Abstract
Human γδ T cells expressing the Vγ2Vδ2 TCR play important roles in immune responses to microbial pathogens by monitoring prenyl pyrophosphate isoprenoid metabolites. Most adult Vγ2Vδ2 cells are memory cytotoxic cells that produce IFN-γ. Recently, murine γδ T cells were found to be major sources of IL-17A in anti-microbial and autoimmune responses. To determine if primate γδ T cells play similar roles, we characterized IL-17A and IL-22 production by Vγ2Vδ2 cells. IL-17A-producing memory Vγ2Vδ2 cells exist at low but significant frequencies in adult humans (1:2,762 T cells) and at even higher frequencies in adult rhesus macaques. Higher levels of Vγ2Vδ2 cells produce IL-22 (1:1,864 T cells) although few produce both IL-17A and IL-22. Unlike adult humans where many IL-17A+ Vγ2Vδ2 cells also produce IFN-γ (Tγδ1/17), the majority of adult macaques IL-17A+ Vδ2 cells (Tγδ17) do not produce IFN-γ. To define the cytokine requirements for Tγδ17 cells, we stimulated human neonatal Vγ2Vδ2 cells with the bacterial antigen, HMBPP, and various cytokines and mAbs in vitro. We find that IL-6, IL-1β, and TGF-β are required to generate Tγδ17 cells in neonates whereas Tγδ1/17 cells additionally required IL-23. In adults, memory Tγδ1/17 and Tγδ17 cells required IL-23, IL-1β, and TGF-β but not IL-6. IL-22-producing cells showed similar requirements. Both neonatal and adult IL-17A+ Vγ2Vδ2 cells expressed elevated levels of RORγt. Our data suggest that, like Th17 αβ T cells, Vγ2Vδ2 T cells can be polarized into Tγδ17 and Tγδ1/17 populations with distinct cytokine requirements for their initial polarization and later maintenance.
Keywords: Interleukin-17A, gamma delta T cell, Vgamma2Vdelta2 T cells, Interleukin-22, human
Introduction
Members of the IL-17 cytokine family (IL-17A through IL-17F) are pro-inflammatory cytokines that possess a diverse array of functions ranging from neutrophil recruitment to induction of wound repair and tissue remodeling. IL-17A induces a plethora of inflammatory cytokines (such as TNF-α, IL-1β, IL-6, GM-CSF, and G-CSF), chemokines (including but not limited to CXCL1, CXCL8, and CXCL10), and matrix metalloproteinases and defensins (1–6). In addition to its role in mediating protection, IL-17A, when dysregulated, has severe pathogenic consequences. Elevated levels of IL-17A have been observed in many autoimmune diseases such as rheumatoid arthritis (7, 8), systemic lupus erythematosus (9, 10), psoriasis (11, 12), and multiple sclerosis (13).
Th17 CD4 αβ T cells have been well described in both humans and mice, and the cytokine requirements for their generation from naïve CD4 T cells have been determined. At present it is believed that IL-6 and/or IL-21 signaling through STAT-3 results in the induction and amplification of Retinoid-related orphan receptor γt4 (RORγt) (rorc) (14) and RORα (rora) in naïve T cells (15). STAT-3, which binds both the Il17A and Il17F promoters (16), then mediates acquisition of IL-17A production capability. IL-6 also induces expression of IL-23R on these developing Th17 precursors (17), thus enabling further STAT-3 signaling through the IL-23R. IL-23/IL-23R signaling through STAT-3 is required by committed Th17 precursors for terminal differentiation of these cells into effector Th17 cells and further maintenance of their phenotype in vivo (18). TGF-β is also required for maximal differentiation of Th17 cells. However, rather than acting directly, TGF-β appears to mediate its effect indirectly by suppressing Th1 and Th2 differentiation by inhibiting STAT-4 and GATA-3, respectively (19). Human Th17 CD4 αβ T cells also appear to require TGF-β for maximal differentiation of Th17 cells (20–22) probably through a similar mechanism (23).
Despite the extensive study of Th17 T cells, IL-17A production is not an exclusive characteristic of CD4 αβ T cells. IL-17A can also be produced by unconventional T cells such as γδ T (reviewed in 24) and αβ NKT (25, 26), as well as macrophages (27) and neutrophils (28). Among unconventional T cells, γδ T cells represent a population of innate-like T cells that developed early in vertebrate phylogeny along with B cells and αβ T cells (29). Much like conventional CD8 αβ T cells, γδ T cells exhibit antigen specificity, robustly proliferate in response to activation, produce pro-inflammatory cytokines (such as TNF-α and IFN-γ), and are highly cytolytic to their targets. However, certain murine γδ T cell subsets are also potent IL-17A producers, and, in some disease settings, γδ T cells constitute a greater fraction of the IL-17A producing cells and secrete IL-17A earlier in disease than conventional CD4 or CD8 αβ T cells (30–35). Furthermore, murine γδ T cells can produce IL-17A, IL-22, and IL-21 in response to IL-23 and IL-1β (36).
Despite their conservation across species, mouse and human γδ T cells demonstrate significant differences. One major difference is the existence of the Vγ2Vδ2 T cell subset (also termed Vγ9Vδ2) in humans and other primates (37), which comprises the majority (up to 90%) of circulating γδ T cells. The orthologous V genes, which rearrange to generate the Vγ2Vδ2 TCR in primates, are absent from mice and other mammals. Vγ2Vδ2 T cells are distinct from conventional αβ T cells in that they are almost exclusively memory cytotoxic T cells producing IFN-γ and TNF-α (38, 39) which can expand to very high levels (commonly >50% of circulating T cells) during in vivo infections with bacteria and protozoa (reviewed in 40, 41). We and others have identified HMBPP, an essential metabolite in isoprenoid synthesis in some bacteria and all Apicomplexan parasites (42–44), as an antigen for Vγ2Vδ2 T cells. By specifically recognizing a common essential microbial metabolite, Vγ2Vδ2 T cells can mount memory responses to many bacterial and parasitic protozoan infections that have never been encountered previously.
Vγ2Vδ2 T cells also recognize, isopentenyl pyrophosphate (IPP), an essential intermediate for isoprenoid synthesis that is common to both microbes and man (45). Under normal circumstances, IPP is sequestered inside host cells at low levels and therefore fails to activate host Vγ2Vδ2 T cells. Certain tumor cells or treatment of human cells with bisphosphonates (46) or alkylamines (47) causes increases in IPP resulting in activation of Vγ2Vδ2 T cells (reviewed in 41). The Vγ2Vδ2 T cell receptor can distinguish foreign HMBPP from self IPP since HMBPP is 30,000-fold more active, stimulating at picomolar concentrations. This recognition by Vγ2Vδ2 γδ T cells allows for immediate memory T cell responses both to microbes and to self IPP when overproduced by malignant cells or after pharmacological treatments.
In contrast to mice, few studies have investigated IL-17A production by human γδ T cells. Human γδ T cells producing IL-17A have been shown to be present in peripheral blood and were slightly increased in patients with active TB infections (48). Similarly, HIV infected patients have an increased frequency of IL-17A-producing Vδ1 T cells (49). However, neither of these studies characterized IL-17A- and IL-22-producing Vγ2Vδ2 T cells in detail or examined the cytokine requirements for IL-17A production by human γδ T cells. Thus, the potential role of γδT cells as sources of IL-17A and IL-22 in human immune responses is unclear.
In this study, we demonstrate that IL-17A and IL-22 producing Vγ2Vδ2 T cells exist at low but significant frequencies in human and non-human primates. Our data suggest that, like Th17 αβ T cells, Vγ2Vδ2 γδ T cells can be polarized into Tγδ17, Tγδ1/17, and Tγδ22 populations with distinct cytokine requirements for their initial polarization and their later maintenance.
Materials and Methods
Antigen and cytokines
HMBPP was synthesized as described (50). Recombinant human IL-6, IL-1β, IL-23, and TGF-β were all purchased from eBioscience. Recombinant human IL-2 (Proleukin) was purchased from Hoffman-La Roche. Neutralizing anti-human IFN-γ, anti-human IL-4, anti-human IL-6, anti-human IL-23 p19, and anti-human IL-1β were purchased from R&D Systems.
Antibodies
FITC-conjugated anti-human Vδ2 TCR (clone B6), allophycocyanin-Cy7-conjugated anti-human CD3 (clone SK7), FITC-conjugated anti-human TCRγδ (clone B1), and PE- or biotin-conjugated anti-human IFN-γ (both clone 4S.B3) were purchased from BD Biosciences. Alexa-Fluor647-conjugated anti-human T-Bet (clone eBio4B10) and Alexa-Fluor647 or PE-conjugated anti-human RORγt (both clone AFKJS-9) were purchased from eBioscience. PerCP-Cy5.5 -conjugated anti-human IL-17A (clone eBio64DEC17) and PE-conjugated anti-human IL-17A (clone eBio64CAP17) were purchased from eBioscience. PE-conjugated anti-human IL-22 (clone 142928) was purchased from R&D Systems. allophycocyanin-conjugated anti-human CD27 (clone O323), biotin-conjugated anti-human CD28 (clone CD28.2), biotin-conjugated anti-human CD4 (clone L3T4) and PE-Cy7 Streptavidin were purchased from eBioscience. For monkey studies, unconjugated anti-human Vδ2 (clone 15D) was purchased from Endogen and FITC-conjugated goat anti-mouse (IgM+IgG Fab fragment) was purchased from Biosource.
Adult PBMC isolation and culture
Normal human or female rhesus macaque peripheral blood was collected by venipuncture and PBMC isolated using Ficoll-Paque Plus from Amersham Biosciences. PBMC in X-VIVO 15 serum free media (BioWhittaker) were cultured at 1×105 cells per well in 96 well round bottom tissue culture plates.
For differentiation and expansion experiments, PBMC were plated as above in X-VIVO 15 serum-free media (unless otherwise stated) and incubated in the presence or absence of 0.316 μM HMBPP, 50 ng/ml rhIL-23, 50 ng/ml rhIL-1β, 50–200 ng/ml rhIL-6, 1 ng/ml rhTGF-β, 10 μg/ml anti-human IL-6, and 10 μg/ml anti-human IL-23. On the third day, 1 nM IL-2 was added to cultures. Cells were cultured for 7–12 days. On the final day, cells were washed, and stimulated with PMA and ionomycin as for ex vivo analyses as described above. Note that the cytokines were titrated in pilot experiments to determine their optimal concentrations. Also, preliminary studies found that the addition of neutralizing anti-IFN-γ and anti-IL-4 were unnecessary for the polarization of IL-17A+ Vγ2Vδ2 T cells and therefore were not added in subsequent polarization experiments (except where otherwise noted). Since there was a high degree of variability between adult human donors, the number of IL-17A+ Vγ2Vδ2 for each donor and condition was normalized to the maximal number of IL-17A+ Vγ2Vδ2 T cells expanded for each particular donor. Such variability was not unexpected as the donors had highly variable frequencies of IL-17A+ Vγ2Vδ2 T cells ex vivo.
