Abstract
Background:
Sunitinib is a multitargeted, oral tyrosine kinase inhibitor with antitumour and antiangiogenic activity. We investigated the safety and pharmacokinetics of sunitinib in combination with irinotecan in patients with advanced, refractory solid tumours.
Methods:
Sunitinib was initially administered once daily at 37.5 mg per day on days 1–14 of a 21-day cycle, in which irinotecan 250 mg m−2 was given on day 1. In a second cohort, the sunitinib dose was reduced to 25 mg per day. Blood samples were collected for pharmacokinetic studies.
Results:
In the sunitinib 37.5 mg per day cohort, 3 out of 10 evaluable patients had objective responses, but dose-limiting toxicities (DLTs) of neutropenia, pneumococcal sepsis, and fatigue were observed. There were no DLTs in the sunitinib 25 mg per day cohort. Paired observations of pharmacokinetic parameter values of sunitinib and irinotecan alone vs the combination did not reveal significant drug–drug interactions. The maximum tolerated dose was defined as sunitinib 25 mg per day (days 1–14) with irinotecan 250 mg m−2 (day 1), but no activity was observed at this dose.
Conclusion:
Although a higher sunitinib dose of 37.5 mg per day (days 1–14) with irinotecan showed preliminary evidence of antitumour activity, this dose was poorly tolerated. Therefore, this particular combination will not be pursued for further studies.
Keywords: sunitinib, irinotecan, combination, advanced solid tumours, pharmacokinetics
The addition of targeted agents to standard chemotherapy is becoming widely investigated in a number of advanced solid tumour types, and has met with success in some malignancies, including colorectal cancer (CRC). The combination of the vascular endothelial growth factor (VEGF)-targeted monoclonal antibody bevacizumab with either irinotecan/5-fluorouracil (5-FU)/leucovorin (LV) (FOLFIRI) or oxaliplatin/5-FU/LV (FOLFOX) has shown increased median progression-free survival and/or overall survival in patients with advanced CRC as compared with chemotherapy alone (Hurwitz et al, 2004, 2005; Giantonio et al, 2007). The optimal role of targeted agents, sequencing of therapies, and primary tumour resistance as well as resistance development are under investigation in advanced CRC (Gravalos et al, 2007), as more effective treatment regimens are still needed.
Sunitinib is an oral tyrosine kinase inhibitor that is known to block the signalling activity of VEGF receptors (VEGFR-1, -2, and -3), platelet-derived growth factor receptors (-α and -β), stem-cell factor receptor (KIT), FMS-like tyrosine kinase 3, colony-stimulating factor-1 receptor, and rearranged during transfection (RET) ligand; glial cell line-derived neurotrophic factor receptor (Abrams et al, 2003; Mendel et al, 2003; Murray et al, 2003; O'Farrell et al, 2003; Kim et al, 2006). It is approved multinationally for the treatment of advanced renal cell cancer and imatinib-resistant/-intolerant gastrointestinal stromal tumours (Goodman et al, 2007). The tolerability profile of sunitinib is well established, and adverse events (mainly diarrhoea, mucositis, skin abnormalities, and altered taste) are generally manageable and reversible (Demetri et al, 2006; Motzer et al, 2007). In addition, hypertension and fatigue have been reported with sunitinib and are, in general, common side effects of VEGF pathway-targeted therapies (Demetri et al, 2006; Hutson et al, 2008; Launay-Vacher and Deray, 2009). Promising single-agent antitumour activity across patients with a range of solid tumour types has been observed in phase I and II trials of sunitinib, including neuroendocrine tumours, breast cancer, hepatocellular cancer, and non-small-cell lung cancer (NSCLC; Burstein et al, 2008; Kulke et al, 2008; Socinski et al, 2008; Faivre et al, 2009). Single-agent sunitinib has also shown modest antitumour activity in patients with metastatic CRC refractory to standard chemotherapy in a phase II trial (Saltz et al, 2007), suggesting that a study of sunitinib in combination with standard chemotherapy for metastatic CRC is warranted.
