laminae (Fig. 6). One limitation of this study is that we cannot confirm the disruption in migration patterns of prematurely-differentiated newborn neurons using co-immunostaining of Tuj1 and BrdU. We showed previously that cocaine interferes with the G1-to-S phase transition of VZ progenitor cells (Lee et al., 2008). This may cause some of the G1-phase arrested progenitors to differentiate earlier than expected into Tuj1-positive neurons, and hence not have the opportunity to incorporate BrdU. A possible future study would be to introduce a GFP gene into the ventricles of rat embryos by in utero electroporation to study how cocaine affects electroporated GFP-expressing neural progenitor cells differentiation and migration during neocorticogenesis.
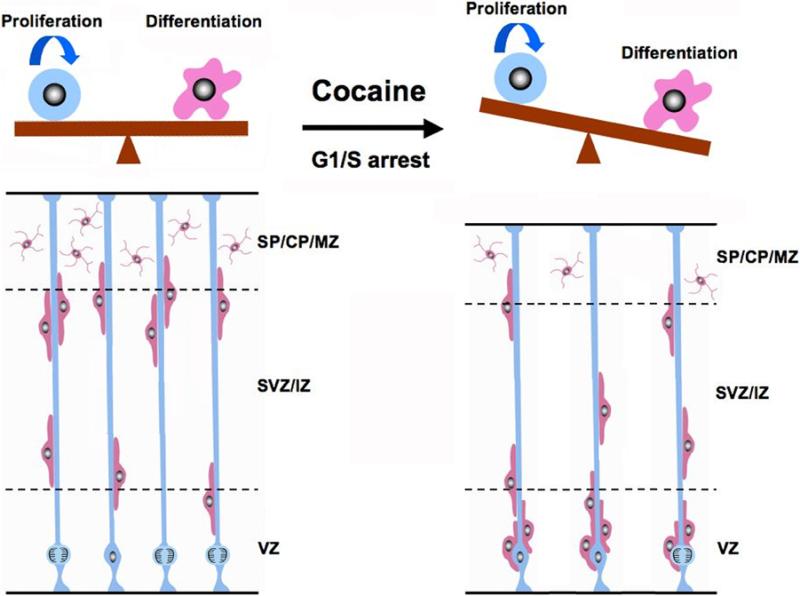
Fig. 6.
Schematic illustrating cocaine's adverse effects on neocortical formation. During neocortical neurogenesis, neural progenitor cells (blue) in the ventricular zone (VZ) have two main fates: continuing proliferation, or exit from the cell cycle and neuronal differentiation (pink). In normal neocortical development (left side), proliferation and differentiation of VZ progenitor cells are well balanced. Cocaine exposure (right side) causes G1/S phase transition arrest, consequently shifting the balance towards differentiation. This premature differentiation not only causes an increase in the number of neurons in the VZ, but also a shortage of radial glial scaffolding which leads to backlog of neurons waiting to travel to their specific cortical layers along radial glia. VZ, ventricular zone; SVZ/IZ, subventricular zone/intermediate zone; SP/CP/MZ, subplate/cortical plate/marginal zone.
In addition, cocaine-induced proliferation inhibition of neuroprogenitor cells (Lee et al., 2008) might be sufficient to cause a shortage of radial glial scaffolding for radial neuronal migration from VZ to the outer neocortical lamina, thereby resulting in the accumulation of postmitotic neurons in the VZ. That is, the simultaneous enhancement of neuronal differentiation in the neocortical VZ might not be necessary to result in a shortage of radial glial scaffolding.
Our observations in Figure 5 illustrating the lack of TH-positive axons in the developing rat neocortex at E15 and E17 are consistent with previous studies that did not detect TH-positive immunostaining in the rat neocortex from E13 to E16 (Iacovitti et al., 1987). These data suggest that dopamine may not play a major role in cocaine-induced disturbances of radial glial scaffolding within the neocortex, since most of the effects induced by cocaine occur during earlier periods of neocortical neurogenesis. In addition, in PC12 cells cocaine modulated the expression of a number of immediate early genes and transcription factors, and these effects were independent of cocaine's effect on dopamine concentrations (Imam et al., 2005).
