Abstract
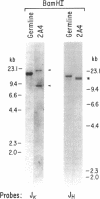
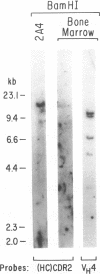
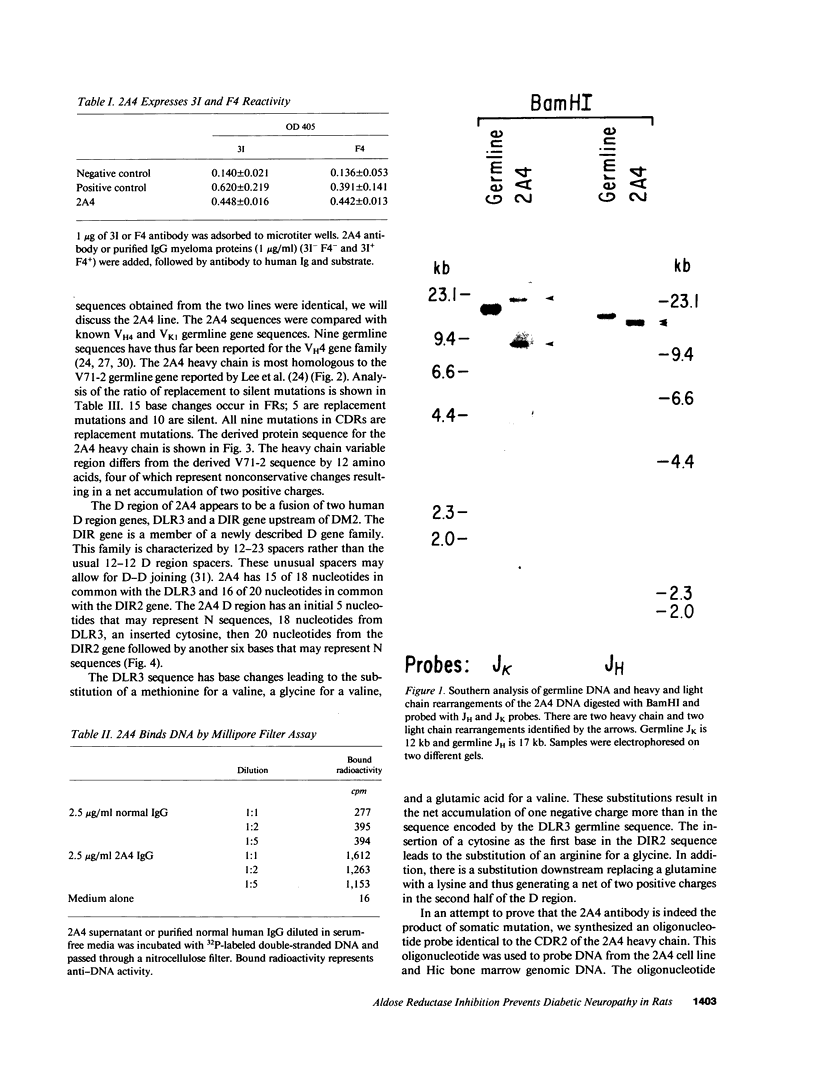
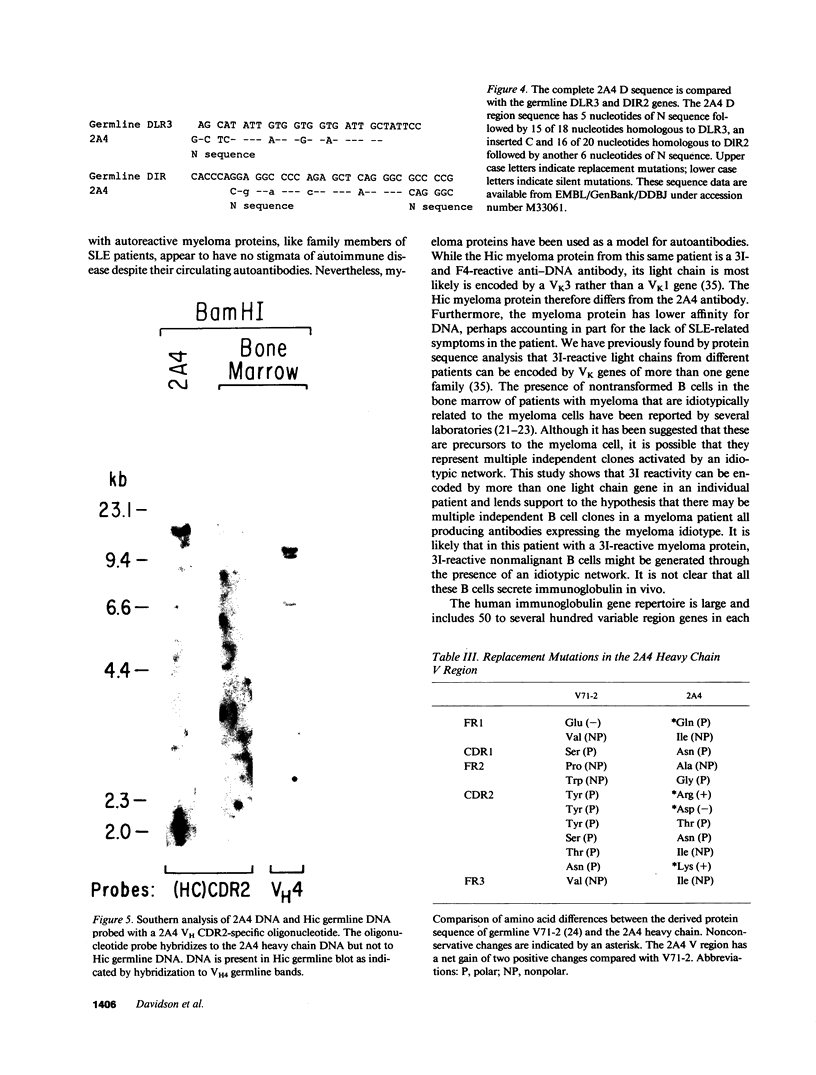
We report the molecular characterization of 2A4, an IgG, DNA-binding antibody bearing the 3I and F4 idiotypes which are associated with anti-DNA antibodies in serum of patients with systemic lupus erythematosus (SLE). The antibody is produced by an EBV-transformed B cell line derived from a patient with multiple myeloma whose myeloma protein is also an IgG, 3I-reactive, F4-reactive, DNA-binding immunoglobulin, although the 2A4 antibody does not itself represent the myeloma protein. The 2A4 heavy chain is encoded by a VH4 gene, a D-D gene fusion and the JH6 gene; the light chain is derived from a Vk1 gene and the Jk2 gene. This is the first human antibody shown to have a CDR3 encoded by a D-D fusion. DNA sequence analysis of the 2A4 VH gene together with a Southern blot of genomic DNA probed with a 2A4 VH-specific oligonucleotide strongly suggest it to be somatically mutated. The data provide evidence that human autoantibodies can be products of somatically mutated genes and suggest that the 2A4 antibody may reflect the selective pressure of antigen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrzejewski C., Jr, Rauch J., Lafer E., Stollar B. D., Schwartz R. S. Antigen-binding diversity and idiotypic cross-reactions among hybridoma autoantibodies to DNA. J Immunol. 1981 Jan;126(1):226–231. [PubMed] [Google Scholar]
- Behar S. M., Scharff M. D. Somatic diversification of the S107 (T15) VH11 germ-line gene that encodes the heavy-chain variable region of antibodies to double-stranded DNA in (NZB x NZW)F1 mice. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3970–3974. doi: 10.1073/pnas.85.11.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human immunoglobulin variable region genes--DNA sequences of two V kappa genes and a pseudogene. Nature. 1980 Dec 25;288(5792):730–733. doi: 10.1038/288730a0. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Goñi F., Fong S., Jirik F., Vaughan J. H., Frangione B., Carson D. A. The majority of human monoclonal IgM rheumatoid factors express a "primary structure-dependent" cross-reactive idiotype. J Immunol. 1985 May;134(5):3281–3285. [PubMed] [Google Scholar]
- Chen P. P., Liu M. F., Sinha S., Carson D. A. A 16/6 idiotype-positive anti-DNA antibody is encoded by a conserved VH gene with no somatic mutation. Arthritis Rheum. 1988 Nov;31(11):1429–1431. doi: 10.1002/art.1780311113. [DOI] [PubMed] [Google Scholar]