The established topoisomerase I inhibitor irinotecan (CPT-11), a derivative of the natural alkaloid camptothecin, prevents repair of single-strand breaks in DNA, resulting in double-strand DNA damage and cell death. Irinotecan is approved for treatment of advanced/metastatic CRC. The drug has also demonstrated antitumor activity in glioblastoma and both small-cell and NSCLC (Fukuoka et al, 1992; Masuda et al, 1992; Friedman et al, 1999). The most common grade 3 or 4 adverse events observed in a phase III trial of single-agent irinotecan administered every 3 weeks were neutropenia, diarrhoea, and vomiting (Fuchs et al, 2003).
The activity of sunitinib and irinotecan, together with their manageable and generally nonoverlapping toxicity profiles, suggests that combining the two agents may be beneficial in a broad range of solid tumours, particularly CRC. Here, we report results from a phase I, dose-finding study of sunitinib and irinotecan in patients with advanced solid tumours and included plasma pharmacokinetics assessed for the drugs alone and combined.
Patients and methods
Study design and treatment regimen
Sunitinib and irinotecan were administered in 3-week cycles, up to a maximum of 12 cycles. Seven days before the start of cycle 1, a single day's dose of sunitinib was administered to allow for the collection of samples for pharmacokinetic analysis. Patients subsequently received sunitinib as once-daily oral doses on days 1–14 followed by a 1-week break, and irinotecan as a 1-h intravenous infusion on day 1.
For combination of sunitinib and irinotecan, starting doses were selected being 75% of single-agent doses, that is, sunitinib 37.5 mg (50 mg daily oral dose as single agent) and irinotecan 250 mg m−2 (350 mg m−2 3-weekly i.v. dose as single agent). The study design allowed for sunitinib a dose escalation to 50 mg and de-escalation to 25 mg, whereas the initial dose of irinotecan could be escalated to 300 or 350 mg m−2. Concomitant medication precluded, among others, potent CYP3A4 inhibitors and inducers. In addition, prophylactic use of hematopoietic growth factors to support neutrophil or platelet counts was not recommended in cycle 1, but could be used in subsequent cycles.
Dose-limiting toxicity (DLT) was defined as any of the following events occurring during the first 2 cycles of treatment that were attributable to the study drug combination: grade 4 neutropenia lasting ⩾7 days; febrile neutropenia (grade 3 or 4 neutropenia and fever ⩾38.5°C); neutropenic infection (grade 3 or 4 neutropenia with ⩾grade 3 infection); either grade ⩾3 thrombocytopenia with bleeding, or grade 4 thrombocytopenia lasting ⩾7 days; grade 3 or 4 nonhaematological toxicities including fatigue lasting ⩾7 days (except for skin or hair discolouration, alopecia, hyperamylasaemia, or hyperlipasaemia without other clinical evidence of pancreatitis, and asymptomatic hyperuricaemia; nausea, vomiting, or diarrhoea had to persist at grade 3 or 4 despite maximal medical therapy to qualify for DLT). MTD was the primary endpoint of the study, defined as the dose level at which none or one out of six patients experienced a DLT, with the next higher dose level having at least two out of three or two out of six patients encountering DLT during the first 2 cycles of combination therapy.
The study was conducted with institutional review board/independent ethics committee approval and in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, as well as applicable local laws and regulatory requirements. All patients provided written informed consent.
