Abstract
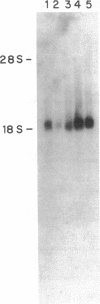
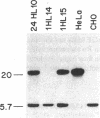
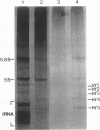
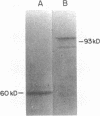
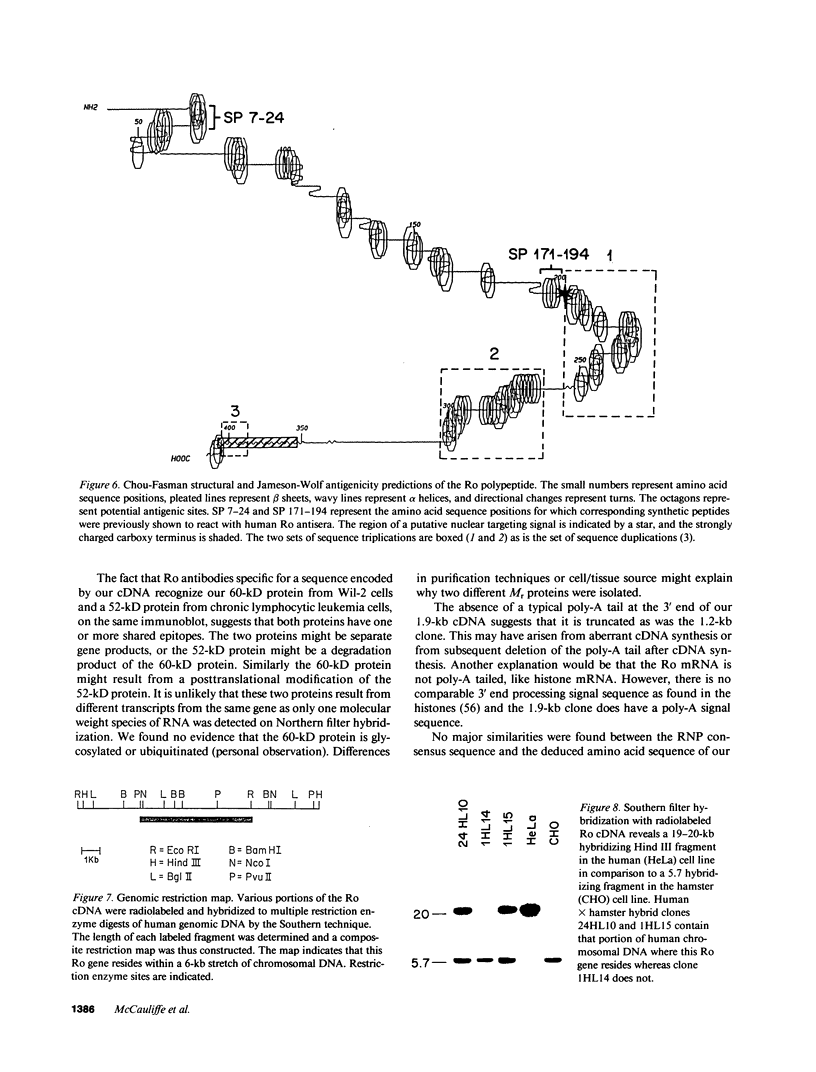
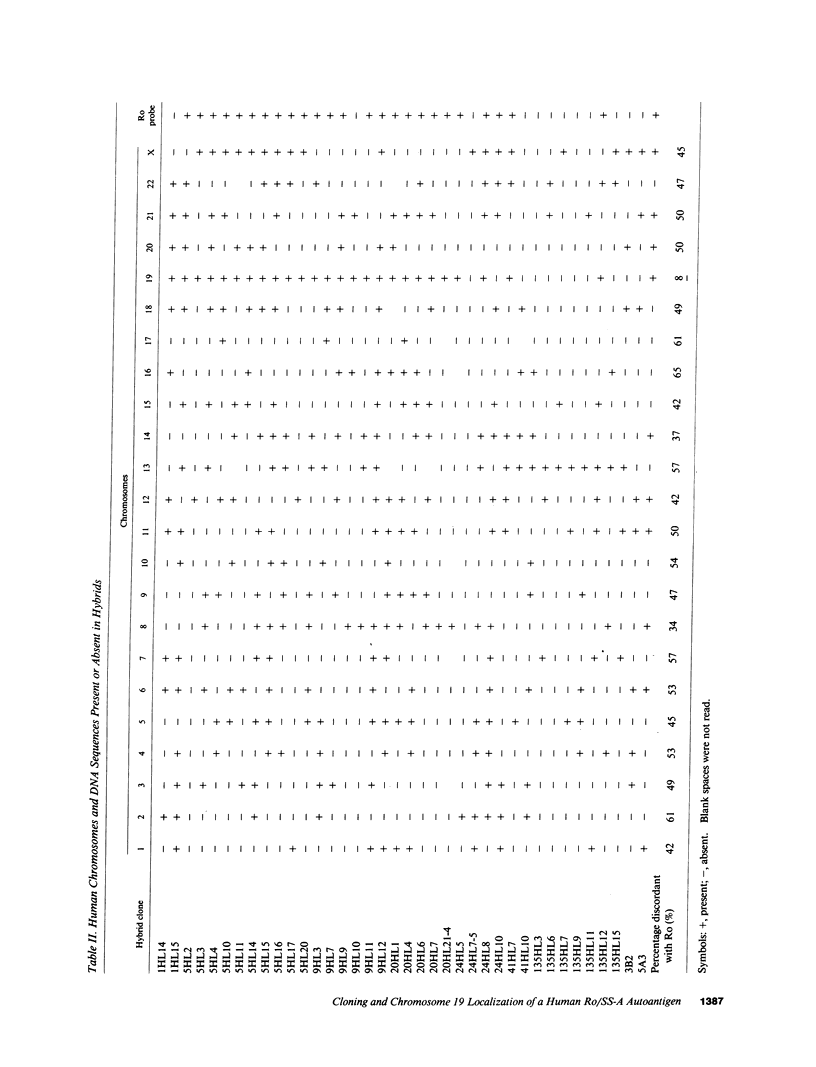
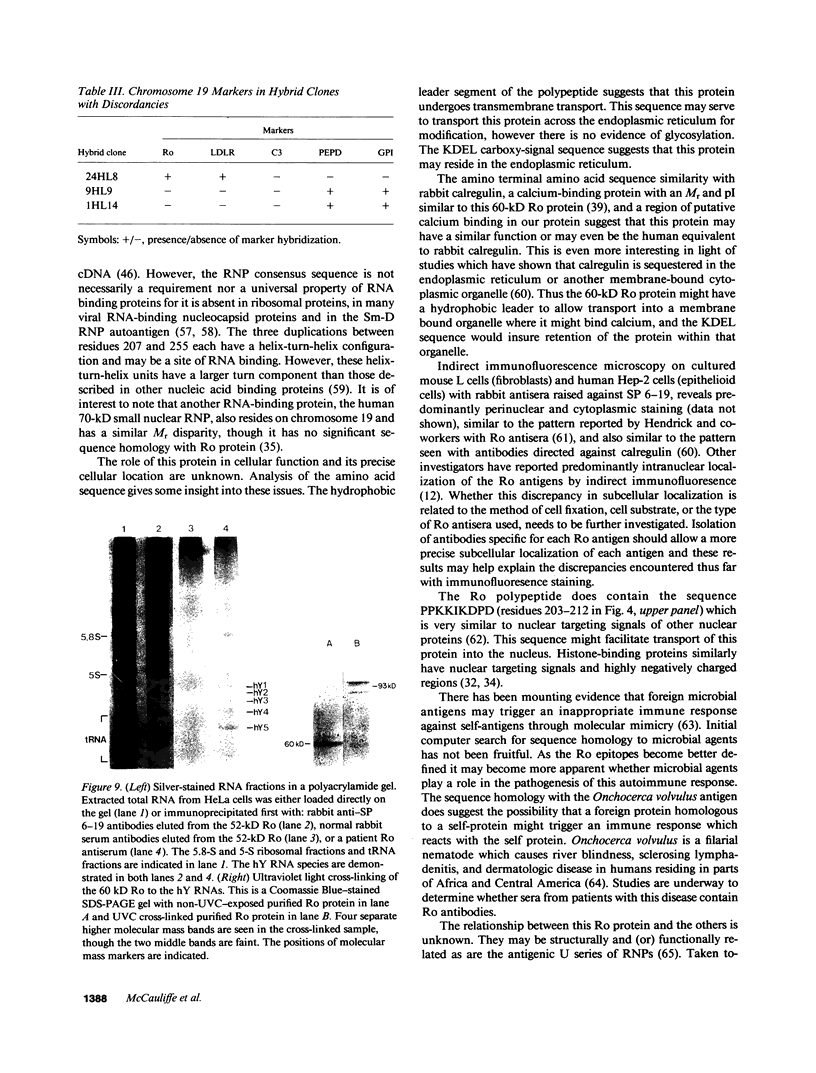
Ro/SS-A antibodies are found in a number of human autoimmune disorders including Sjogren's syndrome and several systemic lupus erythematosus-related disorders. These heterogeneous autoantibodies are known to recognize several distinct cellular antigens. With synthetic oligonucleotides corresponding to amino acid sequence information we have isolated a full-length cDNA clone which encodes a human Ro ribonucleoprotein autoantigen. The 1,890-base pair clone contains an open reading frame that encodes a 417-amino acid, 48-kD polypeptide that migrates aberrantly at 60 kD by SDS-PAGE. Rabbit antibodies raised against this protein's recently described amino-terminal epitope react with a previously identified 52-kD human Ro protein and immunoprecipitate the human cytoplasmic RNAs. Ultraviolet light cross-linking studies suggest that this Ro protein binds each of the four major human cytoplasmic RNAs. The deduced amino acid sequence is 63% homologous to an Onchocerca volvulus antigen. Southern filter hybridization analysis indicates that this gene is not highly polymorphic and exists as a single copy in the human genome. Chromosomal localization studies place this gene on the short arm of chromosome 19 near the gene encoding the low density lipoprotein receptor.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Nakagawa T., Swanson M. S., Woodruff T. K., Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986 Aug;6(8):2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Tan E. M. Antibodies to cellular antigens in Sjögren's syndrome. J Clin Invest. 1975 May;55(5):1067–1073. doi: 10.1172/JCI108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M., Maddison P. Resolution of the identity of certain antigen-antibody systems in systemic lupus erythematosus and Sjögren's syndrome: an interlaboratory collaboration. Arthritis Rheum. 1979 Jul;22(7):796–798. doi: 10.1002/art.1780220719. [DOI] [PubMed] [Google Scholar]