Umbilical cord blood mononuclear cell culture
Umbilical cord blood was obtained from normal term deliveries. Cord blood mononuclear cells (CBMC) were isolated from heparinized cord blood using Ficoll-Paque Plus density gradient centrifugation and frozen in liquid nitrogen until needed. For polarization experiments, CBMC were defrosted and plated in X-VIVO 15 serum free media and cultured with or without 200 μM HMBPP and the same cytokine concentrations as were used for adult PBMC polarizations. IL-2 (1 nM) was added on day 3, and the cells re-stimulated with PMA and ionomycin on day 13 for intracellular cytokine staining. Note that neonatal Vγ2Vδ2 T cells from cord blood require higher concentrations of HMBPP and longer incubation periods than adult Vγ2Vδ2 T cells for expansion.
ELISA for IL-17A
To measure the quantity of IL-17A released from expanded T cell cultures, cells were re-stimulated with 50 ng/ml PMA (Sigma) and 2 μg/ml Ionomycin (Sigma) for four-six hours, after which supernatants were collected. IL-17A was quantified in triplicate using the R & D Systems human IL-17 DuoSet ELISA kit.
Flow Cytometric Staining
To examine cytokine production ex vivo, PBMC were rested overnight and the next day stimulated with 50 ng/ml PMA (Sigma) and 2 μg/ml Ionomycin (Sigma) for four-six hours in the presence of GolgiSTOP (monensin) (BD Biosciences) at the manufacturer’s recommended concentration. PBMC were first stained with Live/Dead Blue (Invitrogen), to exclude dead cells, then stained with allophycocyanin-Cy7-conjugated anti-CD3, FITC conjugated anti-Vδ2 or FITC-conjugated anti-TCRγδ. The cells were then washed, fixed, and permeabilized using the BD Cytofix/Cytoperm Kit and then intracellularly stained with either PE-conjugated anti-IL-17A alone or PE-conjugated anti-IFN-γ or PE-conjugated anti-IL-22 combined with PerCP-Cy5.5-conjugated anti-IL-17A. To determine the memory distribution of IL-17A+ cells, cells were stained as above with Live/Dead Blue, allophycocyanin-Cy7-conjugated anti-CD3 and FITC-conjugated anti-Vδ2, and then stained with biotin-conjugated anti-CD28 and allophycocyanin-conjugated anti-CD27. The cells were then fixed and permeabilized as described above, stained with PE-conjugated anti-IL-17A, and incubated with PE-Cy7 Streptavidin.
Similar ex vivo staining was performed for monkeys. Briefly, PBMC were stimulated as above, then stained with Live/Dead Blue and unconjugated anti-human Vδ2 (clone 15D), followed by detection with FITC-conjugated goat anti-mouse. Because the anti-human CD3 mAb clone SK7 does not cross-react with rhesus macaque CD3, it was not used. The cells were then blocked with normal mouse sera, fixed, and permeabilized with the BD Cytofix/Cytoperm Kit. The monkey cells were then intracellularly stained with PerCP-Cy5.5-conjugated anti-IL-17A, PE-conjugated anti-IFN-γ, and Alexa-Fluor647-conjugated anti-T-bet.
Post expansion, human PBMC and CBMC were re-stimulated with PMA (50 ng/ml) and ionomycin (2 μg/ml) in the presence of GolgiSTOP for four-six hours. The cells were then stained with Live/Dead Blue, followed by surface staining with FITC-conjugated anti-Vδ2, APC-Cy7-conjugated anti-CD3, and biotin-conjugated anti-CD4. The cells were fixed and permeabilized as previously described and then intracellularly stained with either PE-conjugated anti-IFN-γ, Alexa-Fluor647-conjugated anti-IL-22, and PerCP-Cy5.5-conjugated anti-IL-17A or PE-conjugated anti-RORγt, and Alexa-Fluor647-conjugated anti-T-bet, and PerCP-Cy5.5-conjugated anti-IL-17A. Lastly the biotin label was detected with PE-Cy7 Streptavidin.
To assess Vγ2Vδ2 T cells, an anti-Vδ2 mAb was used for all analyses since in adults the vast majority (96.4–100%) of Vδ2 T cells expressVγ2Vδ2 TCRs (51, 52). In fact, 17 out of 36 donors had greater than 99.6% of Vδ2 chains paired with Vγ2 chains (51). The anti-Vδ2 mAb was also used to determine Vγ2Vδ2 T cells after expansion by HMBPP since only γδ T cells expressing Vγ2Vδ2 TCRs respond and expand to prenyl pyrophosphates (53, 54). After expansion of γδ T cells by prenyl pyrophosphates, 100% of Vδ2 T cells express Vγ2Vδ2 TCRs. In neonates prior to prenyl pyrophosphate expansion, about equal proportions of Vδ;2 chains are paired with Vγ2 as paired with Vγ1 (51). However, we did not assess ex vivo production of IL-17A and IL-22 by neonates since the number of Vγ2Vδ2 T cells was too low. Therefore, we have used Vγ2Vδ2 to designate Vδ2 T cells in the text although a very small fraction of the cells may express Vγ1Vδ2 TCRs.
Statistical analyses
Vγ2Vδ2 T cell number was determined by multiplying the frequency of cells within the live cell gate by the total number of live cells in 20 round-bottom wells of a 96 well plate. Cell counts were determined by trypan blue exclusion prior to PMA and ionomycin stimulation. For statistical analyses, the nonparametric Kruskal-Wallis test, followed by Dunn’s post-test was used with p < 0.5 considered statistically significant. Statistical analyses were done in Prism version 4.0c (GraphPad Software).
Results
Frequency of circulating IL-17A- and IL-22-producing Vγ2Vδ2 T cells in normal humans and rhesus macaques
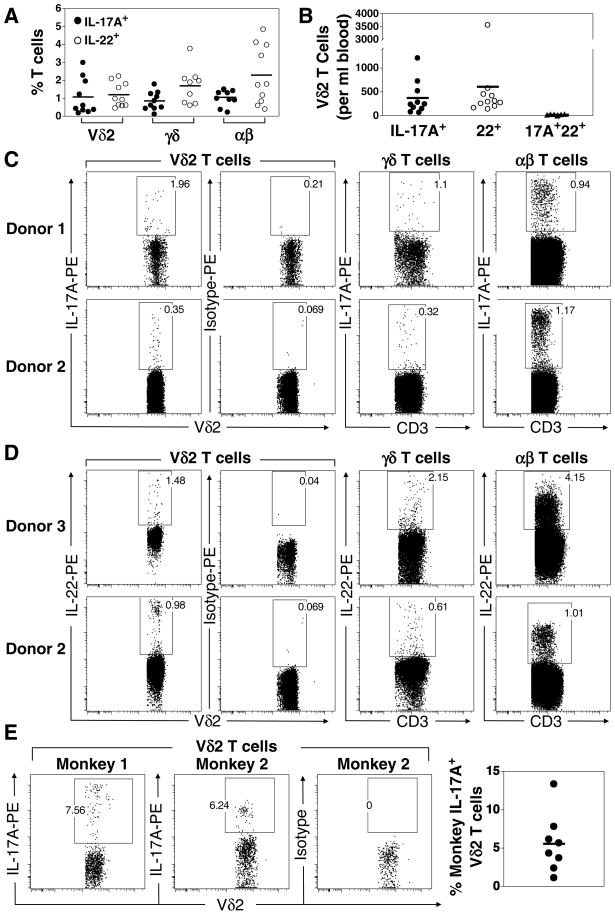
Naïve γδ T cells in mice are epigenetically programmed to be potent IFN- γ-producing Th1-like cells by virtue of constitutive expression of eomesodermin (Eomes) and poor methylation of the IFN-γ locus as compared with naïve CD4 T cells (55, 56). Nonetheless, although most human Vγ2Vδ2 T cells produce IFN-γ, minor populations have been identified that produce IL-4 and IL-10 (57). This suggests that Vγ2Vδ2 T cells, like αβ T cells, can be polarized into different functional lineages. To investigate the existence of Th17-like Vγ2Vδ2 T cells in humans, we isolated peripheral blood mononuclear cells (PBMC) from 10 normal donors, stimulated the PBMC with the mitogen, ionomycin, in the presence of PMA, and performed intracellular cytokine staining. Ex vivo mitogen stimulation of T cells revealed that IL-17A-producing Vγ2Vδ2 T cells were present in most donors although the proportions varied widely ranging from 0.2 to 3% of Vγ2Vδ2 T cells with an average of 1.1 ± 0.3% (Fig. 1A, 1C, and Table 1). No IL-17A production from Vγ2Vδ2 T cells was observed in the absence of ionomycin and PMA stimulation (data not shown). An average of 0.9 ± 0.2% of peripheral blood γδ T cells secreted IL-17A. These proportions were similar to αβ T cells where an average of 1.1 ± 0.1% produced IL-17A (Fig. 1A and 1C). Thus, in one ml of blood an average of 389 ± 112 of Vγ2Vδ2 cells produced IL-17A (Fig. 1B and Table I) and the frequency of IL-17A-producing Vγ2Vδ2 T cells averages 1 out of every 2,762 T cells (Table I).
FIGURE 1. Frequency of Vγ2Vδ2 T cells producing IL-17A and IL-22 in adult human and rhesus macaque donors.
A. PBMC from 10 normal donors were stimulated with PMA and ionomycin and intracellular cytokine staining for IL-17A and IL-22 was performed. Viable T cells were gated using live/dead blue and anti-CD3 after which the different T cell subsets discriminated using anti-Vδ2 (to identify Vγ2Vδ2 T cells) and anti-pan γδ (to identify total γδ T cells). αβT cells were defined as CD3+,γδ−. B. Numbers of IL-17A+ Vγ2Vδ2, IL-22+ Vγ2Vδ2 and IL-17A+, IL-22+ Vγ2Vδ2 T cells per milliliter of blood were calculated (Table 1). C. Representative IL-17A staining for Vγ2Vδ2 T cells (abbreviated Vδ2 T cells), total γδ T cells, and total αβ T cells. D. Representative IL-22 staining for Vγ2Vδ2 T cells (abbreviated Vδ2 T cells), total γδ T cells, and total αβ T cells. E. Representative IL-17A staining and average frequency of IL-17A+ Vδ2 T cells among eight rhesus macaques. Each point refers to one donor and bars depict means.
Table I.