Patient eligibility
Patients eligible for the study had histologically or cytologically proven advanced malignancy refractory to standard therapy, or for which no curative therapy was available, and were suitable for treatment with irinotecan; Eastern Cooperative Oncology Group performance status of 0 or 1, and a life expectancy ⩾12 weeks. Patients were excluded if they had received chemotherapy, radiation therapy, surgery, or investigational agent within 4 weeks before study entry, or previous irradiation to >25% of the bone marrow, grade ⩾2 neuropathy (any cause), uncontrolled brain metastases, myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, congestive heart failure, cerebrovascular accident including transient ischaemic attack, or pulmonary embolus within the 12 months before starting study treatment. Patients with uncontrollable hypertension (>150/100 mm Hg), grade 3 haemorrhage <4 weeks before starting study treatment, cardiac dysrhythmias (grade ⩾2), atrial fibrillation, QTc interval >450 ms (males) or >470 ms (females), or a history of grade 3 or 4 toxicity or severe hypersensitivity reaction associated with previous irinotecan treatment were also excluded.
Patient assessments
The number of patients to be enroled was to be determined by the observed safety profile, which also determined the number of patients per dose level and the number of dose escalations. All patients who received at least one dose of study medication were included in the study analyses.
Patients underwent regular physical examinations (usually on day 1 of each treatment cycle), laboratory tests (blood, usually on days 1 and 15 of each cycle, and urinalysis) and 12-lead electrocardiogram (at screening, at steady state level of sunitinib on cycle 2 day 1, and as clinically indicated in subsequent cycles). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0 (http://ctep.cancer.gov/protocoldevelopment/electronic-applications/docs/ctcaev3.pdf). Tumour measurements were assessed at screening, at every third cycle of chemotherapy, and when disease progression was suspected. Response Evaluation Criteria for Solid Tumors version 1.0 (Therasse et al 2000) was used, requiring repeat imaging studies ⩾4 weeks after the initial documentation of response.
Pharmacokinetics
Pharmacokinetic analysis was carried out in a central laboratory, and pharmacokinetic parameter values were estimated using noncompartmental methods. Human potassium EDTA plasma pharmacokinetic samples were analysed for sunitinib and SU012662 and total drug (sunitinib plus SU012662) concentrations at BASi (West Lafayette, IN, USA) with the use of a validated, sensitive, and specific liquid chromatographic-tandem mass spectrometric method. The performance of the method during validation has been documented in the method validation report (BASi report 1000-05793-1). Plasma specimens were stored at −20°C until assay, and all samples were analysed within the 377 days of established stability. Calibration standard responses met acceptance criteria over the range of 0.100 to 60.0 ng ml−1 for sunitinib and 0.100 to 20.0 ng ml−1 for SU012662 using a quadratic regression with 1/concentration2 weighting. The lower limit of quantitation (LLOQ) for both sunitinib and SU012662 was 0.100 ng ml−1. The between-day assay accuracy, expressed as the ratio (%) of the estimated to the theoretical quality control (QC) concentrations, ranged from −1.1 to 1.3% for the low, medium, and high sunitinib QCs and from −1.0 to 3.6% for the low, medium, and high SU012662 QCs. Assay precision, expressed as the between-day coefficients of variation (%) of the estimated concentrations of QC samples, was ⩽6.5% for the low, medium, and high sunitinib QCs and ⩽6.9% for the low, medium, and high SU012662 QCs.
Human sodium heparin plasma pharmacokinetic samples were analysed for irinotecan and SN-38 concentrations at Eurofins AvTech Laboratories (Portage, MI, USA) using a validated, sensitive, and specific high performance liquid chromatographic method with fluorescence detection. The performance of the method during validation has been documented in the method validation report (Eurofins AvTech Laboratories: report 94-455.07). The plasma specimens were stored at −20°C until assay, and all samples were assayed within the 1860 days (for irinotecan) and 1020 days (for SN-38) of established stability. Calibration standard responses met acceptance criteria over the range of 1.28 to 3840 ng ml−1 for irinotecan and 0.480 to 640 ng ml−1 for SN-38, using a linear regression with 1/concentration weighting. The LLOQ was 1.28 ng ml−1 for irinotecan and 0.480 ng ml−1 for SN-38. The between-day assay accuracy ranged from −0.7 to 3.8% for the low, medium, and high irinotecan QCs and from −8.0 to 3.2% for the low, medium, and high SN-38 QCs. Assay precision was ⩽6.3% for the low, medium, and high irinotecan QCs and ⩽7.1% for the low, medium, and high SN-38 QCs.
