Abstract
We have previously observed that TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) induces acquired TRAIL resistance by increasing Akt phosphorylation and Bcl-xL expression. In this study, we report that Src, c-Cbl, and PI3K are involved in the phosphorylation of Akt during TRAIL treatment. Data from immunoprecipitation and immunblotting assay reveal that Src interacts with c-Cbl and PI3K. Data from immune complex kinase assay demonstrate that Src can directly phosphorylate c-Cbl and PI3K p85 subunit protein. Data from gene knockdown experiments with an RNA interference (RNAi) technique show that c-Cbl is involved in the interaction between Src and PI3K p85 during TRAIL treatment, playing an important role in TRAIL-induced Akt phosphorylation. Taken together, c-Cbl may act as a mediator to regulate the Src-PI3K-Akt signal transduction pathway during TRAIL treatment.
Keywords: c-Cbl, TRAIL, Src-PI3K-Akt
1. Introduction
TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) is a type II integral membrane protein belonging to the TNF family. Like Fas ligand (FasL) and TNF-α, the c-terminal extracellular region of TRAIL (amino acids 114-281) exhibits a homotrimeric subunit structure [1]. However, unlike FasL and TNF-α, TRAIL induces apoptosis in a variety of tumor cell lines more efficiently than in normal cells [2]. The apoptotic signal of TRAIL is transduced by binding to the death receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), which are members of the TNF receptor superfamily. Ligation of TRAIL to its receptors results in trimerization of the receptor and clustering of the receptor’s intracellular death domain (DD), leading to the formation of the death-inducing signaling complex (DISC). Trimerization of the receptors leads to the recruitment of an adaptor molecule, Fas-associated death domain (FADD), and subsequent binding and activation of caspase-8 and -10. Activated caspase-8 and -10 then cleave caspase-3, which in turn leads to cleavage of the death substrate. Despite TRAIL’s potential as an anticancer agent both in vitro and in vivo, some cancer cells that were originally sensitive to TRAIL-induced apoptosis can become resistant after repeated exposure (acquired resistance) [3,4], suggesting that the physiological role of TRAIL is more complex than merely activating caspase-dependent apoptosis of cancer cells [5]. For example, it was reported that TRAIL stimulated the anti-apoptotic PI3K/Akt pathway in endothelial cells [5.6] and fibroblast cells [7] as well as that TRAIL induced PI3K/Akt and NF-κB activation in Jurkat T leukemia cells [8]. These results imply that, depending on circumstances, TRAIL can function as a cytokine of either cell death or cell survival, similar to NF-κB [9]. In the pathway of cell survival, Akt has been known to be activated by phosphorylation at threonine 308 and serine 473 in response to various growth factors through a pathway that requires PI3K-dependent generation of PI(3,4,5)P3 [10]. PIP3 facilitates the recruitment of Akt to the plasma membrane through binding with the pleckstrin homology (PH) domain of Akt. At the plasma membrane, Akt is activated by phosphoinositide-dependent kinase-1 (PDK-1) at threonine 308 and becomes fully activated after phosphorylation within the carboxy-terminus at serine 473 [11,12].
Previously, we reported that DU-145 prostate cancer cells develop acquired TRAIL resistance after TRAIL treatment, and that phosphorylated Akt (pAkt) and its downstream member Bcl-xL are involved in the process of acquired resistance [4]. However, how Akt phosphorylation is increased during development of acquisition of TRAIL resistance has not yet been clearly understood. The main point is that, as stated by Trauzold [13], TRAIL and TRAIL death receptors do not only stimulate apoptosis but also engage non-apoptotic signaling pathways leading to activation of survival-related signals. One of the well known nondeath signaling pathways induced by TRAIL is through TNF receptor-associated protein with death domain (TRADD), receptor-interacting protein (RIP) and TNF receptor-associated factor 2 (TRAF2) [14], which are nondeath signaling modulatory adaptors that interact with the ligand’s homotrimerized receptors, and lead to the activation of kinase cascades resulting in activation of NF-κB and the mitogen-activated protein kinases [15]. However, activation of Akt, upstream of NF-κB [16,17], has not been well defined.
Recently, we observed that acquired TRAIL resistance is developed through degradation of TRAIL receptors as well as increased Bcl-xL expression, demonstrating that degradation of TRAIL receptors is mediated by c-Cbl (Casitas B-lineage lymphoma) during TRAIL treatment [4,18]. However, the mechanism of increased Akt phosphorylation during TRAIL treatment which is another cause of acquired resistance has not yet been clearly explained. Here, we demonstrate that c-Cbl is also responsible for TRAIL-induced Akt phosphorylation through Src-PI3K activation. Src is activated during TRAIL treatment, followed by phosphorylation of PI3K and c-Cbl. c-Cbl may acts as a scaffolding molecule for phosphorylation of PI3K by Src.