Cocaine exposure disrupts the normal distribution of glutamate and GABA neurons
We found a substantial increase in the number of Tuj1-positive cells in the neocortical VZ after cocaine exposure, but a much smaller increase in the number of glutamate-positive cells and no increase in the number of GABA-positive cells in the same region. We also found a large decrease in the number of glutamate-positive cells in the upper cortical laminae, comparable in size to the increase in Tuj1-positive cells in the VZ. This leads us to conclude that the increase in Tuj1-positive cells in the VZ is due to an accumulation of glutamatergic neurons, since most newborn glutamatergic neurons in the VZ do not initially express glutamate, whereas most Tuj-1 positive GABAergic neurons that are undergoing tangential migration to the neocortex show expression of GABA (Haydar et al., 2000; Nadarajah et al., 2002).
Our data show that cocaine causes a decrease in the number of GABA-positive cells in the neocortex; however, the number of GABA-positive cells in the VZ was unchanged. After entering the neocortex, most GABAergic interneurons exhibit ventricle-directed migration, where they first enter the neocortical VZ to obtain positional information, through the chemical environment or via neural interaction, before migrating radially to the upper cortical laminae. Our results suggest that the GABAergic interneurons do not get proper positional information in the VZ after cocaine exposure, as evidenced by the decrease in GABAergic neurons in the outer cortical laminae. Since radial glial scaffolding is necessary for proper passage of both glutamatergic and GABAergic neurons into the cortical plate during neocortical development (Poluch and Juliano, 2007), our proposed model involving radial glia (Fig. 6) can explain the decrease of both these neuronal populations in the outer cortical laminae.
It must be stressed, however, that in contrast to glutamatergic neurons, migration of GABAergic neurons is more complex; interneurons originate in the ganglionic eminences of the ventral telencephalon, not the neocortical VZ, and thus their migration includes both tangential and radial components. Our study reveals that cocaine causes more GABA-positive cells to stay in the subcortical telencephalon, instead of joining the tangential migratory stream in the neocortical SVZ/IZ. These findings are consistent with previous reports that mice treated with 20 or 40 mg/kg/day of cocaine from E8–E15 had fewer GABA neurons migrating tangentially from the ganglionic eminences to the cerebral cortex (Crandall et al., 2004), possibly due to interruption of D1 receptor signaling in the neostriatum (Crandall et al., 2007; Métin et al., 2008).
Chemical signaling of GABAergic migration may also be disrupted by cocaine. CXCL12 is an attractant for CXCR4-expressing interneuron precursors and regulates their tangential migration and cortical layer-specific integration during neocortical development (Li et al., 2008; Stumm et al., 2003). Recently, an in vitro study showed that cocaine (0.1 to 100 μM) interrupted the migratory response of CXCR4-expressing human fetal brain-derived neural precursor cells to CXCL12, which correlated with cocaine-induced down-regulation of CXCR4 expression in these cells (Hu et al., 2006). Therefore, cocaine may disrupt migration pattern of GABAergic interneurons through diminishing CXCL12/CXCR4 signaling in the developing neocortex. It is important to note that Crandall et al. (2004) reported no changes in cell proliferation in the MGE or LGE after cocaine exposure. Indeed, we did not see any differences in the numbers of Tuj1-or GABA-positive cells in the VZ of the MGE or LGE; therefore, unlike in the neocortex, the cellular functions of VZ progenitor cells in the ganglionic eminences might not be sensitive to cocaine.