Sunitinib was assessed on day −7 (i.e., 1 week before cycle 1); samples were taken pre-dose and at 1, 2, 4, 6, 8, 10, and 24 h post-dose. Irinotecan was evaluated on cycle 1 day 1; samples were taken before infusion and at 1, 1.5, 2, 4, 6, 8, 10, and 24 h after the start of treatment. In cycle 1, sunitinib administration started on day 2. Both agents were evaluated in combination on cycle 2 day 1; samples were drawn before drug administration and at 1, 1.5, 2, 4, 6, 8, 10, and 24 hours post-dose.
Pharmacokinetic parameter values were calculated for each subject by noncompartmental analysis of concentration–time data using WinNonlin version 4.1 (http://pharmacy.ucsf.edu/irc/pdfs/wul_users_guide.pdf). Actual sample collection time was used for sunitinib, SU012662, total drug, irinotecan, and SN-38. If pre-dose concentration for an individual was >5% of Cmax, a carryover correction was made as recommended by FDA guidance (FDA, 2003). Summary descriptives of pharmacokinetic values were presented only for paired observations with respect to each analyte. In the case where the dose for one of the paired observations was different from the other observation, dose correction to the intended dose was performed (correction factor: intended dose/actual dose). Dose correction to the MTD was performed. Individual patient trough plasma concentrations were summarised per cycle and study day. All concentrations that were below the limits of quantitation (BLQ) were set to zero before computation of descriptive statistics (BLQ values were excluded from the calculations of geometric means and the associated 95% confidence intervals).
Standard plasma pharmacokinetic parameters were used including the maximum plasma concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration–time curve to the time of the last measurable concentration (AUC0–last) or infinity (AUC0–∞), clearance, and terminal elimination half-life (t1/2). The geometric mean ratio was used to give a robust measurement of differences in exposure in rate (Cmax) and extent (AUC), with values below 0.8 or above 1.25 suggesting differences between reference and test treatment.
Results
Patient baseline characteristics
A total of 21 patients were enroled in the study, which finally comprised two cohorts (Table 1). Patients in each cohort had been pretreated for a variety of tumour types: all had received chemotherapy, and several had received other treatments including radiation therapy (n=11), hormones (n=1), or other agents (n=4).
Table 1. Patient baseline characteristics.
| Cohort 1 sunitinib 37.5 mg per day+irinotecan 250 mg m−2 (n=11) | Cohort 2 sunitinib 25 mg per day+irinotecan 250 mg m−2 (n=10) | |
|---|---|---|
| Median (range) age, years | 50 (42–62) | 51 (32–67) |
| Male/female, n (%) | 8 (73)/3 (27) | 4 (40)/6 (60) |
| ECOG performance status, n (%) | ||
| 0 | 6 (55) | 3 (30) |
| 1 | 5 (45) | 6 (60) |
| 2 | 0 | 1 (10) |
| Tumour types, n | ||
| Colorectal carcinoma | 1 | 3 |
| Non-small-cell lung carcinoma | 2 | 1 |
| Cervical carcinoma | 1 | 2 |
| Head-and-neck tumour | 2 | 0 |
| Breast carcinoma | 0 | 1 |
| Gall bladder carcinoma | 1 | 0 |
| Gastric carcinoma | 1 | 0 |
| Mesothelioma | 1 | 1 |
| Osteosarcoma | 1 | 0 |
| Prostate carcinoma | 0 | 1 |
| Soft tissue sarcoma | 1 | 0 |
| Mediastinal carcinoid | 0 | 1 |
Abbreviation: ECOG, eastern cooperative oncology group.