We recently observed that c-Cbl functions in the degradation of TRAIL receptors as an E3 ligase [18]. In addition to this E3 ligase activity, many cellular events mediated or regulated by c-Cbl protein are dependent on its adaptor functions, suggesting c-Cbl’s diverse and sometimes opposing roles in the regulation of signal transduction in response to different stimulation [19]. For example, association of the distal proline-rich sequences of c-Cbl and the SH3 domain of the p85 subunit of PI3 kinase is responsible for the activation of PI3-kinase by EGF stimulation [20-22[.
In this paper we demonstrate that c-Cbl plays an important role in the TRAIL-induced activation of the Src-PI3K-Akt signal transduction pathway.
2. Materials and Methods
2.1. Cell culture and survival assay
Human prostate adenocarcinoma DU-145 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA) and 26 mM sodium bicarbonate for monolayer cell culture. The cells were maintained in a humidified atmosphere containing 5% CO2 and air at 37°C.
2.2. Reagents and antibodies
Anti-caspase 8, anti-phosphoS473-Akt, anti-Akt, anti-phosphoTyr416-Src, anti-Src, anti-phosphoTyr731-c-Cbl, and anti-c-Cbl were purchased from Cell Signaling (Beverly, MA, USA). Anti-phosphoTyr508 PI3K p85, anti-PI3K p85, anti-Src antibody, and protein G and protein A-agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PP-2 was purchased from Calbiochem (San Diego, CA, USA). Anti-actin was purchased from ICN (Costa Mesa, CA, USA), and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal anti-PARP was purchased from Biomol International, L.P. (Plymouth Meeting, PA, USA). Monoclonal anti-HA (clone 3F10) was purchased from Roche Applied Science (Indianapolis, IN, USA). Monoclonal anti-actin was purchased from ICN (Costa Mesa, CA, USA).
2.3. RNA interference by siRNA c-Cbl or by siRNA caspase-8
To construct siRNA of c-Cbl, pSilencer 2.1-U6 hygro vector (Ambion, Inc., Austin, TX, USA) was used for expressing siRNA for c-Cbl. The insert for hairpin siRNA into pSilencer was prepared by annealing two oligonucleotides. For human c-Cbl siRNA, the top strand sequence was 5′- GATCCGATGGAGACACTTGGAGAATTCAAGAGATTCTCCAAGTGTCTCCATCTTTTTTGGAAA-3′, and the bottom strand sequence was 5′-AGCTTTTCCAAAAAAGATGGAGACACTTGGAGAATCTCTTGAATTCTCCAAGTGTCTCCATCG-3′. The annealed insert was cloned into pSilencer 2.1-U6 hygro digested with BamH I and Hind III. The correct structure of pSilencer 2.1-U6 hygro-c-Cbl was confirmed by nucleotide sequencing. The resultant plasmid, pSilencer-c-Cbl, was transfected into DU-145 cells. The interference of c-Cbl protein expression was confirmed by immunoblot using anti-c-Cbl antibody (Cell Signaling). To construct siRNA of caspase-8, we used the same methods described previously for si c-Cbl except the insert for hairpin siRNA into pSilencer. For human caspase-8 siRNA, the top strand sequence was 5′-GATCCAGGGAACTTCAGACACCAGTTCAAGAGACTGGTGTCTGAAGTTCCCTTTTTTTGGAAA-3′, and the bottom strand sequence was 5′-AGCTTTTCCAAAAAAAGGGAACTTCAGACACCAGTCTCTTGAACTGGTGTCTGAAG TTCCCTG-3′ was used for annealing.
2.4. Protein extracts and polyacrylamide gel electrophoresis
Cells were lysed with 1 × Laemmli lysis buffer (2 % sodium dodecyl sulfate, 10 % glycerol, 0.002 % bromophenol blue, 62.5 mM Tris, pH 6.8) and boiled for 10 min. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). The samples were diluted with 1 × lysis buffer and β-mercaptoethanol was added to be 350 mM, then equal amounts of protein were loaded on 10 % or 15 % sodium dodecyl sulfate (SDS)-polyacrylamide gels. SDS-PAGE analysis was performed according to Laemmli using a Hoefer gel apparatus.
2.5. Immunoblot analysis
Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5 % nonfat dry milk in PBS-Tween-20 (0.1 %, v/v) at 4°C overnight. The membrane was incubated with primary antibody (diluted according to the manufacturer’s instructions) for 2 h. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL, USA).
2.6. In vivo binding
To examine the interaction between Src and c-Cbl or phosphorylated PI3K p85 and the interaction between PI3K p85 and c-Cbl, DU-145 cells in 100-mm culture plates were treated with TRAIL (100 ng/ml) for various times (0-4 h). For immunoprecipitation, cells were lysed in buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 10 mM EDTA, 1 % Triton X-100, 1 % deoxycholate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and protein inhibitor cocktail solution (Sigma-Aldrich). The lysates were incubated with 1 μg of anti-Src or anti-PI3K p85 for 2 h. After the addition of protein G or A agarose, the lysates were incubated for an additional 2 h. The beads were washed three times with the lysis buffer, separated by SDS-polyacrylamide gel electrophoresis (PAGE), and immunoblotted with appropriate antibodies. The proteins were detected with the enhanced chemiluminescence reaction.