- Baudier J., Gérard D. Ions binding to S100 proteins: structural changes induced by calcium and zinc on S100a and S100b proteins. Biochemistry. 1983 Jul 5;22(14):3360–3369. doi: 10.1021/bi00283a009. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Connor D. H., George G. H., Gibson D. W. Pathologic changes of human onchocerciasis: implications for future research. Rev Infect Dis. 1985 Nov-Dec;7(6):809–819. doi: 10.1093/clinids/7.6.809. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Dilworth S. M., Black S. J., Kearsey S. E., Cox L. S., Laskey R. A. Nucleoplasmin cDNA sequence reveals polyglutamic acid tracts and a cluster of sequences homologous to putative nuclear localization signals. EMBO J. 1987 Jan;6(1):69–74. doi: 10.1002/j.1460-2075.1987.tb04720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Forman M. S., Nakamura M., Mimori T., Gelpi C., Hardin J. A. Detection of antibodies to small nuclear ribonucleoproteins and small cytoplasmic ribonucleoproteins using unlabeled cell extracts. Arthritis Rheum. 1985 Dec;28(12):1356–1361. doi: 10.1002/art.1780281207. [DOI] [PubMed] [Google Scholar]
- Harmon C. E., Deng J. S., Peebles C. L., Tan E. M. The importance of tissue substrate in the SS-A/Ro antigen-antibody system. Arthritis Rheum. 1984 Feb;27(2):166–173. doi: 10.1002/art.1780270207. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Jongstra J., Tidmarsh G. F., Jongstra-Bilen J., Davis M. M. A new lymphocyte-specific gene which encodes a putative Ca2+-binding protein is not expressed in transformed T lymphocyte lines. J Immunol. 1988 Dec 1;141(11):3999–4004. [PubMed] [Google Scholar]
- Kephart D. C., Hood A. F., Provost T. T. Neonatal lupus erythematosus: new serologic findings. J Invest Dermatol. 1981 Sep;77(3):331–333. doi: 10.1111/1523-1747.ep12482531. [DOI] [PubMed] [Google Scholar]
- Khanna N. C., Tokuda M., Waisman D. M. Calregulin: purification, cellular localization, and tissue distribution. Methods Enzymol. 1987;139:36–50. doi: 10.1016/0076-6879(87)39073-1. [DOI] [PubMed] [Google Scholar]
- Khanna N. C., Tokuda M., Waisman D. M. Comparison of calregulins from vertebrate livers. Biochem J. 1987 Feb 15;242(1):245–251. doi: 10.1042/bj2420245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Dingwall C., Maier G., Franke W. W. Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 of Xenopus laevis. EMBO J. 1986 Dec 20;5(13):3547–3552. doi: 10.1002/j.1460-2075.1986.tb04681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Jiang M., Steigerwald J. C., Tan E. M. Identification of the SS-A/Ro intracellular antigen with autoimmune sera. J Immunol Methods. 