Treatment, dose reductions, and discontinuations
Because of DLT encountered in two out of six patients at the starting dose level, sunitinib was de-escalated to 25 mg per day. In a later stage the protocol was amended to expand cohorts with patients who had received no more than two previous chemotherapy regimens and had aspartate aminotransferase and alanine aminotransferase serum levels <2.5 × upper limit of normal (ULN; was <5 × ULN).
All patients received at least one full cycle of treatment, either at the sunitinib 37.5 mg per day (cohort 1) or at the sunitinib 25 mg per day (cohort 2) dose level. Those in cohort 1 received a median of six cycles of both sunitinib and irinotecan, whereas patients in cohort 2 received a median of 3 cycles of each agent. The majority of discontinuations from the study were due to lack of efficacy (n=8 in cohort 1; n=6 in cohort 2). Discontinuation due to adverse events during cycles 1 and 2 occurred in one patient, who experienced grade 3 febrile neutropenia and grade 5 pneumococcal sepsis.
During cycles 1 and 2, dose delay of sunitinib for ⩾1 week was required in three patients in cohort 1 and was not required in cohort 2; delays were required for irinotecan in four and one patients in cohorts 1 and 2, respectively.
Dose reductions of sunitinib occurred in three patients in cohort 1 during cycles 1 and 2, and were not required in cohort 2; no patients required more than one sunitinib dose reduction. For irinotecan, six patients in cohort 1 and one patient in cohort 2 required dose reduction during the first two cycles of treatment.
DLTs, safety, and tolerability
In the sunitinib 37.5 mg per day group, including the expansion of this cohort, a total of four patients experienced a grade 3 or 4 adverse event during the first 2 cycles of treatment that was categorised as DLT: two patients had grade 4 neutropenia, one patient had grade 5 pneumococcal sepsis, and one patient had grade 3 fatigue. At the de-escalated dose of sunitinib 25 mg per day in cohort 2, no DLTs were observed, and the MTD was therefore defined as this dose level.
One patient in the sunitinib 37.5 mg per day group discontinued from the study because of a treatment-related adverse event (reactivation of hepatitis B). There were four deaths during the study, of which three were due to adverse events that were considered to be related to treatment with irinotecan and sunitinib: neurological disorder (n=1; cohort 2), septic shock (n=1; cohort 1), and pneumococcal infection and neutropenic fever (n=1; cohort 1).
The most frequent adverse events of any cause during cycles 1 and 2 (all seen in ⩾60% of patients overall) were vomiting, diarrhoea, and neutropenia. Events occurring in >25% of patients overall during cycles 1 and 2 are shown in Table 2.
Table 2. Adverse events (AEs) occurring in >25% of patients during cycles 1 and 2 and during all cycles (all grades, all causalities).
|
Cohort 1 sunitinib 37.5 mg per day+irinotecan 250 mg m−2
(n=11) |
Cohort 2 sunitinib 25 mg per day+irinotecan 250 mg m−2
(n=10) |
|||
|---|---|---|---|---|
| AE, n (%) | Cycles 1 and 2 | All cycles | Cycles 1 and 2 | All cycles |
| Neutropenia | 11 (100) | 11 (100) | 6 (60) | 6 (60) |
| Nausea | 8 (73) | 8 (73) | 4 (40) | 9 (90) |
| Vomiting | 6 (55) | 8 (73) | 8 (80) | 9 (90) |
| Leukopenia | 9 (82) | 10 (91) | 4 (40) | 5 (50) |
| Diarrhoea | 9 (82) | 9 (82) | 6 (60) | 6 (60) |
| Anorexia | 7 (64) | 8 (73) | 2 (20) | 4 (40) |
| Asthenia | 5 (45) | 6 (55) | 4 (40) | 5 (50) |
| Abdominal pain | 6 (55) | 6 (55) | 4 (40) | 5 (50) |
| Alopecia | 5 (45) | 7 (64) | 4 (40) | 4 (40) |
| Fatigue | 4 (36) | 4 (36) | 3 (30) | 5 (50) |
| Headache | 3 (27) | 4 (36) | 4 (40) | 5 (50) |
| Dyspnoea | 3 (27) | 3 (27) | 4 (40) | 6 (60) |
| Anaemia | 3 (27) | 6 (55) | 3 (30) | 3 (30) |
| Mucosal inflammation | 5 (45) | 5 (45) | 1 (10) | 2 (20) |
| Thrombocytopenia | 3 (27) | 4 (36) | 2 (20) | 2 (20) |
Adverse events in patients receiving sunitinib 25 mg per day with irinotecan were predominantly grade 1 or 2 and nonhaematological in nature. In these patients, the most common grade ⩾3 all-causality adverse events in cycles 1 and 2 were neutropenia (n=2) and vomiting (n=2; Table 3). In patients receiving sunitinib 37.5 mg per day with irinotecan, the most common grade ⩾3 adverse events during the first two cycles were haematological: neutropenia (n=8) and leukopenia (n=6; Table 3).