Frequency of adult IL-17A- and IL-22-producing human Vγ2Vδ2 T cells1
| Donor | PBMC per ml blood | % Vδ2 among CD3 T cells | % IL-17A+ among Vδ2 | % IL-22+ among Vδ2 | Frequency IL-17A+ Vδ2 among CD3 T cells | IL-17A+ Vδ2 per ml blood | Frequency IL-22+ Vδ2 among CD3 T cells | IL-22+ Vδ2 per ml blood |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.70×106 | 3.99 | 0.61 | 0.64 | 1:4,109 | 239 | 1:3,916 | 256 |
| 2 | 2.36×106 | 1.81 | 1.96 | 1.60 | 1:2,819 | 283 | 1:3,456 | 142 |
| 3 | 1.45×106 | 2.04 | 2.29 | 2.24 | 1:2,141 | 522 | 1:2,188 | 421 |
| 4 | 1.93×106 | 3.58 | 0.44 | 0.93 | 1:6,348 | 147 | 1:3,004 | 309 |
| 5 | 3.33×106 | 4.18 | 0.19 | 0.46 | 1:12,591 | 67 | 1:5,201 | 167 |
| 6 | 1.89×106 | 6.88 | 0.26 | 0.62 | 1:5,590 | 246 | 1:2,344 | 454 |
| 7 | 3.57×106 | 23.9 | 0.35 | 0.98 | 1:1,195 | 1213 | 1:427 | 3567 |
| 8 | 1.23×106 | 3.68 | 3.00 | 2.10 | 1:906 | 728 | 1:1,294 | 580 |
| 9 | 2.25×106 | 1.24 | 0.38 | 1.81 | 1:21,222 | 80 | 1:4,456 | 293 |
| 10 | 2.82×106 | 1.23 | 1.23 | 0.59 | 1:6,610 | 367 | 1:13,780 | 203 |
| Mean±SEM | 2.25±2.46×106 | 5.25±2.14 | 1.07±0.32 | 1.20±0.21 | 1:2,762±1:9,047 | 389±112 | 1:1,864±1:4,775 | 639±328 |
PBMC were harvested from normal adult donors, counted, and stimulated with 50 ng/ml PMA and 2 μg/ml Ionomycin for 4–6 h in the presence of GolgiSTOP (monensin). PBMC were then stained with Live/Dead Blue (Invitrogen) followed by APC-Cy7-conjugated anti-CD3, FITC conjugated anti-Vδ2 or FITC-conjugated anti-TCR γδ. The cells were then washed, fixed, and permeabilized using the BD Cytofix/Cytoperm Kit and then intracellularly stained with either PE-conjugated anti-IL-17A alone or PE-conjugated anti-IFN-γ or PE-conjugated anti-IL-22 combined with PerCP-Cy5.5-conjugated anti-IL-17A.
Because the Vγ2Vδ2 TCR is exclusively expressed in primates and not expressed by murine γδ T cells, the rhesus macaque (Macaca mulatta) is a useful animal model to study Vγ2Vδ2 T cells in vivo (58). Therefore, we next asked whether Vγ2Vδ2 T cells in rhesus macaques produce IL-17A. PBMC were isolated from 8 macaques, stimulated with ionomycin in the presence of PMA, and cytokine production determined by intracellular staining. The frequency of peripheral blood IL-17A-producing Vδ2 T cells ex vivo was increased with a mean frequency of 5.6 ± 1.3% (ranging from 1.1 to 13.4%, see Fig. 1E) compared to 1.1 ± 0.3% in humans. We noted similar frequencies in splenic T cells from another 2 rhesus macaques (data not shown). Taken together, these results demonstrate that an IL-17A+ Vδ2 T cell population, parallel to the Th17 αβ T cell subset, exists in humans and that this population is conserved in non-human primates albeit at higher levels.
IL-22 is believed to be produced by Th17-lineage T cells and is thought to help epithelial healing (59) and to mediate epithelial inflammation since it is elevated in the skin of patients with psoriasis (60, 61) and in the colonic mucosa of patients with Crohn’s disease (62). We found that IL-22-producing Vγ2Vδ2 T cells were a separate subset of cells distinct from IL-17A-producing Vγ2Vδ2 T cells since only 2.7% of IL-22-producing cells also produced IL-17A (Fig. 2A, 3B, 4B, Supplemental Fig. 1). The frequency of Vγ2Vδ2 T cells producing IL-22 averaged 1.2 ± 0.2% (ranging from 0.5 to 2.2%) of peripheral blood Vγ2Vδ2 T cells. The frequency of αβ T cells producing IL-22 among these same donors averaged 2.3 ± 0.5%. When the absolute cell numbers were calculated (Table I), there were 1.6-fold more IL-22-producing Vγ2Vδ2 T cells than IL-17A-producing Vγ2Vδ2 T cells (639 ± 328 cells/ml producing IL-22 versus 389 ± 112 cells/ml producing IL-17A) or 1 out of every 1,864 total T cells. Very few produced both IL-17A and IL-22 (17 ± 4 cells/ml). Thus, our results show that IL-17A- and IL-22-producing cells are separate populations of Vγ2Vδ2 T cells.
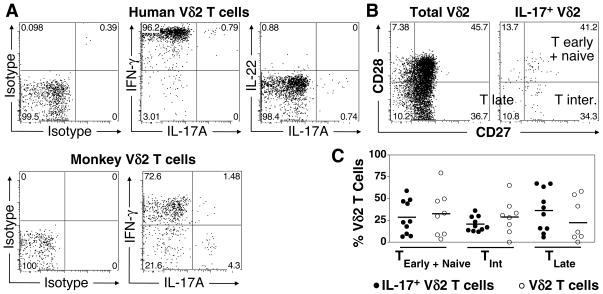
FIGURE 2. Cytokine profile and memory phenotype of IL-17A+ Vγ2Vδ2 T cells from adult human and monkey donors.
A. IFN-γ production by IL-17A-producing Vδ2 T cells. PBMC were stimulated with PMA and ionomycin and stained intracellularly for IL-17A, IFN-γ, and IL-22. Shown is a representative human (top) and monkey (bottom) donor. B. Representative surface staining for memory markers, CD27 and CD28 on total Vγ2Vδ2 T cells or IL-17A+ gated Vγ2Vδ2 T cells. C. Frequency of total human Vγ2Vδ2 T cells or IL-17A+ gated Vγ2Vδ2 T cells belonging to T early + naïve (CD27+, CD28+), T intermediate (CD27+, CD28−) or T late (CD27−, CD28−) memory subsets. Each point represents one donor and bars depict means.
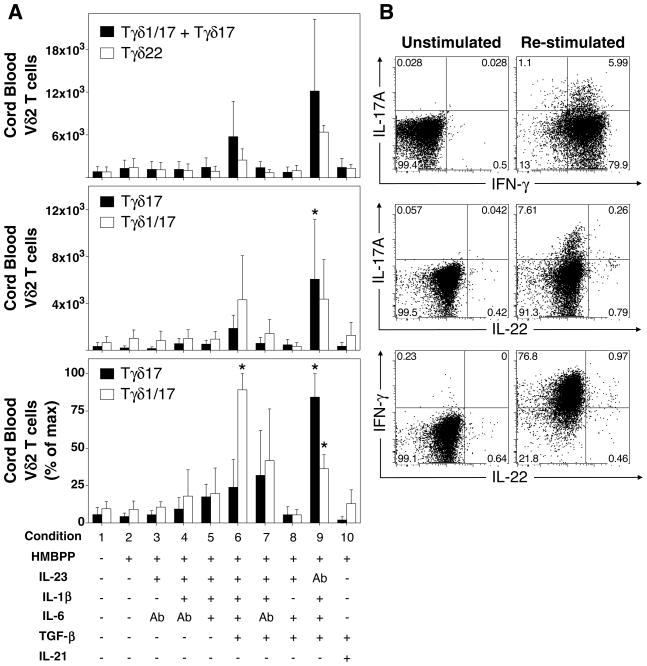
FIGURE 3. IL-1β, TGF-β, and IL-6 induce maximal polarization of IL-17A+ neonatal CD4− Vγ2Vδ2 T cells upon antigen stimulation with HMBPP.
Umbilical cord blood mononuclear cells were expanded in the presence or absence of HMBPP, IL-23, IL-1β, TGF-β, IL-6, neutralizing anti-IL-6, or neutralizing anti-IL-23 for 13 days (n = 8 individuals for IL-17A data and n = 4 for IL-22 data). IL-2 was added on day 3. On the final day, cells were restimulated with PMA and ionomycin and intracellular staining for IL-17A, IL-22, and IFN-γ performed. Expanded cord blood CD4− Vγ2Vδ2 T cells (defined as Vδ2+, CD3+, CD4−) were divided into IFN-γ−, IL-17A+ Vγ2Vδ2 T cells (termed Tγδ17), IFN-γ+, IL-17A+ Vγ2Vδ2 T cells (termed Tγδ1/17), IFN-γ+, IL-17A− Vγ2Vδ2 T cells (termed Tγδ1), and IFN-γ+/−, IL-22+ Vγ2Vδ2 (termed Tγδ22). A. (Top) Median number of total IL-17A+ CD4− Vγ2Vδ2 T cells (combined Tγδ1/17 and Tγδ17) or Tγδ22 Vγ2Vδ2 T cells among total CD4− Vγ2Vδ2 T cells for each cytokine condition. (Middle) Median number of Tγδ17 or Tγδ1/17 CD4− Vγ2Vδ2 T cells among total CD4− Vγ2Vδ2 T cells for each condition. (Bottom) Median percent of maximum Tγδ17 or Tγδ1/17 CD4− Vγ2Vδ2 T cells expanded for each condition. B. Representative cytokine staining on viable CD4− Vγ2Vδ2 T cells expanded in the presence of HMBPP, IL-1β, TGF-β, IL-6, and anti-IL-23, either unstimulated (left) or restimulated with PMA and ionomycin (right). Bars depict medians and error bars depict median absolute error. *p < 0.05, Kruskal-Wallis comparison with condition 2.
FIGURE 4. IL-23 is required for expansion of adult IL-17+ Vγ2Vδ2 T cells.
IL-17A-producing Vγ2Vδ2 T cells, in serum-supplemented media, were measured in PBMC after expansion with HMBPP, IL-1β, IL-6, neutralizing anti-IL-4, and neutralizing anti-IFN-γ in the presence or absence of IL-23. IL-2 was added on day 3. On day 12, cells were re-stimulated with PMA and ionomycin, after which the supernatants and cells were harvested for analysis. Expanded Vγ2Vδ2 T cells were defined as Vδ2+, CD3+. Representative of 2 donors. A. Cytokine profile of expanded Vγ2Vδ2 T cells. Intracellular staining for IL-17A, IL-22, and IFN-γ, (or isotype control) in the presence or absence of IL-23 is shown. B. Percent and total number of IL-17+ Vγ2Vδ2 T cells in the presence or absence of exogenous IL-23 (top two panels). Total expanded Vγ2Vδ2 T cells on day 12 (third panel). Total IL-17A protein released into culture as determined by ELISA (bottom panel).