2.7. Immune complex kinase assay
For the immune complex kinase assay, DU-145 cells in 100-mm culture plates were treated with TRAIL (100 ng/ml) for various times (0-4 h). For immunoprecipitation, DU-145 cells were lysed in a buffer solution containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EGTA, 10 mM NaF, 1% Triton X-100, 0.5 % deoxycholate, 2 mM DTT, 1 mM sodium orthovanadate, 1mM PMSF and protein inhibitor cocktail solution (Sigma-Aldrich). Cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with 1 μg of anti-Src antibody by incubating for 2 h. After the addition of protein A-agarose (Santa Cruz Biotechnology), the lysates were incubated for an additional 2 h. The beads were washed twice with a solution containing 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 5 mM EGTA, 2 mM DTT, 1 mM sodium orthovanadate, 1 mM PMSF and protein inhibitor cocktail solution, and washed once with the kinase buffer solution, and then they were subjected to kinase assays. 1 μg of PI3K (p110β/p85α) human recombinant protein (Calbiochem) was incubated with immunoprecipitated Src in kinase buffer containing 20 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 1 mM sodium orthovanadate, 2 mM DTT, and 20 μM ATP at 30 °C for 1 h. Finally, the reaction was stopped by adding 2X Laemmli buffer. Phosphorylated proteins were resolved by SDS-PAGE and analyzed by phospho-PI3K p85 antibody.
For the purification of soluble c-Cbl fragment containing the 731 phosphorylation site, carboxy (from 691 a.a. to end) domain of c-Cbl digested with BamHI/XhoI was subcloned into the BamHI/XhoI site of pGEX4T-1 after PCR of cytoplasmic domain of c-Cbl. The sense primer was 5′-GCTCGGATCCCAATGTGAGGGTGAAGAGGAC-3′, and the antisense primer was 5′-CAGTCTCGAGCTAGGTAGCTACATGGGCAGG-3′. pGEX-4T-1/c-Cbl was transformed into JM109, and expression of c-Cbl corresponding to cytoplasmic domain was purified by using glutathione-Sepharose 4B (Amersham Biosciences). To examine whether the Src phosphorylates the c-Cbl at the 731 tyrosine site, 1 μg of purified GST-c-Cbl fragment protein was used as a substrate of Src for in vitro kinase assay. The kinase assay was performed according to the procedure as described for Src-PI3K p85.
3. Results
3.1. Akt and Src phosphorylation during TRAIL treatment
We previously reported that acquired TRAIL resistance is developed by multimode such as increasing Akt phosphorylation and Bcl-xL expression [4]. It is well known that Bcl-xL expression is upregulated by activated (phosphorylated) Akt [23]. However, the remaining question is how TRAIL treatment increases phosphorylation of Akt. In this study, we hypothesized that Src, one of the non receptor tyrosine kinase, is responsible for Akt phosphorylation during TRAIL treatment. To examine this possibility, cells were treated with 100 ng/ml TRAIL for various times (0-4 h) and then analyzed. As shown in Fig. 1, Akt and Src phosphorylation as well as PARP and caspase 8 cleavage, hallmarks for apoptosis, were increased during TRAIL treatment.
Figure 1. TRAIL-induced Akt and Src activation.
DU-145 cells were treated with 100 ng/ml TRAIL for various times (0-4 h). Cells were lysed, and lysates were analyzed for the detection of PARP, caspase 8, phosphorylated Akt, Akt, phosphorylated Src and Src. Actin was used to confirm the equal amount of proteins loaded in each lane.
3.2. Src as an upstream kinase responsible for Akt phosphorylation during TRAIL treatment
To investigate whether Src activation is responsible for Akt phosphorylation during TRAIL treatment, PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), a potent and selective inhibitor of the Src-family tyrosine kinase, was used. As shown in Fig 2A, Akt phosphorylation induced by TRAIL treatment was markedly suppressed by pretreatment with PP2. These results suggest that Src is an upstream kinase responsible for Akt phosphorylation. Interestingly, TRAIL-induced PARP cleavage was increased in PP2-pretreated cells. Similar results were observed by morphological feature studies (Fig. 2B) and cell survival determinations (Fig. 2C). These results suggest that inhibition of TRAIL-induced Akt phosphorylation promotes TRAIL-induced cytotoxicity. We further examined the effect of PP2 on PI3K and c-Cbl which are known to be substrates of Src [24,25]. Figure 3 shows that TRAIL-induced phosphorylation of both PI3K and c-Cbl was inhibited in the presence of PP2.