Table 3. Most frequent (occurred in ⩾2 patients) adverse events (AEs), grade ⩾3, all causalities.
|
Cohort 1 sunitinib 37.5 mg per day+irinotecan 250 mg m−2
(n=11) |
Cohort 2 sunitinib 25 mg per day+irinotecan 250 mg m−2
(n=10) |
|||
|---|---|---|---|---|
| AE, n (%) | Cycles 1 and 2 | All cycles | Cycles 1 and 2 | All cycles |
| Haematological | ||||
| Neutropenia | 8 (73) | 8 (73) | 2 (20) | 3 (30) |
| Leukopenia | 6 (55) | 6 (55) | 1 (10) | 1 (10) |
| Anaemia | 1 (9) | 2 (18) | 1 (10) | 1 (10) |
| Thrombocytopenia | 0 | 1 (9) | 1 (10) | 1 (10) |
| Nonhaematological | ||||
| Asthenia | 2 (18) | 3 (27) | 1 (10) | 3 (30) |
| Vomiting | 1 (9) | 2 (18) | 2 (20) | 3 (30) |
| Fatigue | 1 (9) | 1 (9) | 1 (10) | 2 (20) |
| Nausea | 1 (9) | 1 (9) | 0 | 1 (10) |
| Abdominal pain | 0 | 1 (9) | 1 (10) | 1 (10) |
| Gamma glutamyl transferase increase | 1 (9) | 2 (18) | 0 | 0 |
Efficacy
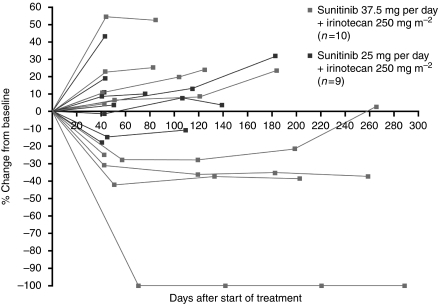
The majority of patients (10 out of 11 patients in the sunitinib 37.5 mg per day group and all 10 patients in the 25 mg per day group) had measurable disease at baseline, and were available for assessment of tumour response (Table 4). Out of 10 evaluable patients, 3 (30%) in cohort 1 had a confirmed partial response: one patient with submandibular cancer, one patient with oropharyngeal cancer who experienced complete regression of the target lesion in the presence of persisting non-target lesions, and one patient with relapsed NSCLC. Figure 1 shows durable tumour shrinkage in the patient with NSCLC. A further two patients in cohort 1 experienced stable disease for ⩾12 weeks (one patient each with CRC and leiomyosarcoma). In cohort 2, two patients (one patient each with prostate cancer and cervical cancer) had a best response of stable disease for ⩾12 weeks. Tumour response assessments (percentage change from baseline in sum of target lesions) are shown for individual patients in Figure 2.