1984 Jul 6;71(2):217–228. doi: 10.1016/0022-1759(84)90068-1. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Newkirk M. M., Arnett F. C., Lee L. A., Deng J. S., Capra J. D., Sontheimer R. D. A major autoepitope is present on the amino terminus of a human SS-A/Ro polypeptide. J Autoimmun. 1989 Aug;2(4):367–374. doi: 10.1016/0896-8411(89)90165-0. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Newkirk M. M., Capra J. D., Sontheimer R. D. Molecular characterization of human Ro/SS-A antigen. Amino terminal sequence of the protein moiety of human Ro/SS-A antigen and immunological activity of a corresponding synthetic peptide. J Clin Invest. 1988 Jul;82(1):96–101. doi: 10.1172/JCI113607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison P. J., Provost T. T., Reichlin M. Serological findings in patients with "ANA-negative" systemic lupus erythematosus. Medicine (Baltimore) 1981 Mar;60(2):87–94. doi: 10.1097/00005792-198103000-00002. [DOI] [PubMed] [Google Scholar]
- Martinez-Lavin M., Vaughan J. H., Tan E. M. Autoantibodies and the spectrum of Sjögren's syndrome. Ann Intern Med. 1979 Aug;91(2):185–190. doi: 10.7326/0003-4819-91-2-185. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Zmudzka B. Z., Wilson S. H. Chromosomal location of the human gene for DNA polymerase beta. Proc Natl Acad Sci U S A. 1987 Jan;84(2):503–507. doi: 10.1073/pnas.84.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauliffe D. P., Lux F. A., Lieu T. S., Sanz I., Hanke J., Newkirk M. M., Siciliano M. J., Sontheimer R. D., Capra J. D. Ro/SS-A and the pathogenic significance of its antibodies. J Autoimmun. 1989 Aug;2(4):375–381. doi: 10.1016/0896-8411(89)90166-2. [DOI] [PubMed] [Google Scholar]
- Meyer O., Hauptmann G., Tappeiner G., Ochs H. D., Mascart-Lemone F. Genetic deficiency of C4, C2 or C1q and lupus syndromes. Association with anti-Ro (SS-A) antibodies. Clin Exp Immunol. 1985 Dec;62(3):678–684. [PMC free article] [PubMed] [Google Scholar]
- Mowry K. L., Steitz J. A. Identification of the human U7 snRNP as one of several factors involved in the 3' end maturation of histone premessenger RNA's. Science. 1987 Dec 18;238(4834):1682–1687. doi: 10.1126/science.2825355. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Provost T. T., Arnett F. C., Reichlin M. Homozygous C2 deficiency, lupus erythematosus, and anti-Ro (SSA) antibodies. Arthritis Rheum. 1983 Oct;26(10):1279–1282. doi: 10.1002/art.1780261017. [DOI] [PubMed] [Google Scholar]