Figure 2. Effect of PP2 on TRAIL-induced Akt activation and cytotoxicity.
DU-145 cells were pretreated with PP2 (20 μM, 1 h) and treated with TRAIL (100 ng/ml) for various times (0-4 h). After treatment, cells were harvested and western blot analysis was performed for detecting PARP, phosphorylated Akt and Akt (A). Morphological features were analyzed with a phase-contrast inverted microscope (B). Cell survival was determined by trypan blue exclusion assay (C). DMSO: 1% dimethyl sulfoxide treated sham control. T-1, T-2, and T-4 represent TRAIL treatment for 1 h, 2 h, and 4 h, respectively.
Figure 3. Effect of PP2 on TRAIL-induced phosphorylation of c-Cbl and PI3k p85.
DU-145 cells were pretreated with PP2 (20 μM, 1 h) and treated with TRAIL (100 ng/ml) for 2 h. After treatment, cells were harvested and cell lysates were subjected to immunoblotting for phosphorylated c-Cbl, c-Cbl, phosphorylated PI3K p85, PI3K p85, phosphorylated Akt and Akt. Actin was used to confirm the equal amount of proteins loaded in each lane.
3.3. Involvement of c-Cbl in TRAIL-induced Akt phosphorylation
c-Cbl protein is a multi-functional adaptor protein. Recently, we have shown that the c-Cbl molecule mediates the degradation of TRAIL receptors DR4 and DR5 as an E3 ligase (submitted). Among its many structural domains, c-Cbl protein also has a PI3K p85 binding site. It is known that PI3K p85 binds to c-Cbl after phosphorylation at the 731 site of c-Cbl. To examine whether c-Cbl is involved in the phosphorylation of Akt, an RNA interference (RNAi) technique was employed to suppress endogenous c-Cbl gene expression. DU-145 cells were stably transfected with either pSilencer sham vector (Si neg) or pSilencer vector containing c-Cbl siRNA (Si c-Cbl). Stably transfected clones were pooled and the gene silencing effect was assessed by measuring the endogenous level of c-Cbl (Fig. 4A). Interestingly, figure 4B shows that a pool of si c-Cbl clones in comparison to pSilencer control plasmid transfectant was more sensitive to TRAIL cytotoxicity. TRAIL-induced morphological alterations, cell death, and proteolytic PARP cleavage were increased in the pool of si c-Cbl clones. To investigate the role of c-Cbl in TRAIL-induced Akt phosphorylation, Si c-Cbl transfectants were treated with 10 ng/ml TRAIL, which is the isosurvival dose to 100 ng/ml TRAIL treated control Si neg cells. Figure 4A clearly shows that knockdown of c-Cbl expression led to inhibition of Akt phosphorylation during TRAIL treatment. A question, which has been unanswered, is how c-Cbl is involved in the phosphorylation of Akt. As described above, phosphorylation of Tyr-731 residue of c-Cbl is essential for c-Cbl-PI3K p85 interaction. We hypothesized that phosphorylation of Tyr-731 residue is required for the downstream phosphorylation of Akt. To test this possibility, Tyr-731 was replaced with phenylalanine (Y731F) by employing site-directed mutagenesis techniques. Figure 4C shows that overexpression of mutant-type c-Cbl (Y731F) suppressed Akt phosphorylation during treatment with 200 ng/ml TRAIL. We further examined whether these observations are consistent in challenged TRAIL treatment. For this study, cells were treated with 200 ng/ml TRAIL for 4 h and incubated for various times (0-24 h) before being challenged to 200 ng/ml TRAIL for 4 h. Data from western blot analysis show that overexpression of mutant-type c-Cbl (Y731F) effectively suppressed Akt phosphorylation (Fig. 4C). However, data from survival assay and PARP cleavage assay show that acquired TRAIL resistance development was partially suppressed by overexpression of mutant-type c-Cbl (Y731F) (Fig. 4C). These results suggest that phosphorylation of Akt contributes to, but is not essential for, the development of acquired TRAIL resistance.
Figure 4. Role of c-Cbl in TRAIL-induced Akt activation and acquired TRAIL resistance development.