- Datta S. K., Naparstek Y., Schwartz R. S. In vitro production of an anti-DNA idiotype by lymphocytes of normal subjects and patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1986 Mar;38(3):302–318. doi: 10.1016/0090-1229(86)90240-0. [DOI] [PubMed] [Google Scholar]
- Davidson A., Preud'homme J. L., Solomon A., Chang M. D., Beede S., Diamond B. Idiotypic analysis of myeloma proteins: anti-DNA activity of monoclonal immunoglobulins bearing an SLE idiotype is more common in IgG than IgM antibodies. J Immunol. 1987 Mar 1;138(5):1515–1518. [PubMed] [Google Scholar]
- Davidson A., Smith A., Katz J., Preud'Homme J. L., Solomon A., Diamond B. A cross-reactive idiotype on anti-DNA antibodies defines a H chain determinant present almost exclusively on IgG antibodies. J Immunol. 1989 Jul 1;143(1):174–180. [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Diamond B., Solomon G. A monoclonal antibody that recognizes anti-DNA antibodies in patients with systemic lupus. Ann N Y Acad Sci. 1983;418:379–385. doi: 10.1111/j.1749-6632.1983.tb18087.x. [DOI] [PubMed] [Google Scholar]
- Eilat D., Webster D. M., Rees A. R. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J Immunol. 1988 Sep 1;141(5):1745–1753. [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H. Characteristics of pathogenic subpopulations of antibodies to DNA. Arthritis Rheum. 1982 Jul;25(7):747–752. doi: 10.1002/art.1780250706. [DOI] [PubMed] [Google Scholar]
- Halpern R., Davidson A., Lazo A., Solomon G., Lahita R., Diamond B. Familial systemic lupus erythematosus. Presence of a cross-reactive idiotype in healthy family members. J Clin Invest. 1985 Aug;76(2):731–736. doi: 10.1172/JCI112028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S., Schwaber J. Specificity analysis of human anti-DNA antibodies. J Immunol. 1986 Feb 1;136(3):892–897. [PubMed] [Google Scholar]
- Holm G., Mellstedt H., Pettersson D., Biberfeld R. Idiotypic immunoglobulin structures on blood lymphocytes in human plasma cell myeloma. Immunol Rev. 1977;34:139–164. doi: 10.1111/j.1600-065x.1977.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988 Dec 20;7(13):4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenichen H. R., Pech M., Lindenmaier W., Wildgruber N., Zachau H. G. Composite human VK genes and a model of their evolution. Nucleic Acids Res. 1984 Jul 11;12(13):5249–5263. doi: 10.1093/nar/12.13.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stepwise intraclonal maturation of antibody affinity through somatic hypermutation. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8206–8210. doi: 10.1073/pnas.85.