Phenotype of IL-17A+ Vγ2Vδ2 T cells
Classically defined murine Th17 have been reported to produce IL-17A, IL-17F, and IL-22 but not IFN-γ (63). Nonetheless, CD4 T cells producing both IL-17A and IFN-γ (64–66) and CD4 T cells producing IL-17A without IL-22 have been observed (67–70). Regardless of the other cytokines co-produced, both CD4 and CD8 αβ T cells producing IL-17A have been exclusively detected within memory subsets (64, 65, 68, 71, 72). We therefore determined the spectrum of cytokines co-produced by IL-17A+ Vγ2Vδ2 T cells and the memory phenotype of IL-17A+ Vγ2Vδ2 T cells. After stimulation with PMA and ionomycin, most IL-17A+ Vγ2Vδ2 T cells co-produced IFN-γ, almost none co-produced IL-22 (Fig. 2A), IL-4, or IL-10 (data not shown). In contrast, fewer rhesus macaque IL-17A+ Vγ2Vδ2 T cells dual produced IFN-γ, with most being IL-17A single producers (representative staining in Fig. 2A). Consistent with published work (55, 57, 73, 74), and their memory-like phenotype, we observed that the vast majority of human peripheral blood Vγ2Vδ2 T cells produced IFN-γ (> 90%) and a small fraction (< 5%) produced IL-4 (data not shown and 55, 57, 73, 74).
Unlike αβ T cells, Vγ2Vδ2 T cells transition very early in life into phenotypically memory cells leaving few naïve Vγ2Vδ2 T cells in the adult circulation (< 2% naïve Vγ2Vδ2, C. Jin and C. T. Morita, unpublished observations and 75, 76). The process by which this occurs is not fully understood but probably is the result of stimulation by the ubiquitous foreign and self prenyl pyrophosphate antigens. Reminiscent of CD8 αβ T cells, Vγ2Vδ2 T cells can be subdivided into memory subsets based on their expression of the CD27 and CD28 costimulatory receptors. Analogous to CD8 T cell development, Vγ2Vδ2 T cells can be divided into CD27+, CD28+ early memory cells (central memory), CD28−, CD27+ intermediate memory cells, and CD27−, CD28− late memory cells (CD45RA+ effector memory) (77). Naïve Vγ2Vδ2 T cells represent < 2% of adult Vγ2Vδ2 T cells and constitute only a negligible proportion of CD27+ CD28+ Vγ2Vδ2 T cells that are distinguishable from central memory cells by their lack of CD45RO and their high level expression of CD45RA (C Jin and C. T. Morita, unpublished observations). To characterize the memory status of IL-17A+ Vγ2Vδ2 T cells, we performed staining for CD27 and CD28 on human PBMC after PMA and ionomycin re-stimulation. We observed similar proportions of Tearly (+ Tnaïve), Tintermediate, and TCD45RA late cells in IL-17A+ Vγ2Vδ2 as found in total Vγ2Vδ2 T cells (Fig. 2B and 2C). Thus, in contrast to IL-17A+ Tc17 CD8 αβ T cells that are almost exclusively restricted to the Tearly and Tintermediate subsets (72), IL-17A+ Vγ2Vδ2 T cells were found within all three memory subsets without skewing.
Cytokine requirements for the differentiation and expansion of neonatal IL-17A+ Vγ2Vδ2 T cells
Unlike CD4 and CD8 αβ T cells, little is known about the cytokines required to differentiate naïve γδ T cells to produce IL-17A. Although umbilical cord blood represents the best source for naïve Vγ2Vδ2 T cells, even in cord blood only ~50% of the Vγ2Vδ2 T cells are phenotypically naïve (C Jin and C. T. Morita, unpublished observations). Given the low frequency of Vγ2Vδ2 T cells in cord blood (< 1% of T cells (51)), isolating pure naïve Vγ2Vδ2 T cells for in vitro polarization studies was not feasible. Therefore, we studied the polarization of total neonatal cord blood Vγ2Vδ2 T cells where ~50% have a naïve surface phenotype and none have been exposed to foreign antigens. Because a high proportion of Vγ2Vδ2 T cells react to the HMBPP antigen without prior selection (41), we were able to specifically expand Vγ2Vδ2 T cells directly from cord blood without purification. To determine the cytokine requirements for polarization of Vγ2Vδ2 T cells into IL-17A-producing cells, we cultured Vγ2Vδ2 T cells from 8 different donors in serum free media for 13 days with HMBPP and various combinations of the classical Th17 polarizing cytokines, IL-6, IL-1β, IL-21, IL-23, and TGF-β and neutralizing cytokine antibodies. IL-2 was added on the third day. On the final day, cells were re-stimulated with PMA and ionomycin, surface stained for CD3 and Vδ2 (to identify Vγ2Vδ2 T cells, see Methods) and intracellularly stained for IL-17A, IFN-γ, and IL-22 as well as the transcription factors, RORγt and T-bet. We hypothesized that naïve Vγ2Vδ2 T cells, like naïve CD4 αβ T cells, can be polarized under similar cytokine conditions (namely TGF-β, IL-6, IL-21, and IL-1β) into IL-17A-producing Tγδ17 Vγ2Vδ2 T cells.
Expansion of cord blood Vγ2Vδ2 T cells in response to antigen stimulation with HMBPP ranged from 8% to more than 20% of total CD3 T cells (Supplemental Fig. 2). Expanded Vγ2Vδ2 T cells were divided into IL-17A+ IFN-γ− (Tγδ17), IL-17A+ IFN-γ+ (Tγδ1/17), IL-22+ IFN-γ+/− (Tγδ22) subsets and their total numbers plotted (Fig. 3A). Representative staining for IL-17A, IFN-γ, and IL-22 is shown in Fig. 3B for condition 9. Because each donor differed in the magnitude of expansion, the number of IL-17A+ Vγ2Vδ2 for each donor and condition was normalized to the maximal number of IL-17A+ Vγ2Vδ2 T cells expanded for each donor (Fig. 3A, bottom panel).
At baseline in the presence or absence of HMBPP (Fig. 3A, conditions 1 and 2), very few IL-17A+ (Tγδ17 and Tγδ1/17) and IL-22+ (Tγδ22) Vγ2Vδ2 T cells were observed. IL-23 alone and IL-23 plus IL-1β had minimal effects on the expansions of Tγδ1/17 and Tγδ 17 Vγ2Vδ2 T cells (Fig. 3A, conditions 3 and 4). The combined effect of IL-23, IL-6, and IL-1β also had little effect on the numbers of Tγδ17 or Tγδ1/17 (condition 5). However, when IL-23, IL-6, and IL-1β were combined with TGF-β, a statistically significant increase in the number of Tγδ1/17 cells (normalized to each donor’s maximal response) was observed (Fig. 3A, bottom panel, compare condition 5 lacking TGF-β with condition 6 containing TGF-β). When endogenous IL-6 was neutralized in the presence of IL-23, IL-1β, and TGF-β, the number of Tγδ1/17 returned to moderate levels (Fig. 3A, compare conditions 6 and 7), suggesting an important role for IL-6 in the expansion of Tγδ1/17 Vγ2Vδ2 T cells. However, exogenous IL-1β, in combination with IL-6, IL-23, and TGF-β, was critical for expansion of both Tγδ17 and Tγδ1/17 (Fig. 3A, compare conditions 7 and 8). Taken together, these data suggest that neonatal Tγδ17 and Tγδ1/17 populations similarly require IL-1β and TGF-β but that the Tγδ1/17 population additionally requires IL-6.
Because IL-23 is considered a maintenance cytokine for memory Th17 T cells (78), we hypothesized that IL-23 would not be required for initial polarization of naïve cord blood Vγ2Vδ2 T cells into Tγδ17 (or Tγδ1/17) T cells. To test this hypothesis, we polarized Vγ2Vδ2 T cells in the presence of IL-1β, IL-6, TGF-β, and neutralizing anti-IL-23. As predicted, we observed a statistically significant expansion in Tγδ17 and Tγδ1/17 Vγ2Vδ2 T cells even when IL-23 was neutralized (Fig. 3A, bottom, condition 9). These results support our hypothesis that IL-23 is not required for initial polarization of cord blood Vγ2Vδ2 into Tγδ17, but that IL-1β, IL-6, and TGF-β are required. Furthermore, our results suggest that exogenous IL-23 may actually inhibit Tγδ17 development since more Tγδ17 cells were found after its neutralization. Similar results were noted for a second small subset of cord blood Vγ2Vδ2 T cells expressing CD4. These cells showed very similar responses to cytokines with optimal expansion with IL-1β, IL-6, TGF-β, and neutralization of IL-23 (Supplemental Fig. 1 and 2). In contrast, the effect of IL-23 on Tγδ1/17 cells was different. This subset required IL-23 with IL-1β, IL-6, and TGF-β for optimal expansion (compare condition 6 to condition 9). Thus, IL-23 favors the differentiation/and or expansion of neonatal Vγ2Vδ2 T cells as Tγδ1/17 cells rather than Tγδ17 cells.
IL-21 and TGF-β also polarize naïve CD4 αβ T cells to a Th17 phenotype (22). Therefore, we tested whether IL-21 and TGF-β would similarly polarize neonatal Vγ2Vδ2 T cells into Tγδ17 and/or Tγδ1/17 cells. This combination (Fig. 3A, condition 10) failed to increase the number and/or percent of Tγδ17 or Tγδ1/17 Vγ2Vδ2 T cells, despite robust proliferation of Vγ2Vδ2 T cells (Supplemental Fig. 2, condition 10). Thus, unlike CD4 αβ T cells, IL-21 and TGF-β were insufficient to support the development of IL-17A-producing Vγ2Vδ2 T cells.
Like IL-17A-producing Vγ2Vδ2 T cells, the numbers of Tγδ22 Vγ2Vδ2 T cells tended to increase in the presence of IL-1β, IL-6, and TGF-β (Fig. 3A, top, condition 9), although this increase did not reach statistical significance. Thus, our data suggest that although the Tγδ22 cell population is distinct from Tγδ1/17 and Tγδ17 cell populations (Fig. 3B), Tγδ22 Vγ2Vδ2 T cells require similar cytokines as those observed for Tγδ17 cells.