The mechanisms underlying the regional selectivity of the actions of cocaine are not yet known. One possibility is that it involves the different effects of cocaine on dopamine receptor subtypes in the proliferative zones of ganglionic eminences, which are adjacent to the dopamine-rich neostriatum region of the developing brain. Specifically, activation of dopamine D1 receptors causes an inhibition of G1-to-S phase transition of neural progenitor cells, while activation of D2 receptors cause the opposite effect (Ohtani et al., 2003). Another possibility is the difference in sensitivity to cocaine is related to variations in the timing of neurogenesis across different brain regions (Anthony et al., 2004).
There is evidence that prenatal cocaine exposure is also able to affect the development of the serotoninergic system (Akbari et al., 1992; Yan, 2002). Serotonergic axons enter the developing neocortex without accessing the proliferating ventricular progenitors when the cortical plates are forming in the rat (Lidov and Molliver, 1982). Depletion of embryonic serotonin affects the integration of GABAergic interneurons in the cortical plate and differentiation of cortical neurons (Vitalis et al., 2007). Prenatal cocaine exposure has been shown to decrease S-100β, a cortical trophic factor that plays a part in neuronal cytoskeletal stabilization and differentiation, in the developing cerebral cortex, and can subsequently lead to microcephaly (Azmitia, 2002; Akbari et al.,1994). These effects of cocaine could be reversed by treatment with 5-HT1A agonists (Akbari et al.,1994). Therefore, it is possible that changes in serotonergic function are involved in cocaine-induced changes in neocortical development.
In addition to the cocaine-induced disruption of radial migration of glutamate and GABA neurons within the neocortex, previous studies have suggested that cocaine induces cell death in the primate fetal cerebral wall (He et al. 1999). We therefore cannot exclude the possibility that the decrease in glutamate and GABA neurons is due to cocaine-induced cell death. Even though we did not observe an increase in cell death of neuroprogenitor cells in the neocortical VZ (Lee et al., 2008), future studies on possible apoptotic effects of cocaine on glutamate and GABA neurons in the outer cortical laminae of neocortex would be needed.
Glutamate and GABA influence early fetal brain development and later cocaine addiction-related behaviors
Traditionally, roles of glutamate and GABA have been thought to be restricted to synaptic neurotransmission. Recently, several studies indicate that both are involved in the regulation of neocortical development. Both glutamate and GABA have been shown to depolarize neocortical VZ progenitor cells, and regulate progenitor cell proliferation and neurogenesis (Haydar et al., 2000; LoTurco et al., 1995). In addition, both glutamate and GABA are suggested to be able to modulate the migratory behaviors of newly-generated neurons in the cerebral cortex. For example, both glutamate and GABA signaling are involved in the radial migration of postmitotic cortical neurons (Behar et al., 1999; Behar et al., 2000). GABA, through GABAA receptors, is required for ganglionic eminence-derived interneurons to cross the corticostriatal junction on their tangential migration to the cortex (Cuzon et al., 2006). Once migratory neurons reach their destinations in the developing cortex, both glutamate and GABA continue to be involved in ensuing neuronal maturation and synaptogenesis (Luján et al., 2005). Therefore, glutamate and GABA not only carry out their specific neurotransmitter functions, but also influence neocortical development.
In addition, the displacement of glutamate neurons in the prefrontal cortex may also disturb glutamate homeostasis, i.e., the balance which exists between synaptic and non-synaptic glutamate. Glutamate imbalance has been shown to negatively affect communication along the corticostriatal projections, which are involved in regulating the control of drug-seeking behavior. Decreased numbers of cortical glutamate neurons and disruption in their normal distribution due to prenatal cocaine exposure may diminish projections to the nucleus accumbens from the prefrontal cortex, disrupting glutamate homeostasis and decreasing control of drug-seeking behaviors (Kalivas, 2009).