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira M., Kinashi T., Umemura I., Matsuda F., Noma T., Ono Y., Honjo T. Organization and evolution of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1986 Aug 20;190(4):529–541. doi: 10.1016/0022-2836(86)90239-1. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Vogler L. B., Capra J. D., Conrad M. E., Lawton A. R., Cooper M. D. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979 Oct 1;150(4):792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preud'homme J. L., Klein M., Labaume S., Seligmann M. Idiotype-bearing and antigen-binding receptors produced by blood T lymphocytes in a case of human myeloma. Eur J Immunol. 1977 Dec;7(12):840–846. doi: 10.1002/eji.1830071204. [DOI] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Sanz I., Capra J. D. The genetic origin of human autoantibodies. J Immunol. 1988 May 15;140(10):3283–3285. [PubMed] [Google Scholar]
- Sanz I., Dang H., Takei M., Talal N., Capra J. D. VH sequence of a human anti-Sm autoantibody. Evidence that autoantibodies can be unmutated copies of germline genes. J Immunol. 1989 Feb 1;142(3):883–887. [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Gefter M. L., Wysocki L. J., Margolies M. N. Recurrent somatic mutations in mouse antibodies to p-azophenylarsonate increase affinity for hapten. J Immunol. 1989 Jan 15;142(2):596–601. [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A., Rauch J., Madaio M. P., Stollar B. D., Schwartz R. S. Idiotypic cross-reactions of monoclonal human lupus autoantibodies. J Exp Med. 1983 Sep 1;158(3):718–730. doi: 10.1084/jem.158.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon G., Schiffenbauer J., Keiser H. D., Diamond B. Use of monoclonal antibodies to identify shared idiotypes on human antibodies to native DNA from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1983 Feb;80(3):850–854. doi: 10.1073/pnas.80.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Faiferman I., Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977 Jan;59(1):90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeff R. A., Geier S. S., Nathenson S. G. Molecular loss variants of the murine major histocompatibility complex: nonexpression of H-2K antigens associated with marked reduction in H-2K mRNA as determined by oligonucleotide hybridization analysis. J Immunol. 1986 Aug 15;137(4):1366–1370. [PubMed] [Google Scholar]
- Zouali M., Eyquem A. Idiotype restriction in human autoantibodies to DNA in systemic lupus erythematosus. Immunol Lett. 1984;7(4):187–190. doi: 10.1016/0165-2478(84)90041-5. [DOI] [PubMed] [Google Scholar]