- Rader M. D., O'Brien C., Liu Y. S., Harley J. B., Reichlin M. Heterogeneity of the Ro/SSA antigen. Different molecular forms in lymphocytes and red blood cells. J Clin Invest. 1989 Apr;83(4):1293–1298. doi: 10.1172/JCI114014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Haselby J. A., Hoch S. O. Molecular cloning of a cDNA encoding the human Sm-D autoantigen. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4832–4836. doi: 10.1073/pnas.85.13.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Maddison P. J., Reichlin M., Jordon R. E., Stastny P., Gilliam J. N. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Strunk K., Surowy C. S., Hoch S. O., Barton D. E., Francke U. The human U1-70K snRNP protein: cDNA cloning, chromosomal localization, expression, alternative splicing and RNA-binding. Nucleic Acids Res. 1987 Dec 23;15(24):10373–10391. doi: 10.1093/nar/15.24.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings R. L., Olson E., Strauss A. W., Thompson L. H., Bachinski L. L., Siciliano M. J. Human creatine kinase genes on chromosomes 15 and 19, and proximity of the gene for the muscle form to the genes for apolipoprotein C2 and excision repair. Am J Hum Genet. 1988 Aug;43(2):144–151. [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Nakagawa T. Y., LeVan K., Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol Cell Biol. 1987 May;7(5):1731–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnasch T. R., Gallin M. Y., Soboslay P. T., Erttmann K. D., Greene B. M. Isolation and characterization of expression cDNA clones encoding antigens of Onchocerca volvulus infective larvae. J Clin Invest. 1988 Jul;82(1):262–269. doi: 10.1172/JCI113581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon A. P., De Groot R. J., De Haan M., Dekker A., Grivell L. A. The DNA sequence of the nuclear gene coding for the 17-kd subunit VI of the yeast ubiquinol-cytochrome c reductase: a protein with an extremely high content of acidic amino acids. EMBO J. 1984 May;3(5):1039–1043. doi: 10.1002/j.1460-2075.1984.tb01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]