Cytokine requirements for the expansion of adult IL-17A+ Vγ2Vδ2 T cells
We next asked whether IL-17A+ Vγ2Vδ2 T cells could be expanded from adult peripheral blood, and if similar cytokines were required to those required for neonatal IL-17A+ Vγ2Vδ2 T cells. Because the vast majority of adult Vγ2Vδ2 T cells are memory cells and since IL-23R expression is restricted to memory CD4 T cells (79), we hypothesized that, like memory Th17 αβ T cells, expansion of adult IL-17A+ Vγ2Vδ2 T cells would require IL-23. IL-1β might also be required since it is important for the expansion of human CD4 Th17 T cells (80, 81), and since the combination of IL-23 and IL-1β induces IL-17A production by murine γδ T cells (36). To determine the role of IL-23, we expanded adult Vγ2Vδ2 T cells in PBMC with HMBPP, IL-1β, IL-6, neutralizing anti-IL-4, and neutralizing anti-IFN-γ in the presence or absence of IL-23 (Fig. 4). On the twelfth day, cells were restimulated with PMA and ionomycin and stained for IL-17A, IL-22, and IFN-γ intracellular cytokines. The addition of IL-23 to IL-6 and IL-1β increased the frequency of IL-17A+ Vγ2Vδ2 T cells from 1.1% (roughly the same frequency of IL-17A+ Vγ2Vδ2 present ex vivo) to 9.4% (Fig. 4) due to an 8-fold increase in the number of IL-17A+ Vγ2Vδ2 T cells (Fig. 4B). Similarly, IL-17A levels increased 5.2-fold from 276 pg/ml in the absence to 1431 pg/ml in the presence of IL-23 (Fig. 4B). In these cultures, TGF-β was likely provided by serum included in the media. Thus, exogenous IL-23 can increase the numbers of IL-17A-producing Vγ2Vδ2 T cells.
To study this in more depth, Vγ2Vδ2 T cells from 10 adult donors were stimulated with HMBPP in the presence or absence of different cytokines to determine the cytokine requirements for the expansion of IL-17A-producing Vγ2Vδ2 T cells. To determine if TGF-β was required, serum free media was used. We found that the expanded adult Vγ2Vδ2 T cells, like neonatal Vγ2Vδ2 T cells, could be divided into IL-17A+ IFN-γ+ (Tγδ1/17), IL-17A+ IFN-γ− (Tγδ17), and IL-22+ IFN-γ+/− (Tγδ22) populations (representative staining for IL-17A and IFN-γ is shown in Fig. 4A and 5B). The vast majority of IL-17A+ Vγ2Vδ2 T cells expanded from adult blood were Tγδ1/17 producing both IFN-γ and IL-17A (Fig. 5B). Fewer IL-17A+ IFN-γ− (Tγδ17) Vγ2Vδ2 T cells were detected in adults with only 4 out of 10 adult donors exhibiting expansions of both Tγδ17 and Tγδ1/17 Vγ2Vδ2 T cells (compared with Tγδ1/17 cells in Supplemental Fig. 3). These in vitro results were consistent with the ex vivo results since only these same four donors had detectable Tγδ17 cells after stimulation.
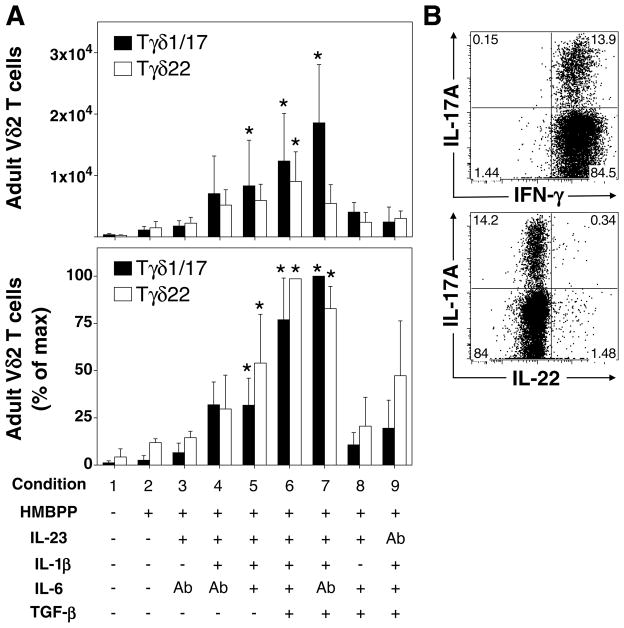
FIGURE 5. IL-23, IL-1β, and TGF-β are sufficient for polarization of adult IL-17A+ Vγ2Vδ2 T cells after stimulation with HMBPP.
Total PBMC, from 10 donors, were cultured in the presence or absence of HMBPP, IL-23, IL-1β, TGF-β, IL-6, neutralizing anti-IL-6, or neutralizing anti-IL-23 for seven days. IL-2 was added on day 3. On the seventh day, cells were re-stimulated with PMA and ionomycin and intracellular staining for IL-17A, IL-22, and IFN-γ was performed. Expanded PBMC Vγ2Vδ2 T cells (defined as Vδ2+, CD3+, CD4−) could be divided into IFN-γ+, IL-17A+ Vγ2Vδ2 T cells (termed Tγδ1/17), IFN-γ+, IL-17A− Vγ2Vδ2 T cells (termed Tγδ1), and IFN-γ+/−, IL-22+ Vγ2Vδ2 (termed Tγδ22). No IFN-γ −, IL-17A+ Vγ2Vδ2 T cells (Tγδ17) were detected in these adult donors. A. (Top) Median number of Tγδ1/17 or Tγδ22 Vγ2Vδ2 T cells among total Vγ2Vδ2 T cells for each cytokine condition. (Bottom) Median percent of maximum Tγδ1/17 Vγ2Vδ2 T cells expanded for each cytokine condition. B. Representative cytokine staining on Vγ2Vδ2 T cells expanded in the presence of HMBPP, IL-23, IL-1β, TGF-β, and anti-IL-6, and restimulated with PMA and ionomycin. Bars depict medians and error bars depict median absolute error. * p < 0.05, Kruskal Wallis comparison with condition 2.
IL-17A+ (Tγδ1/17) or IL-22+ (Tγδ22) Vγ2Vδ2 T cells were not preferentially expanded in the presence of HMBPP and IL-2 only (Fig. 5A, condition 2). Addition of IL-23 alone had minimal effect on the expansion of Tγδ1/17 Vγ2Vδ2 T cells (Fig. 5A, condition 3) but did increase expansion of total Vγ2Vδ2 T cells from 12.7% to 25.1% of CD3 T cells (Supplemental Fig. 4A). In contrast to neonatal Vγ2Vδ2 T cells, there were moderate increases in Tγδ1/17 cells with IL-23 and IL-1β (condition 4). The addition of IL-6 to IL-23 and IL-1β had little effect on Tγδ1/17 Vγ2Vδ2 T cell numbers (Fig. 5A, top, condition 5) or the proportion of Tγδ1/17 cells (Fig. 5A, bottom). However, the addition of TGF-β to IL-23 and IL-1β further increased the expansion of Tγδ1/17 Vγ2Vδ2 T cells (Fig. 5A, conditions 6 and 7, p < 0.5). Again the presence (condition 6) or absence (condition 7) of IL-6 had little effect on IL-17A+ cell numbers or in the expansion of total Vγ2Vδ2 T cells (Supplemental Fig. 4).
Like neonatal Vγ2Vδ2 T cells, IL-1β appeared to also be critical for the expansion of adult Tγδ1/17 Vγ2Vδ2 T cells, because in its absence (Fig. 5A, condition 8) the number of Tγδ1/17 Vγ2Vδ2 T cells fell to low levels. However, unlike neonatal Vγ2Vδ2 T cells, neutralization of IL-23 in the presence of IL-1β, IL-6, and TGF-β caused Tγδ1/17 Vγ2Vδ2 T cell numbers to also drop to low levels (compare Fig. 5A, condition 9 to Fig. 3A, condition 9). These results support part of our hypothesis – that the expansion of Tγδ1/17 Vγ2Vδ2 T cells from adult PBMC required IL-23 and IL-1β – however we were surprised to see that TGF-β was also important for expansion of these cells.
A minority of adult donors (4 out of 11) had detectable expansions in Tγδ17 Vγ2Vδ2 T cells. These individuals were analyzed separately, and the cytokine requirements for Tγδ17 were compared with those for Tγδ1/17 (Supplemental Fig. 3). As with the expansions of Tγδ1/17 from the other adult donors, statistically significant increases in Tγδ17 cells in these four donors were observed for the combination IL-23, IL-1β, and TGF-β in the presence or absence of IL-6. This same combination (IL-23, IL-1β, and TGF-β, in the absence of IL-6) also optimally expanded Tγδ1/17 cells in these donors. Together, these results suggest that adult peripheral blood IL-17A+ Vγ2Vδ2 T cells, be they Tγδ17 or Tγδ1/17, have similar requirements for IL-23, IL-1β, and TGF-β.
Although we were unable to expand significant numbers of Tγδ22 Vγ2Vδ2 T cells from cord blood, significant numbers of Tγδ22 Vγ2Vδ2 T cells were easily expanded from adult peripheral blood. IL-23, IL-1β, and IL-6 were the minimal cytokines required for statistically significant expansion of Tγδ22 Vγ2Vδ2 T cells (Fig. 5A, bottom, condition 5 and Fig. 5B). Addition of TGF-β to these cytokines enhanced expansion of Tγδ22 Vγ2Vδ2 T cells (Fig. 5A, bottom, condition 6). As with neonatal Vγ2Vδ2 T cells after in vitro expansion and adult Vγ2Vδ2 T cells ex vivo, Vγ2Vδ2 T cells producing both IL-22 and IL-17A were extremely rare among expanded Vγ2Vδ2 T cells (Fig. 5B, bottom panel). These results provide further evidence that Tγδ22 are a separate population distinct from the Tγδ1/17 and Tγδ17 populations.