(A) DU-145 cells were stably transfected with pSilencer control plasmid (Si neg) or pSilencer-si c-Cbl (Si c-Cbl) plasmid. Cells were treated with isosurvival dose of TRAIL (100 ng/ml for Si neg or 10 ng/ml for Si c-Cbl) for 4 h and cell lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho Akt, anti-Akt or anti-c-Cbl antibody. Actin was used to confirm the equal amount of proteins loaded in each lane. (B) Control plasmid (si neg) or a pool of pSilencer-si c-Cbl stably transfected clones (si c-Cbl pool) were treated with TRAIL (100 ng/ml) for 4 h, and morphological features were analyzed with a phase-contrast inverted microscope (upper panels), or cell survival and PARP cleavage were determined by tryphan blue exclusion assay and immunoblot analysis, respectively (lower panels). Error bars represent the S.E. from three separate experiments. Cell lysates were subjected immunoblotting for PARP, c-Cbl or actin. (C) DU-145 cells were transfected with plasmid containing HA-tagged wild-type c-Cbl (wild type: pSRαneo-HA-Cbl) or mutant-type (Y731F Cbl) HA-c-Cbl cDNA. After 48 h incubation, cells were first treated with TRAIL (100 ng/ml) for 4 h, and then detached cells were removed by washing out with PBS. After removal of detached cells, fresh media were added onto the remaining attached cells and cells were incubated for the time indicated (0 h, 12 h, 24 h), and then treated a second time with TRAIL (100 ng/ml) for 4 h. Cell survival was determined by trypan blue exclusion assay (upper panel) and cell lysates were subjected to immunoblotting for PARP, HA-Cbl or phosphorylated Akt (lower panels). Con, untreated control cells. Error bars represent the S.E. from three separate experiments.
3.4. Src acts as a direct kinase to phosphorylate Cbl during TRAIL treatment
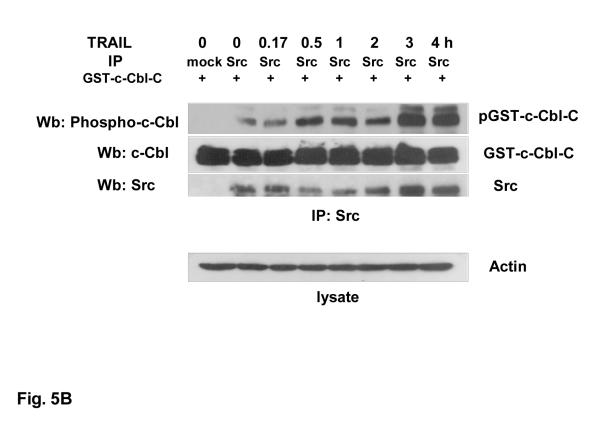
Odai et al. [26] reported an interaction between c-Cbl and v-Src during epidermal growth factor treatment in v-Src-transformed fibroblasts. We further investigated whether Src is responsible for phosphorylation of c-Cbl. As a first step, we examined whether Src interacts with c-Cbl during TRAIL treatment. Cells were treated with 100 ng/ml TRAIL for various times (0-4 h) and the interaction between Src and c-Cbl was examined by immunoprecipitation. Figure 5A shows that Src interacted with c-Cbl during TRAIL treatment. Next, we investigated whether Src directly phosphorylates c-Cbl. For this study, immune complex kinase assay was perfomed. GST-tagged fusion carboxy domain of c-Cbl (GST-c-Cbl-C) was used as a substrate of Src. Figure 5B shows that phosphorylation of Tyr-731 residue of c-Cbl by Src was increased during TRAIL treatment. These data suggest that Src directly phosphorylates c-Cbl during TRAIL treatment.
Figure 5. Interaction between Src and c-Cbl during TRAIL treatment and phosphorylation of c-Cbl by Src.
(A) DU-145 cells were treated with TRAIL (100 ng/ml) for various times (0-4 h) and lysed. Cell lysates were immunoprecipitated with anti-Src antibody and immunoblotted with anti-c-Cbl or anti-Src antibody (upper panels). The presence of c-Cbl in the lysates was verified by immunoblotting (lower panels). Actin was used to confirm the equal amount of proteins loaded in each lane. (B) DU-145 cells were treated with TRAIL (100 ng/ml) for various times (0-4 h) and lysed. Cell lysates were immunoprecipitated with anti-Src antibody. Src catalytic activity in in vitro was determined by using GST-carboxy Cbl protein as substrate (upper panels). phosphorylated GST-carboxy Cbl, GST-carboxy Cbl or Src was detected with anti-phospho Cbl , anti-Cbl, or anti-Src antibody, respectively. Cell lysates (lower panel) were immunoblotted with anti-actin antibody.