In this study, we identified novel actions of cocaine on radial migration of postmitotic neurons within the neocortex. Cocaine may shift the balance away from proliferation of neocortical VZ progenitors and towards differentiation, possibly leading to a reduction of the radial glia scaffolding available for migration, thus disturbing the normal distribution of glutamatergic and GABAergic neurons in the developing neocortex. Since glutamate and GABA play important roles for the establishment of normal neocortical organization, changes in the distribution of glutamatergic and GABAergic neurons in the developing fetal cortex alter the cortical chemical microenvironment and cytoarchitecture. Structural changes of this type may be irreversible (Berger-Sweeny and Hohmann, 1997) and can lead to later cortical dysfunction and related behavioral effects. We previously showed that cocaine causes down-regulation of cyclin A, as well as of cytoskeleton-related genes such as ezrin, in neocortical neural progenitor cells (Lee et al., 2008; Lee et al., 2009), which may lead to disturbances in neural differentiation and migration. Initial changes in neural progenitor cells due to cocaine exposure are likely to underlie subsequent developmental problems: altered distribution of glutamatergic and GABAergic neuronal populations can lead to disturbances in the neurochemical environment and in cortical organization. In the future, we plan to examine the long-term fate of these dislocated neurons in the postnatal cortex, and to study cortical function-related behavioral changes induced by cocaine. Elucidating the adverse actions of cocaine on neural progenitor cells could lead to the design of new therapies to prevent or treat effects of cocaine on cortical development.
Supplementary Material
Supplementary Fig. 1. Immunostaining of Tuj1- and GABA-positive or glutamate-positive cells in the neocortex during the middle period of neocortical neurogenesis using 40x objective lens. (A) Immunostaining for Tuj1 (green) and GABA (red) in the VZ/SVZ of the neocortex of the control fetus. Yellow arrows indicate the counted signals; yellow arrowheads indicate the excluded signals. Note the GABA-positive cells are overlapped with Tuj1-positive cells in the VZ (yellow arrows in the merged figure). Scale bar = 50 μm. (B) Immunostaining for Tuj1 (green) and glutamate (red) in the SP/CP/MZ of the neocortex of the control fetus. Yellow arrows indicate the counted signals; yellow arrowheads indicate the excluded signals. Scale bar = 50 μm.
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Tsung-Ping Su and Teruo Hayashi for discussion of the data and proof reading, as well as Cindy Ambriz for preparing the manuscript.
REFERENCES
- Akbari HM, Kramer HK, Whitaker-Azmitia PM, Spear LP, Azmitia EC. Prenatal cocaine exposure disrupts the development of the serotonergic system. Brain Res. 1992;572:57–63. doi: 10.1016/0006-8993(92)90450-n. [DOI] [PubMed] [Google Scholar]
- Akbari HM, Whitaker-Azmitia PM, Azmitia EC. Prenatal cocaine decreases the trophic factor S-100 beta and induced microcephaly: reversal by postnatal 5-HT1A receptor agonist. Neurosci Lett. 1994;170:141–4. doi: 10.1016/0304-3940(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Alessandri SM, Bendersky M, Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev Psychol. 1998;34:565–573. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Arendt R, Angelopoulos J, Salvator A, Singer L. Motor development of cocaine-exposed children at age two years. Pediatrics. 1999;103:86–92. doi: 10.1542/peds.103.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. Cajal's hypotheses on neurobiones and neurotropic factor match properties of microtubules and S-100 beta. Prog Brain Res. 2002;136:87–100. doi: 10.1016/s0079-6123(02)36010-2. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA, Barker JL. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci. 1999;19:4449–4461. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C, Massocco D, Serra G. Prenatal cocaine exposure and the expanding spectrum of brain malformations. Arch Intern Med. 2000;160:2393. doi: 10.1001/archinte.160.15.2393. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav Brain Res. 1997;86:121–142. doi: 10.1016/s0166-4328(96)02251-6. [DOI] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol. 2009;20:395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Brust JC, Bateman D, Hauser WA. Dose-response effect of fetal cocaine exposure on newborn neurologic function. Pediatrics. 