Regulation of Vγ2Vδ2 T cells by RORγt and T-bet transcription factors
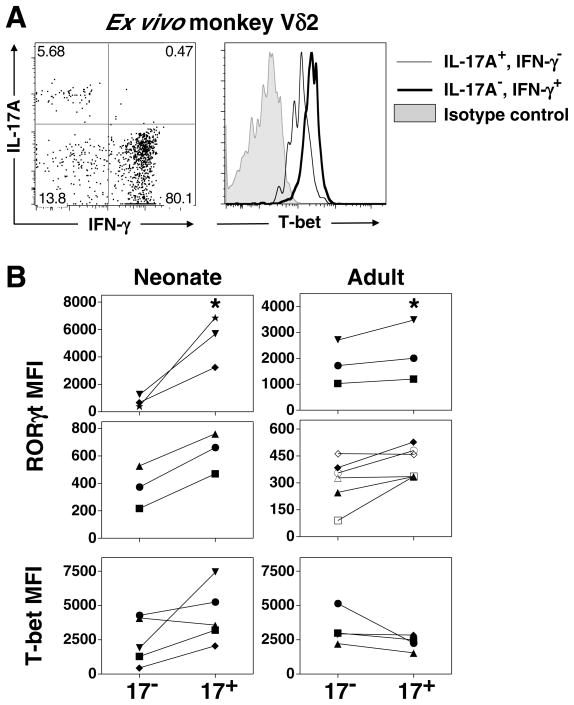
Differentiation of Th17 cells involves the coordinated upregulation of the key transcription factors, RORγt (RORC2) and RORα, as well as the corresponding epigenetic changes to reinforce these genes and suppress others (82–84). Murine IL-17A-producing γδ T cells are virtually absent from RORγt deficient mice, suggesting that IL-17A production by murine γδ T cell requires this transcription factor. We therefore determined whether human IL-17A+ Vγ2Vδ2 T cells similarly express RORγt. We hypothesized that IL-17A+ Vγ2Vδ2 T cells have increased expression of RORγt and decreased expression of T-bet. To test this hypothesis, we performed intracellular staining for RORγt and T-bet on total IL-17A+ Vγ2Vδ2 T cells polarized from human neonates and adults. Because the ex vivo population of human IL-17A+ Vγ2Vδ2 T cells is relatively infrequent, we were not able to perform RORγt or T-bet staining on non-expanded Vγ2Vδ2 T cells from humans. However, because macaque blood contains a higher frequency of IL-17A+ Vδ2 T cells, we were able to analyze T-bet expression within monkey IL-17A+ Vδ2 T cells. Due to lack of confirmed antibody cross-reactivity for monkey RORγt we did not assess the expression of RORγt in monkey IL-17A+ Vδ2 T cells. Shown in Fig. 6A, is the staining from one representative macaque out of three studied demonstrating that IL-17A+, IFN-γ− Vδ2 T cells have somewhat decreased intracellular T-bet levels relative to IL-17A−, IFN-γ+ Vδ2 T cells.
FIGURE 6. Expression of RORγt and T-bet by IL-17A+ Vγ2Vδ2 T cells.
A. Representative staining for T-bet on monkey peripheral blood Vδ2 T cells, segregated into IL-17A+, IFN-γ− Vδ2 T cells (Tγδ17) or IL-17A−, IFN-γ+ Vδ2 T cells (Tγδ1). Represents one of three monkeys examined. PBMC were isolated and stimulated with PMA and ionomycin and intracellular staining for IL-17A, IFN-γ, and T-bet performed. B. (Neonate, left) Cord blood mononuclear cells were polarized with HMBPP for 13 days in the presence of IL-1β, IL-6, TGF-β, and anti-IL-23, and (Adult, right) adult PBMC were polarized with HMBPP for 7 days in the presence of IL-1β, IL-23, TGF-β, and anti-IL-6. On the final day, cells were restimulated with PMA and ionomycin, surface stained for Vδ2 and CD3, and intracellularly stained for IL-17A, RORγt, and T-bet. Vγ2Vδ2 T cells were segregated into IL-17A+ and IL-17A− and the MFI for each transcription factor minus the MFI of the respective isotype control is shown. Because donors had variable baseline RORγt staining, the donors are segregated into two graphs to accommodate the different magnitudes exhibited. Note that cells were not segregated based on IFN-γ production, therefore the neonatal IL-17A+ fraction refers to the sum of Tγδ1/17 and Tγδ17. * p < 0.05, Kruskal Wallis comparison with IL-17A− group.
Next we wished to compare the expression RORγt and T-bet within expanded human IL-17A+ Vγ2Vδ2 T cells from cord blood. We chose to examine RORγt and T-bet expression on cord blood Vγ2Vδ2 T cells cultured under condition 9 (anti-IL-23, IL-1β, IL-6, and TGF-β) because this combination yielded significant expansions of both Tγδ17 and Tγδ1/17 T cells (Fig. 3B, bottom). Total neonatal IL-17A+ cells (Tγδ17 and Tγδ1/17), expressed significantly more RORγt than IL-17A− Vγ2Vδ2 T cells under the same culture conditions (Fig. 6B, left panels). We failed to detect statistically significant differences in the expression of T-bet by total IL-17A+ Vγ2Vδ2 T cells from cord blood. Similarly, total adult IL-17A+ Vγ2Vδ2 T cells cultured under condition 7 (IL-23, IL-1β, anti- IL-6, TGF-β) expressed significantly more RORγt than IL-17A− Vγ2Vδ2 T cells expanded from the same donors under the same culture conditions (Fig. 6B, right panels). And, as before, the expression of T-bet did not significantly differ between adult IL-17A+ and IL-17A− Vγ2Vδ2 T cells. These data suggest that like murine IL-17A+ γδ T cells, human IL-17A+ Vγ2Vδ2 T cells up-regulate the RORγt transcription factor consistent with it playing a role in IL-17A production by human γδ T cells.
Discussion
Although γδ T cells are a major source of IL-17A in mice, the role of γδ T cells in IL-17A production in humans has been unclear. In this study, we show that significant numbers of adult human blood Vγ2Vδ2 T cells produce IL-17A or IL-22 ex vivo although few produce both. IL-17A-producing adult Vγ2Vδ2 T cells are primarily memory cells distributed among early (central), intermediate, and late (effector) subsets similar to Tγδ1 Vγ2Vδ2 T cells. Differentiation of IL-17A-producing cells from neonatal naïve Vγ2Vδ2 T cells required the inflammatory cytokines, IL-1β and IL-6, coupled with TGF-β but not IL-23. The addition of IL-23 favored differentiation to IL-17A-producing cells that also produced IFN-γ. Adult memory Vγ2Vδ2 T cells required IL-23 for maximal expansion of IL-17A-producing cells but did not require IL-6. For both neonatal and adult Vγ2Vδ2 T cells, cells producing IL-17A had higher levels of RORγt compared with cells that did not produce IL-17A, establishing a role for RORγt in IL-17A production. Although the frequency of IL-17A- and IL-22-producing Vγ2Vδ2 T cells are low, they could be significant sources of IL-17A and IL-22.
Consistent with this hypothesis, in mice, γδ T cells and other unconventional T cells are important sources of IL-17A and IL-22. Murine γδ T cells are rapidly mobilized and secrete IL-17A in response to a range of different pathogens including Mycobacterium tuberculosis (32), M. bovis BCG (85), Listeria monocytogenes (34), Escherichia coli (33), and Salmonella enterica serovar Enteritidis (86). The production of IL-17A by murine γδ T cells precedes that of Th17 CD4 T cells (33) and IL-17A-producing γδ T cells are protective in most infections (34, 85). Besides their protective roles, murine IL-17A-producing γδ T cells play pathologic roles in collagen-induced arthritis (87), experimental autoimmune uveitis (88), and experimental autoimmune encephalitis (36).
In mice, IL-17A-producing γδ TCR subsets include invariant Vγ6Vδ1 T cells in the peritoneum of mice with bacterial infections (33), oligoclonal Vγ4Vδ4 T cells in collagen-induced arthritis (87), and Vγ4+ T cells in experimental autoimmune encephalitis (36). None of the antigens are known for these γδ subsets. Additionally, some T10/T22-specific γδ T cells, which express TCRs containing a CDR3δ motif (89), produce IL-17A. Murine γδ T cells acquire IL-17A potential in the neonatal thymus (90). For T10/T22-specific γδ T cells, this was dependent on not encountering T10/22 in the thymus (89). In the periphery, γδ T cells can be rapidly induced by IL-23 and IL-1β (but not IL-6 and/or TGF-β in the presence or absence of TLR/Dectin-1 ligands to produce IL-17A, IL-21, and/or IL-22 (32, 33, 36, 91, 92). Although this is without apparent γδ TCR triggering, this ability to release IL-17A could be due to the constant low-level activation of these cells by an endogenous TCR ligand since their antigen specificity is unknown. Nonetheless, IL-17A-production by murine γδ T cells appears independent of exogenous antigen.
A second type of unconventional T cell is the invariant NKT cell (iNKT). Murine iNKT producing IL-17A develop in the adult thymus, independently of IL-6 and constitute <1% of iNKT cells in the spleen and liver but are greatly enriched in lymph nodes (25, 26, 93). IL-17A release by iNKT17 cells is stimulated by exposure to exogenous lipid antigens. However, similar to murine γδ T cells, IL-23 alone (but possibly with self lipid antigens) also stimulates IL-17A. The combination has a synergistic effect (25). Human blood iNKT cells also produce IL-17A in response to IL-23 and agonistic anti-CD3 (25).
Unlike αβ T cells which can recognize only a single pathogen’s peptide/MHC complex, Vγ2Vδ2 T cells are specific for many pathogens by virtue of their recognition of essential prenyl pyrophosphates. Almost all adult Vγ2Vδ2 T cells recognize prenyl pyrophosphate antigens (for example, 91 out of 94 (97%) adult Vγ2Vδ2 T cell clones responded (53, 94, 95)) due to the extensive use of germline encoded regions of the Vγ2Vδ2 TCR for antigen recognition (96) and the selection for Jγ1.2 and a hydrophobic Vδ2 CDR3 residue that occurs during infancy (51, 94, 97–99). The frequency of CD4 or CD8 αβ T cells specific for a particular peptide/MHC complex among naïve cells is usually very low – 1:158,000–1:1,875,000 for CD4 (100) and 1:33,000–1:164,000 (4/6 were >1:142,000) for CD8 (101). In contrast, because all Vγ2Vδ2 T cells respond to prenyl pyrophosphates, the frequency of reactive cells is high at 1 in 19 T cells (Table I). On average, 1.1% of Vγ2Vδ2 T cells (1 in 2,762 T cells) produce IL-17A. The frequency of IL-17A- and IL-22-producing Vγ2Vδ2 T cells maybe much higher in peripheral lymph nodes, in mucosa, or in the peritoneum as has been found in mice (35). Thus, for primary infections, Vγ2Vδ2 T cells and other unconventional T cells may be important sources of early IL-17A and IL-22 until naïve CD4 and CD8 αβ T cells can be expanded and differentiated into memory Th17/Tc17 and Th22/Tc22 cells. This suggests that γδ T cells, like NK cells, may help bridge the gap between early innate and later adaptive immune responses.
IL-22 is an important cytokine produced by conventional and unconventional T cells in the Th17 lineage. We now describe Vγ2Vδ2 T cells that produce IL-22 but not IL-17A. The existence of IL-22 single positive (Th22 cells) and IL-17A single positive cells has been described for CD4 αβ T cells (59, 69, 70, 102–104). The differentiation of T cells that exclusively produce IL-22 likely reflects the priming conditions during initial antigen exposure. For instance, naïve CD4 T cells primed by skin Langerhans cells or dermal dendritic cells preferentially differentiate into cells that exclusively produce IL-22 and not IL-17A or IFN-γ (102). Plasmacytoid dendritic cells also preferentially differentiate naïve CD4 T cells to Th22 cells (70). Although both αβ and Vγ2Vδ2 T cells have subsets that produce exclusively IL-17A or IL-22, only 2.7% of IL-22-producing Vγ2Vδ2 T cells co-produce IL-17A whereas 10.4–18.2% of IL-22-producing CD4 αβ T cells co-produce IL-17A (70) suggesting increased specialization for blood Vγ2Vδ2 T cells.