3.5. c-Cbl acts as a mediator of Src-induced PI3K p85 phosphorylation during TRAIL treatment
To investigate the role of c-Cbl in the Src-PI3K-Akt signal transduction pathway, we systematically analyzed the involvement of c-Cbl in Src-induced PI3K phosphorylation. First, we examined phosphorylation of PI3K during TRAIL treatment. It is well known that PI3K consists of a regulatory subunit (p85) which binds to an activated growth factor/cytokine receptor and undergoes phosphorylation. Phosphorylation of p85 subunit results in the activation of its catalytic subunit (P110) [27]. Figure 6A shows that Tyr-508 residue of PI3K p85 was phosphorylated during treatment with 100 ng/ml TRAIL. Next, we examined whether phosphorylated PI3K p85 interacts with Src. For this study, cells were treated with 100 ng/ml TRAIL for various times (0-4 h) and the interaction between the two molecules was examined by immunoprecipitation. Figure 6B shows that Src associated with PI3K p85 during TRAIL treatment. We further examined whether Src directly phosphorylates PI3K by using immune complex kinase assay. Src was immunoprecipitated from TRAIL treated cells and its kinase activity was determined by incubation with purified PI3K p110/p85α protein. Figure 6C shows that Src directly phosphorylated PI3K p85 during TRAIL treatment. Our studies reveal that Src interacts with both c-Cbl and PI3K p85. A question, which remains unanswered, is how c-Cbl is involved in the phosphorylation of PI3K p85. We hypothesized that c-Cbl interacts with PI3K p85 and mediates the interaction between Src and PI3K p85. To test this possibility, we examined the interaction between c-Cbl and PI3K p85 by immunoprecipitation assay. Figure 7A demonstrates that c-Cbl associated with PI3K p85 during TRAIL treatment. Next, we investigated the role of c-Cbl in the interaction of PI3K p85 by Src during TRAIL treatment. For this study, we attempted to silence c-Cbl expression by using siRNA technique. Cells were stably transfected with either pSilencer control plasmid (Si neg) or pSilencer-sic-Cbl vector (Si c-Cbl). We pooled stable transfectants for further studies (Fig. 7B). Figure 7B shows that the expression of c-Cbl was effectively suppressed in the Si c-Cbl transfected cells. To examine the role of c-Cbl in the interaction between Src and PI3K p85, cells were treated with 100 ng/ml TRAIL for 2 h and immunoprecipitated with anti-Src antibody. Figure 7B shows that TRAIL-induced interaction between Src and p-PI3K p85 as well as PI3K p85 was suppressed in Si c-Cbl transfected cells, but not in Si neg cells.
Figure 6. Phosphorylation of PI3K p85, interaction between Src and phosphorylated PI3K p85, and activation of Src kinase activity during TRAIL treatment.
DU-145 cells were treated with TRAIL (100 ng/ml) for various times (0-4 h) and lysed. (A) Cell lysates were immunoblotted with anti-phospho-PI3K p85 or anti-PI3K p85 antibody. (B) Cell lysates were immunoprecipitated with anti-mouse Src antibody and immunoblotted with anti-phospho PI3K p85 antibody or anti-rabbit Src antibody (upper panels). Cell lysates (lower panel) were immunoblotted with anti-actin antibody. (C) Cell lysates were immunoprecipitated with anti-Src antibody. Src catalytic activity in in vitro was determined by using PI3K p110β/p85α protein as substrate (upper panels). Phosphorylated PI3K p85, PI3K p85 or Src was detected with anti-phospho-PI3K p85, anti-PI3K p85, or anti-Src antibody, respectively. Cell lysates (lower panel) were immunoblotted with anti-actin antibody.
Figure 7. Role of c-Cbl in the interaction between Src and PI3K p85 during TRAIL treatment.
(A) DU-145 cells were treated with TRAIL (100 ng/ml) for various times (0-4 h) and lysed. Cell lysates were immunoprecipitated with anti-PI3K p85 antibody and immunoblotted with anti-c-Cbl or anti-PI3K p85 antibody (upper panels). The presence of c-Cbl in the lysates was verified by immunoblotting with anti-c-Cbl (lower panels). Actin was used to confirm the equal amount of proteins loaded in each lane. (B) Control plasmid (Si neg) transfected cells and a pool of pSilencer-si c-Cbl stably transfected clones (Si c-Cbl pool) were treated with TRAIL (100 ng/ml) for 4 h, and cell lysates were immunoprecipitated with anti-Src antibody. Phosphorylated PI3K p85 or Src was detected with anti-phospho PI3K p85 or anti-Src antibody, respectively (upper panels), and cell lysates were subjected to immunoblotting for c-Cbl or actin (lower panels).
Discussion
In this study we observed that Src is responsible for TRAIL-induced activation of the PI3K-Akt signal transduction pathway. Interestingly, c-Cbl acts as a mediator in the Src-PI3K-Akt signal transduction pathway. Previous studies have shown that c-Cbl has multiple functions in the cells [28]. One of functions is to be involved in the degradation of TRAIL receptors [18]; c-Cbl acts as an E3 ligase and plays an important role in the development of acquired TRAIL resistance through the degradation of TRAIL receptors, DR4 and DR5. In this study, we observed another function of c-Cbl in the development of acquired TRAIL resistance. Namely, c-Cbl acts as mediator in Src-induced activation of the PI3K-Akt signal transduction pathway through regulating phosphorylation of PI3K (Figs. 6 and 7). Data from immune complex kinase assay reveal that Src can directly phosphorylate PI3K p85 subunit protein in vitro (Fig. 6C). However, data from c-Cbl knock down experiments suggest that c-Cbl is required for the interaction between Src and phosphorylated PI3K p85 (Fig. 7B). Thus, our observations from Figs. 6C and 7B are somewhat contradictory. We can speculate that this discrepancy is a result of differences in analytical methods (immune complex kinase assay vs immunoprecipitation assay). In in vitro kinase assay, we added excess amounts of PI3K as substrate which may allow Src and PI3K to interact in a cell-free system. However, in in vivo condition, c-Cbl might act as a typical scaffold protein for Src and PI3K p85, increasing their chance of interaction in the cytoplasm. Phosphorylation at 731 tyrosine site of c-Cbl by Src kinase during TRAIL treatment may lead to conformational alteration. Phosphorylated c-Cbl would be able to easily recruit the PI3K p85 [28] to Src and then PI3K p85 would be phosphorylated by Src subsequently. Indeed, previous studies reveal that c-Cbl can serve as an enhancer of proliferation and survival through the PI3K-dependent pathway after cytokine treatment [29].