1999;103:79–85. doi: 10.1542/peds.103.1.79. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA. Fetal alcohol and drug effects. Neurologist. 2003;9:267–279. doi: 10.1097/01.nrl.0000094941.96358.d1. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Kuhn L, Wasserman GA. Prenatal cocaine exposures and dose-related cocaine effects on infant tone and behavior. Neurotoxicol Teratol. 2007;29:323–330. doi: 10.1016/j.ntt.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex. 2004;14:665–675. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Cheng Q, Yeh HH. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex. 2006;16:1377–1388. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–1864. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Savatier P, Cortay V, Kennedy H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ostrea E, Jr, Romero A, Baker D, Tagle MT, Nordstrom-Klee B, Silvestre MA, Angelilli ML, Hack C, Long J. Prenatal cocaine and neonatal outcome: evaluation of dose-response relaionship. Pediatrics. 1996;98:735–740. [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Zuckerman BS. Neonatal neurobehavioral and neuroanatomic correlates of prenatal cocaine exposure. Problems of dose and confounding. Ann N Y Acad Sci. 1998;846:40–50. doi: 10.1111/j.1749-6632.1998.tb09725.x. [DOI] [PubMed] [Google Scholar]
- Gaultney JF, Gingras JL, Martin M, DeBrule D. Prenatal cocaine exposure and infants' preference for novelty and distractibility. J Genet Psychol. 2005;166:385–406. doi: 10.3200/GNTP.166.4.385-406. [DOI] [PubMed] [Google Scholar]
- Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Song ZM, Lidow MS. Cocaine induces cell death within the primate fetal cerebral wall. Neuropathol Appl Neurobiol. 1999;6:504–512. doi: 10.1046/j.1365-2990.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- Heng JI, Moonen G, Nguyen L. Neurotransmitters regulate cell migration in the telencephalon. Eur J Neurosci. 2007;26:537–546. doi: 10.1111/j.1460-9568.2007.05694.x. [DOI] [PubMed] [Google Scholar]
- Hodge RD, D'Ercole AJ, O'Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Cheeran MC, Sheng WS, Ni HT, Lokensgard JR, Peterson PK. Cocaine alters proliferation, migration, and differentiation of human fetal brain-derived neural precursor cells. J Pharmacol Exp Ther. 2006;318:1280–1286. doi: 10.1124/jpet.106.103853. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Lee J, Joh TH, Reis DJ. Expression of tyrosine hydroxylase in neurons of cultured cerebral cortex: evidence for phenotypic plasticity in neurons of the CNS. J Neurosci. 1987;7:1264–1270. doi: 10.1523/JNEUROSCI.07-04-01264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, Duhart HM, Skinner JT, Ali SF. Cocaine induces a differential dose-dependent alteration in the expression profile of immediate early genes, transcription factors, and caspases in PC12 cells: A possible mechanism of neurotoxic damage in cocaine addiction. Ann NY Acad Sci. 2005;1053:482–490. doi: 10.1111/j.1749-6632.2005.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hida H, Jung CG, Miura Y, Nishino H. Treatment with deferoxamine increases neurons from neural stem/progenitor cells. Brain Res. 2006;1092:1–15. doi: 10.1016/j.brainres.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee CT, Chen J, Hayashi T, Tsai SY, Sanchez JF, Errico SL, Amable R, Su TP, Lowe RH, Huestis MA, Shen J, Becker KG, Geller HM, Freed WJ. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008;5:e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Lehrmann E, Hayashi T, Amable R, Tsai SY, Chen J, Sanchez JF, Shen J, Becker KG, Freed WJ. Gene expression profiling reveals distinct cocaine-responsive genes in human fetal CNS cell types. J Addict Med. 2009 doi: 10.1097/ADM.0b013e318199d863. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BA, Singer LT, Short EJ, Minnes S, Arendt R, Weishampel P, Klein N, Min MO. Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicol Teratol. 2004;26:617–627. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21:332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Bozian D, Song ZM. Cocaine affects cerebral neocortical cytoarchitecture in primates only if administered during neocortical neuronogenesis. Brain Res Dev Brain Res. 2001;128:45–52. doi: 10.1016/s0165-3806(01)00139-0. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001;435:263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luján R, Shigemoto R, López-Bendito G. Glutamate and GABA receptor signaling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Kennedy H, Dehay C. Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J Neurosci. 2002;22:6610–6622. doi: 10.1523/JNEUROSCI.22-15-06610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Métin C, Vallee RB, Rakic P, Bhide PG. Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci. 2008;28:11746–11752. doi: 10.1523/JNEUROSCI.3860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Loncar C, Lester BM, Seifer R, Lagasse LL, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Bigsby R, Liu J. Predictors of motor development in children prenatally exposed to cocaine. Neurotoxicol Teratol. 2005;27:213–220. doi: 10.1016/j.ntt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Mirochnick M, Frank DA, Cabral H, Turner A, Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. J Pediatr. 1995;126:636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- Miyama S, Takahashi T, Nowakowski RS, Caviness VS., Jr A gradient in the duration of the G1 phase in the murine neocortical proliferative epithelium. Cereb Cortex. 1997;7:678–689. doi: 10.1093/cercor/7.7.678. [DOI] [PubMed] [Google Scholar]
- Morrow CE, Vogel AL, Anthony JC, Ofir AY, Dausa AT, Bandstra ES. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J Pediatr Psychol. 2004;29:543–554. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5:18–224. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3167–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluch S, Juliano SL. A normal radial glial scaffold is necessary for migration of interneurons during neocortical development. Glia. 2007;55:822–830. doi: 10.1002/glia.20488. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Malanga CJ, Tabit E, Kosofsky BE. Neuropathological consequences of prenatal cocaine exposure in the mouse. Int J Dev Neurosci. 2004;22:309–320. doi: 10.1016/j.ijdevneu.2004.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL. Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Katikaneni LP, McArthur PD, Ibrahim HM, Nesbitt L, Sethuraman G. Head growth in cocaine-exposed infants: relationship to neonate hair level. J Dev Behav Pediatr. 1995;16:77–81. [PubMed] [Google Scholar]
- Sheen VL, Ganesh VS, Topcu M, Sebire G, Bodell A, Hill RS, Grant PE, Shugart YY, Imitola J, Khoury SJ, Guerrini R, Walsh CA. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36:69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, Kliegman R. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995;15:6058–6068. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyssen Van Beveren T, Little BB, Spence MJ. Effects of prenatal cocaine exposure and postnatal environment on child development. Am J Hum Biol. 2000;12:417–428. doi: 10.1002/(SICI)1520-6300(200005/06)12:3<417::AID-AJHB12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Frank DA, Cabral H, Mirochnick M, Zuckerman B. Late dose-response effects of prenatal cocaine exposure on newborn neurobehavioral performance. Pediatrics. 1996;98:76–83. [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26:331–44. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Yan QS. Reduced serotonin release and serotonin uptake sites in the rat nucleus accumbens and striatum after prenatal cocaine exposure. Brain Res. 2002;929:59–69. doi: 10.1016/s0006-8993(01)03378-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Immunostaining of Tuj1- and GABA-positive or glutamate-positive cells in the neocortex during the middle period of neocortical neurogenesis using 40x objective lens. (A) Immunostaining for Tuj1 (green) and GABA (red) in the VZ/SVZ of the neocortex of the control fetus. Yellow arrows indicate the counted signals; yellow arrowheads indicate the excluded signals. Note the GABA-positive cells are overlapped with Tuj1-positive cells in the VZ (yellow arrows in the merged figure). Scale bar = 50 μm. (B) Immunostaining for Tuj1 (green) and glutamate (red) in the SP/CP/MZ of the neocortex of the control fetus. Yellow arrows indicate the counted signals; yellow arrowheads indicate the excluded signals. Scale bar = 50 μm.