Since essentially all adult Vγ2Vδ2 T cells are memory cells, the relatively high frequency of Tγδ22 Vγ2Vδ2 T cells (1 out of every 1,864 T cells) suggests that they make significant contributions to early IL-22 production during infections. In this role, they may function like innate NKp44+ IL-22+ NK cells that are enriched at mucosal surfaces (105, 106). Production of IL-22 mediates mucosal host defense against bacteria (107, 108). Binding of IL-22 to IL-22 receptors expressed by epithelial cells of the digestive tract, skin, and lungs induces antimicrobial peptides, acute phase reactants, and matrix-metalloproteinases (109, 110). Th22 T cells also produce fibroblast growth factors, CCL7 and CCL15 chemokines, and express the skin homing receptor, CCR10 (59). Similarly, Vγ2Vδ2 T cells have been shown to produce FGF-7 (111) and connective tissue growth factor (112). Murine γδ dendritic epidermal cells are primary sources of keratinocyte growth factor and IGF-1 and the presence of murine γδ T cells speed wound healing in the skin and gut (113–116). These findings demonstrate parallels between murine and human γδ T cells and suggest that subsets of both function as specialized Tγδ22 cells.
In contrast to humans, adult rhesus macaques exhibited ~5-fold higher frequencies of IL-17A-producing Vγ2Vδ2 cells than humans. Moreover, the majority of rhesus macaque Vγ2Vδ2 T cells exclusively produced IL-17A without IFN-γ. One possible explanation for this finding is the difference in γδ TCR repertoires between humans and rhesus macaques. Unlike adult humans where Vγ2Vδ2 T cells predominate (54% Vγ2Vδ2 versus 15% Vδ1 T cells), adult rhesus macaques exhibit a predominance of Vδ1 T cells (24% Vγ2Vδ2 versus 33% Vδ1 T cells) (58). This is similar to neonatal human γδ T cells where human Vγ1 T cells constitute 40% of total γδ T cells while Vγ2Vδ2 T cells only constitute 9% (51). Between 1–10 years of age, an expansion of human Vγ2Vδ2 T cells occurs due to environment factors causing a predominance of the Vγ2Vδ2 subset (75). Since the maturity of the rhesus monkeys and humans studied were similar, this lack of predominance of Vγ2Vδ2 T cells in rhesus macaques may reflect their housing in specific pathogen free environments where they are sheltered from significant infectious agents. As a consequence, their Vγ2Vδ2 T cells may be less antigen experienced allowing the persistence of naïve and early memory Vγ2Vδ2 T cells that can be differentiated or maintained as Tγδ17 cells rather than converted to Tγδ1/17 or Tγδ1 cells.
Unexpectedly, the addition of exogenous IL-23 to IL-1β, IL-6, and TGF-β appeared to inhibit Tγδ17 development in neonates. The likely explanation for this observation is that IL-23 promotes the conversion of Tγδ17 cells into Tγδ1/17 cells. A similar effect has been seen for Th17 CD4 clones where IL-23 converted a subset of Th17 cells into Th1/17 cells (117). The same study found an even higher degree of conversion to Th1/17 with IL-12. Further studies are needed to confirm that Tγδ17 Vγ2Vδ2 T cells give rise to Tγδ1/17 in the presence of IL-23/IL-12. If this is so, then decreasing exposure to IL-12 or IL-23 may suppress conversion to Tγδ1/17 and help maintain the Tγδ17 phenotype.
Adult Vγ2Vδ2 T cells required IL-23 in addition to IL-1β and TGF-β but not IL-6 for maximal expansion of Tγδ1/17 (and in some donors, Tγδ17) cells. This is in contrast to neonatal Vγ2Vδ2 T cells which required IL-6 with IL-1β and TGF-β but not IL-23. The difference in cytokine requirements is consistent with the hypothesis that naïve Vγ2Vδ2 T cells (present in neonates), like naïve CD4 T cells, require IL-6 for initial up-regulation of IL-23R, RORγt, and RORα (14, 15, 118), whereas memory IL-23R+ Vγ2Vδ2 T cells (present in adults) require only IL-23, IL-1β, and TGF-β for re-expression of IL-17A. Similarly, production of IL-17A by murine IL-23R+ memory γδ T cells has been shown to require only IL-23 and IL-1β in the presence of fetal bovine serum, which contains TGF-β (36). TGF-β is likely not directly required for Tγδ17 or Tγδ22 cell differentiation but instead functions to inhibit differentiation to Tγδ1 and Tγδ2 lineages (19). In both neonates and adults, Vγ2Vδ2 T cells producing IL-17A had higher levels of RORγt compared with cells not producing IL-17A suggesting a role for RORγt in IL-17A production by γδ T cells whereas levels of the Th1 transcription factor, T-bet did not significantly differ between IL-17A+ and IL-17A− cells.
The conditions favoring IL-17A-producing Vγ2Vδ2 T cells also favored IL-22-producing Vγ2Vδ2 T cells although few produced both IL-17A and IL-22 (Fig. 3A, 4A, and S1). Th22 CD4 αβ T cells optimally differentiate from naïve precursors in the presence of IL-6 and TNF-α. The combination of IL-1β, IL-6, and TNF-α favors development of IL-22+, IL-17A+ CD4 αβ T cells (70) whereas adding TGF-β to these cytokine combinations inhibits Th22 differentiation (70, 119). In contrast to Th17, Th22 cells were characterized as expressing FOXO4 and lower levels of RORγt, high levels of the skin homing receptors, CCR4, CCR6, and CCR10, and cytokines/chemokines such as FGFs, CCL7, and CCL15 (59, 70). Unlike some Th17 cells, Th22 T cells retained their ability to produce IL-22 upon repeated cell division (59, 70) suggesting that the Th22 subset is a distinct functional subset that may be more stable than the Th17 subset. Our data suggests that Tγδ22 and Tγδ17 T cells, like some Th17 and Th22 CD4 αβ T cells (59, 70, 120), belong to separate subsets that do not produce both cytokines. However, both Tγδ17 and Tγδ22 T cells can acquire IFN-γ production. Future experiments are needed to characterize the plasticity of Tγδ22 and Tγδ17 Vγ2Vδ2 T cells.
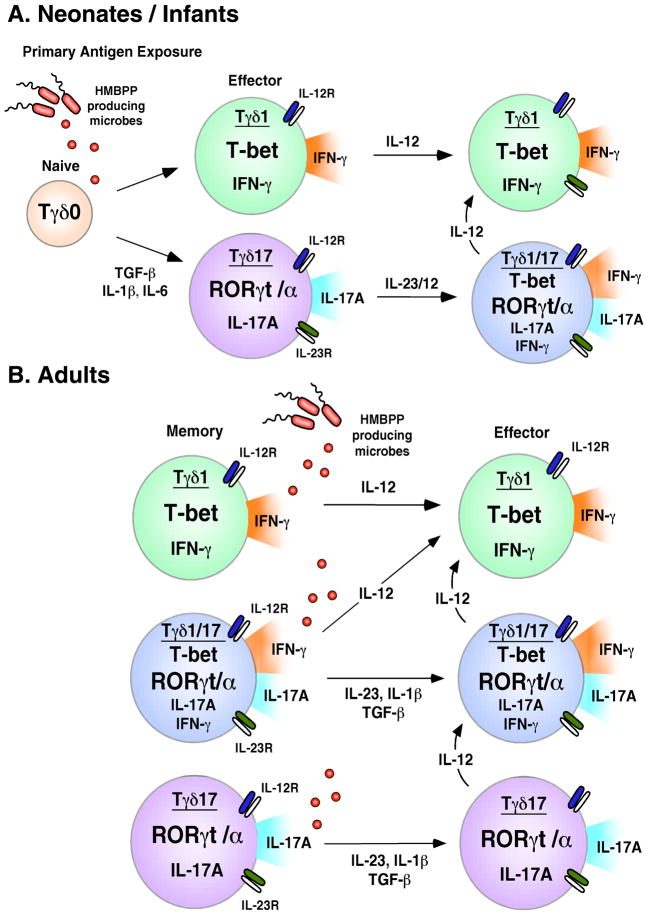
Based on our findings, we propose the following model for the development of Tγδ17 and Tγδ1/17 Vγ2Vδ2 T cells (Fig. 7). In neonates and infants, microbial infections polarize some Vγ2Vδ2 T cells into memory Tγδ17 through the actions of innate cell derived IL-6, IL-1β, and TGF-β. Several studies have demonstrated that neonatal innate cells, including professional antigen presenting cells, produce insufficient levels of IL-12 to program Th1 effector T cells (121–123), and instead make IL-23, IL-1β, and IL-6 (124, 125). We propose that these cytokines, in combination with bacteria or parasite derived HMBPP, differentiate naïve Vγ2Vδ2 T cells into Tγδ17 T cells that produce IL-17A and express RORγt, IL-23R, and IL-12R. Our data suggests that the presence of IL-23 (or possibly IL-12) causes Tγδ17 Vγ2Vδ2 T cells to acquire IFN-γ production through the upregulation of T-bet, thereby converting to Tγδ1/17 cells. Since IL-12 production increases with age, responses to subsequent childhood infections are dominated by IL-12, converting most of the responding Tγδ17 to memory Tγδ1/17 by early adulthood (Fig. 7).
FIGURE 7. Steps in the differentiation and expansion of neonatal and adult Tγδ17 and Tγδ1/17 Vγ2Vδ2 T cells.
A. Neonates/Infants. Naïve Vγ2Vδ2 T cells present in neonates are polarized to the Tγδ17 phenotype by antigen activation in the presence of IL-6, IL-1β, and TGF-β. These early Tγδ17 cells are characterized by elevated expression of RORγt, IL-17A production, and minimal expression of IFN-γ and T-bet. The Tγδ17 cells up-regulate IL-23R (and likely IL-12R) enabling them to maintain their Tγδ17 phenotype in the presence of IL-23, IL-1β, and TGF-β or to convert to a Tγδ1/17 phenotype in the presence of IL-23 or IL-12. B. Adults. Most adult Vγ2Vδ2 T cells are memory cells and include small but significant populations of Tγδ1/17 and Tγδ17 cells. Expansion of adult memory Tγδ1/17 and Tγδ17 cells by HMBPP requires IL-23 in addition to IL-1β and TGF-β but not IL-6. Tγδ1/17 and Tγδ17 likely have limited persistence and are either short-lived effector populations or are converted to Tγδ1 through the effects of IL-12.