A fundamental question, which needs to be answered, is how Src is activated during TRAIL treatment. We previously reported that caspases (-3, -7, and -8) are involved in TRAIL-induced activation of the MAPK superfamily through cleavage of Mst1 (mammalian sterile 20-like kinase 1) kinase [30]. It is possible that activated caspase 8 also plays an important role in the phosphorylation of Src. We examined this possibility by employing an RNA interference (RNAi) technique to suppress endogenous caspase 8 gene expression. Knockdown of caspase 8 expression does not block the activation of Src and Akt during TRAIL treatment (data not shown). Thus, we ruled out the possibility of involvement of caspase 8 in the activation of Src and Akt during TRAIL treatment. Another possibility is that TRAIL receptors are directly/indirectly involved in activation of Src. It is well known that Src kinase is activated by receptor tyrosine kinases such as EGFR [28, 31-34]. In many cases, Src kinase is activated by cell surface receptors which contain relatively short cytoplasmic domains, thereby lacking intrinsic catalytic activity [24]. The mechanism of Src activation has been explained by involvement of TRAF6 with the SH3 domain of Src, converting Src from the autoinhibited state to an open conformation facilitating autophosphorylation [24]. Thereby, Src can act like a catalytic domain of receptors by binding to its receptors. TRAIL receptors such as DR4 and DR5 are not exceptional. In vitro kinase activity of DR4 and DR5 tested with GST-Src (Jena Bioscience, Axxora, LLC, San Diego, CA, USA) was not detected during TRAIL treatment (data not shown), suggesting that DR4 and DR5 do not have their own kinase activity. Recent studies reveal that TRAF6 acts as an E3 ligase and regulates Akt ubiquitination, membrane recruitment, and phosphorylation during treatment with growth factor [35]. However, unlike growth factor, TRAIL does not induce ubiquitination of Akt (data not shown). Currently, we can only speculate that an adaptor or adaptors is/are present. These molecules may mediate interaction between TRAIL receptors and Src. Obviously, further studies are necessary to understand the mechanism of activation of Src during TRAIL treatment. Our model will provide a framework for future studies.
Acknowledgments
This work was supported by the following grants: NCI grant funds (CA121395 and CA140554: J. J. S., Y.J. L.) and (CA113263: M.A.A., D.L.B., Y.J. L.), and this research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0071809; J.J. Song) and a faculty research grant of Yonsei University College of Medicine for 2009-0113; J.J. Song). a grant from the Ministry of Commerce, Industry, and Energy of Korea (990-14-05, to J-H. Kim).
Abbreviations used in this paper
- c-Cbl
Casitas B-lineage lymphoma
- DD
death domain
- DISC
death-inducing signaling complex
- DMEM
Dulbecco’s modified Eagle’s medium
- DR4
TRAIL-R1
- DR5
TRAIL-R2
- DTT
dithiothreitol
- EGF
epidermal growth factor
- EGFR
EGF receptor
- EGTA
ethylene glycol tetraacetic acid
- FADD
Fas-associated death domain
- FasL
Fas/APO-1 ligand
- FBS
fetal bovine serum
- GST
glutathione-S transferase
- PAGE
polyacrylamide gel electrophoresis
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PDK-1
phosphoinositide-dependent kinase-1
- PH
pleckstrin homology
- PMSF
phenylmethylsulfonyl fluoride
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- RIP
receptor-interacting protein
- RNAi
RNA interference
- SDS
sodium dodecyl sulfate
- TNF
tumor necrosis factor
- TRADD
TNF receptor-associated protein with death domain
- TRAF2
TNF receptor-associated factor 2
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. J.Biol.Chem. 1996;271:12687. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- [2].Ashkenazi A, Dixit VM. Science. 1998;281:1305. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- [3].Zhu H, Zhang L, Huang X, Davis JJ, Jacob DA, Teraishi F, Chiao P, Fang B. Mol.Ther. 2004;9:666. doi: 10.1016/j.ymthe.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [4].Song JJ, An JY, Kwon YT, Lee YJ. J.Biol.Chem. 2007;282:319. doi: 10.1074/jbc.M608065200. [DOI] [PubMed] [Google Scholar]
- [5].Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D, Zauli G. Circulation. 2003;107:2250. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- [6].Secchiero P, Zerbinati C, Rimondi E, Corallini F, Milani D, Grill V, Forti G, Capitani S, Zauli G. Cell.Mol.Life Sci. 2004;61:1965. doi: 10.1007/s00018-004-4197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morel J, Audo R, Hahne M, Combe B. J. Biol. Chem. 2005;280:15709. doi: 10.1074/jbc.M414469200. [DOI] [PubMed] [Google Scholar]
- [8].Zauli G, Sancilio S, Cataldi A, Sabatini N, Bosco D, Di Pietro R. J.Cell.Physiol. 2005;202:900. doi: 10.1002/jcp.20202. [DOI] [PubMed] [Google Scholar]
- [9].Baetu TM, Hiscott J. Cytokine Growth Factor Rev. 2002;13:199. doi: 10.1016/s1359-6101(02)00006-0. [DOI] [PubMed] [Google Scholar]
- [10].Vivanco I, Sawyers CL. Nat.Rev.Cancer. 2002;2:489. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- [11].Stephens L, Williams R, Hawkins P. Curr.Opin.Pharmacol. 2005;5:357. doi: 10.1016/j.coph.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [12].Osaki M, Oshimura M, Ito H. Apoptosis. 2004;9:667. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- [13].Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Roder C, Kalthoff H, Wajant H. Oncogene. 2006;25:7434. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- [14].Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG. Mol.Cell.Biol. 2000;20:6638. doi: 10.1128/mcb.20.18.6638-6645.2000. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Malhi H, Gores GJ. Oncogene. 2006;25:7333. doi: 10.1038/sj.onc.1209765. [DOI] [PubMed] [Google Scholar]
- [16].Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. Nature. 1999;401:82. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- [17].Kane LP, Shapiro VS, Stokoe D, Weiss A. Curr.Biol. 1999;9:601. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- [18].Song JJ, Szczepanski MJ, Kim SY, Kim JH, An JY, Kwon YT, Lee YJ. 2009. Submitted.
- [19].Dikic I, Szymkiewicz I, Soubeyran P. Cell Mol.Life Sci. 2003;60:1805. doi: 10.1007/s00018-003-3029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. J.Biol.Chem. 2001;276:4872. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- [21].Elly C, Witte S, Zhang Z, Rosnet O, Lipkowitz S, Altman A, Liu YC. Oncogene. 1999;18:1147. doi: 10.1038/sj.onc.1202411. [DOI] [PubMed] [Google Scholar]
- [22].Soltoff SP, Cantley LC. J.Biol.Chem. 1996;271:563. doi: 10.1074/jbc.271.1.563. [DOI] [PubMed] [Google Scholar]
- [23].Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. J.Exp.Med. 2000;191:1721. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schlessinger J. Cell. 2000:293. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- [25].Yokouchi M, Kondo T, Sanjay A, Houghton A, Yoshimura A, Komiya S, Zhang H, Baron R. J.Biol.Chem. 2001;276:35185. doi: 10.1074/jbc.M102219200. [DOI] [PubMed] [Google Scholar]
- [26].Odai H, Sasaki K, Hanazono Y, Ueno H, Tanaka T, Miyagawa K, Mitani K, Yazaki Y, Hirai H. Cancer Res. 1995;86:1119. doi: 10.1111/j.1349-7006.1995.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodriguez-Viciana P, Marte B, Warne P, Downward J. Philos.Trans.R.Soc.Lond.B.Biol.Sci. 1996;351:225. doi: 10.1098/rstb.1996.0020. [DOI] [PubMed] [Google Scholar]
- [28].Kassenbrock CK, Hunter S, Garl P, Johnson GL, Anderson SM. J.Biol.Chem. 2002;277:24967. doi: 10.1074/jbc.M201026200. [DOI] [PubMed] [Google Scholar]
- [29].Ueno H, Sasaki K, Honda H, Nakamoto T, Yamagata T, Miyagawa K, Mitani K, Yazaki Y, Hirai H. Blood. 1998;91:46. [PubMed] [Google Scholar]
- [30].Song JJ, Lee YJ. Cell Signal. 2008;20:892. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Proc.Natl.Acad.Sci.U.S.A. 1999;96:1415. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. J.Biol.Chem. 1999;274:8335. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- [33].Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Proc.Natl.Acad.Sci.U.S. 1995;92:6981. doi: 10.1073/pnas.92.15.6981. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman TJ. Oncogene. 1997;15:3083. doi: 10.1038/sj.onc.1201496. [DOI] [PubMed] [Google Scholar]
- [35].Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, Lin HK. Science. 2009;325:1134. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]