Vγ2Vδ2 T cells are of considerable interest because many infections lead to large expansions of these cells (reviewed in 41) and cancer immunotherapies specifically expanding Vγ2Vδ2 T cells have shown effectiveness against various tumors (126–129). Vγ2Vδ2 T cells are attractive agents for cancer immunotherapy because they are not MHC-restricted like conventional T cells so a single vaccine can be used in all individuals regardless of MHC haplotype. Moreover, Vγ2Vδ2 T cells are specifically stimulated by prenyl pyrophosphates and bisphosphonates; both of which are well tolerated in vivo. Once activated, Vγ2Vδ2 T cells are broadly reactive to cancer cells of many tissue origins and to bacterially- and protozoan-infected cells while sparing normal cells. Vγ2Vδ2 T cells have traditionally been considered Th1-like cytotoxic T cells. However, we now show that Vγ2Vδ2 T cells can differentiate into Tγδ17 and Tγδ22 lineage cells. Others have demonstrated Vγ2Vδ2 T cells with characteristics of follicular homing CD4 αβ T cells (57, 130) and regulatory CD25+ CD4 αβ T cells (131). Taken together, these findings indicate that Vγ2Vδ2 T cells exhibit more functional plasticity than previously appreciated. Understanding their plasticity will enable researchers to optimize existing therapies for the treatment of cancers and infections and to develop new therapies utilizing these alternative functional subsets.
Supplementary Material
Acknowledgments
We thank Hong Wang and Grefachew Workalemahu for critical review of the manuscript. We thank Amy M. Raker and Zhimei Fang for technical assistance.
Footnotes
This work was supported by grants from the NIH National Institute of Arthritis and Musculoskeletal and Skin Disease (AR45504), the National Institute of Allergy and Infectious Diseases (Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, AI057160), and the National Cancer Institute (CA113874) to C.T.M.
Abbreviations used in this paper: CBMC, cord blood mononuclear cell; HMBPP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate; iNKT, invariant NKT cells; IPP, isopentenyl pyrophosphate; MFI, mean fluorescence intensity; ROR, retinoid-related orphan receptor
Disclosures
The authors have no financial conflict of interest
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 2.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh BE, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 4.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 6.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001;3:168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 10.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 12.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Graber JJ, Allie SR, Mullen KM, Jones MV, Wang T, Krishnan C, Kaplin AI, Nath A, Kerr DA, Calabresi PA. Interleukin-17 in transverse myelitis and multiple sclerosis. J Neuroimmunol. 2008;196:124–132. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor β is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 21.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, Annunziato F. TGF-β indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien RL, Roark CL, Born WK. IL-17-producing γδ T cells. Eur J Immunol. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel M-L, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, Eberl G, Leite-de-Moraes MC. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci USA. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 28.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 29.Rast JP, Anderson MK, Strong SJ, Luer C, Litman RT, Litman GW. α, β, γ, and δ T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity. 1997;6:1–11. doi: 10.1016/s1074-7613(00)80237-x. [DOI] [PubMed] [Google Scholar]
- 30.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. γδ T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing γδ T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 32.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 33.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 34.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O’Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010 doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Sicard H, Ingoure S, Luciani B, Serraz C, Fournié JJ, Bonneville M, Tiollier J, Romagné F. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 38.García VE, Sieling PA, Gong JH, Barnes PF, Tanaka Y, Bloom BR, Morita CT, Modlin RL. Single cell cytokine analysis of γδ T cell responses to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- 39.Morita CT, Verma S, Aparicio P, Martinez-A C, Spits H, Brenner MB. Functionally distinct subsets of human γ/δ T cells. Eur J Immunol. 1991;21:2999–3007. doi: 10.1002/eji.1830211215. [DOI] [PubMed] [Google Scholar]
- 40.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–218. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 41.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 42.Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Märker-Hermann E, Pasa-Tolic L, Nieves E, Giner JL, Kuzuyama T, Morita CT. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int Immunol. 2007;19:657–673. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- 43.Altincicek B, Moll J, Campos N, Foerster G, Beck E, Hoeffler JF, Grosdemange-Billiard C, Rodríguez-Concepción M, Rohmer M, Boronat A, Eberl M, Jomaa H. Human γδ T cells are activated by intermediates of the 2-C-methyl-D-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J Immunol. 2001;166:3655–3658. doi: 10.4049/jimmunol.166.6.3655. [DOI] [PubMed] [Google Scholar]
- 44.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 46.Sanders JM, Ghosh S, Chan JMW, Meints G, Wang H, Raker AM, Song Y, Colantino A, Burzynska A, Kafarski P, Morita CT, Oldfield E. Quantitative structure-activity relationships for γδ T cell activation by bisphosphonates. J Med Chem. 2004;47:375–384. doi: 10.1021/jm0303709. [DOI] [PubMed] [Google Scholar]
- 47.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 48.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, Li BQ. Interleukin 17-producing γδ T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, Murdaca G, Zocchi MR. VδT lymphocytes producing IFN-γ and IL-17 are expanded in HIV-1 infected patients and respond to Candida albicans. Blood. 2009;113:6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- 50.Giner JL. A convenient synthesis of (E)-4-hydroxy-3-methyl-2-butenyl pyrophosphate and its [4–13C]-labeled form. Tetrahedron Lett. 2002;43:5457–5459. [Google Scholar]
- 51.Morita CT, Parker CM, Brenner MB, Band H. T cell receptor usage and functional capabilities of human γδ T cells at birth. J Immunol. 1994;153:3979–3988. [PubMed] [Google Scholar]
- 52.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka Y, Sano S, Nieves E, De Libero G, Roca D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCRγ chain junctional region in prenyl pyrophosphate antigen recognition by γδ T cells. J Immunol. 1998;161:286–293. [PubMed] [Google Scholar]
- 55.Chen L, He W, Kim ST, Tao J, Gao Y, Chi H, Intlekofer AM, Harvey B, Reiner SL, Yin Z, Flavell RA, Craft J. Epigenetic and transcriptional programs lead to default IFN-γ production by γδ T cells. J Immunol. 2007;178:2730–2736. doi: 10.4049/jimmunol.178.5.2730. [DOI] [PubMed] [Google Scholar]
- 56.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-γ by γδ T cells. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 57.Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, Dieli F, Salerno A. CXCR5 identifies a subset of Vγ9Vδ2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, Nam KH, Morita CT. Conservation of nonpeptide antigen recognition by rhesus monkey Vγ2Vδ2 T cells. J Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- 59.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 61.Caproni M, Antiga E, Melani L, Volpi W, Del Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol. 2009;29:210–214. doi: 10.1007/s10875-008-9233-0. [DOI] [PubMed] [Google Scholar]
- 62.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkühn T, Göke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 63.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 64.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 65.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 66.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol. 2008;180:7948–7957. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H, Wu C. Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol. 2009;39:1472–1479. doi: 10.1002/eji.200838811. [DOI] [PubMed] [Google Scholar]
- 70.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 71.Sato W, Aranami T, Yamamura T. Human Th17 cells are identified as bearing CCR2+CCR5− phenotype. J Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- 72.Kondo T, Takata H, Matsuki F, Takiguchi M. Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182:1794–1798. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 73.Dagna L, Iellem A, Biswas P, Resta D, Tantardini F, Fortis C, Sabbadini MG, D’Ambrosio D, Manfredi AA, Ferrarini M. Skewing of cytotoxic activity and chemokine production, but not of chemokine receptor expression, in human type-1/-2 γδ T lymphocytes. Eur J Immunol. 2002;32:2934–2943. doi: 10.1002/1521-4141(2002010)32:10<2934::AID-IMMU2934>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 74.Yin Z, Zhang DH, Welte T, Bahtiyar G, Jung S, Liu L, Fu XY, Ray A, Craft J. Dominance of IL-12 over IL-4 in γδ T cell differentiation leads to default production of IFN-γ: failure to down-regulate IL- 12 receptor β2-chain expression. J Immunol. 2000;164:3056–3064. doi: 10.4049/jimmunol.164.6.3056. [DOI] [PubMed] [Google Scholar]
- 75.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Roederer M. Ontogeny of γδ T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 77.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 78.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell A-M, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 80.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 81.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 83.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O’Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 86.Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, γδ T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 87.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 91.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:3–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 93.Doisne JM, Becourt C, Amniai L, Duarte N, Le Luduec JB, Eberl G, Benlagha K. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor γt+ and respond preferentially under inflammatory conditions. J Immunol. 2009;183:2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 94.Davodeau F, Peyrat MA, Hallet MM, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human γδ T cells and expression of V9JPC1γ/V2DJCδ-encoded T cell receptors. J Immunol. 1993;151:1214–1223. [PubMed] [Google Scholar]
- 95.De Libero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γ/δ T cells. J Exp Med. 1991;173:1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang H, Fang Z, Morita CT. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol. 2010;184:6209–6222. doi: 10.4049/jimmunol.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human γδ T cells. Int Immunol. 2003;15:1301–1307. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 99.Davodeau F, Peyrat MA, Hallet MM, Houde I, Vie H, Bonneville M. Peripheral selection of antigen receptor junctional features in a major human γδ subset. Eur J Immunol. 1993;23:804–808. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 100.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 104.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 109.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 110.Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 111.Workalemahu G, Foerster M, Kroegel C. Expression and synthesis of fibroblast growth factor-9 in human γδ T-lymphocytes. Response to isopentenyl pyrophosphate and TGF-β1/IL-15. J Leukoc Biol. 2004;75:657–663. doi: 10.1189/jlb.0902471. [DOI] [PubMed] [Google Scholar]
- 112.Workalemahu G, Foerster M, Kroegel C, Braun RK. Human γδ-T lymphocytes express and synthesize connective tissue growth factor: effect of IL-15 and TGF-β1 and comparison with αβ-T lymphocytes. J Immunol. 2003;170:153–157. doi: 10.4049/jimmunol.170.1.153. [DOI] [PubMed] [Google Scholar]
- 113.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 114.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 115.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 117.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 119.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 120.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 Cells are increased in psoriasis. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arulanandam BP, V, Van Cleave H, Metzger DW. IL-12 is a potent neonatal vaccine adjuvant. Eur J Immunol. 1999;29:256–264. doi: 10.1002/(SICI)1521-4141(199901)29:01<256::AID-IMMU256>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 122.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 123.Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 124.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siegemund S, Schutze N, Schulz S, Wolk K, Nasilowska K, Straubinger RK, Sabat R, Alber G. Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis. Int Immunol. 2009;21:555–565. doi: 10.1093/intimm/dxp025. [DOI] [PubMed] [Google Scholar]
- 126.Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 127.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laurent G. A phase I/II open label study of IPH1101 (with low dose of IL-2) in combination with rituximab re-treatment in patients with follicular lymphoma. ECCO/ESMO Congress; Berlin, Germany. 2009. [Google Scholar]
- 130.Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, Jomaa H, Hayday AC, Eberl M. Distinct cytokine-driven responses of activated blood γδ T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–4314. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M. TGF-β1 and IL-15 induce FOXP3+ γδ regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–3577. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.