Table 4. Patients' best tumour response to treatment according to RECIST 1.0.
| Patients, n (%) | Cohort 1 sunitinib 37.5 mg per day+irinotecan 250 mg m−2 (n=10) | Cohort 2 sunitinib 25 mg per day+irinotecan 250 mg m−2 (n=10) |
|---|---|---|
| Partial response | 3 (30) | 0 |
| Stable disease ⩾12 weeks | 2 (20) | 2 (20) |
| Progressive disease | 3 (30) | 4 (40) |
| Not evaluable | 2 (20) | 4 (40) |
Abbreviation: RECIST 1.0=Response Evaluation Criteria in Solid Tumors version 1.0.
Figure 1.
Partial response in a patient with inoperable non-small-cell lung cancer receiving sunitinib 37.5 mg per day and irinotecan. CT scans are shown at baseline (A), after 7 weeks (B) and 6 months (C) on treatment.
Figure 2.
Percentage change from baseline in sum of target lesions (mm) in patients assessed for treatment response.
Pharmacokinetics
A summary of paired observations with respect to each analyte is shown in Table 5. Data for the 25 mg per day and 37.5 mg per day dose levels of sunitinib were reported in combination after dose correction to the MTD (25 mg for sunitinib and 250 mg m−2 for irinotecan). Where doses differed within paired observations, dose corrections to the intended dose were also performed. Based on this analysis, changes in pharmacokinetic values for sunitinib, total drug (sunitinib+SU012662), irinotecan, and SN-38 were within the range of variability of the data. SU012662 presented higher geometric mean ratios, which was mostly due to an apparent lower plasma exposure with the first dose (day −7; sunitinib alone).
Table 5. Summary of pharmacokinetic values (arithmetic mean (coefficient of variation)) for sunitinib, SU012662, sunitinib+SU012662, irinotecan, and its active metabolite SN-38 for all doses combined (paired observations only).
| Pharmacokinetic parameter | Sunitinib alone C0D–7, mean (CV%) | Irinotecan alone C1D1, mean (CV%) | Sunitinib+irinotecan C2D1, mean (CV%) | Geometric mean ratio (C2D1/C0D–7 or C2D1/C1D1) |
|---|---|---|---|---|
| Sunitinib (n=18)a | ||||
| Tmax (h)b | 8 (4–24) | NA | 8 (2–29) | |
| Tlast (h)c | 24.0 | NA | 24.0 | |
| Cmax (ng l−1) | 16.3 (39.2) | NA | 13.0 (31.8) | 0.82 |
| AUC0–last (ng h ml−1) | 266.5 (41.2) | NA | 225.5 (29.5) | 0.88 |
| SU012662 (n=18)a | ||||
| Tmax (h)b | 7 (2–24) | NA | 24 (1.8–29) | |
| Tlast (h)c | 24.0 | NA | 24.0 | |
| Cmax (ng ml−1) | 2.4 (51.4) | NA | 3.6 (32.5) | 1.6 |
| AUC0–last (ng h ml−1) | 38.6 (54.4) | NA | 53.2 (30.9) | 1.48 |
| Total drug (n=18)a | ||||
| Tmax (h)b | 8 (4–24) | NA | 9 (2–29) | |
| Tlast (h)c | 24.0 | NA | 24.0 | |
| Cmax (ng ml−1) | 18.5 (40.3) | NA | 16.2 (29.7) | 0.90 |
| AUC0–last (ng h ml−1) | 305.2 (42.1) | NA | 278.2 (28.7) | 0.95 |
| Irinotecan (n=18–20)c | ||||
| Tmax (h)b | NA | 1 (1–1.7) | 1 (0.9–1.7) | |
| Tlast (h)c | NA | 24.0 | 24.0 | |
| Cmax (μg ml−1) | NA | 2.9 (21.3) | 3.6 (26.8) | 1.21 |
| AUC0–last (μg h ml−1) | NA | 14.9 (27.7) | 16.9 (33.3) | 1.12 |
| AUC0–∞ (μg h ml−1) | NA | 15.9 (29.3) | 18.1 (34.0) | 1.13 |
| CL (l h−1) | NA | 31.9 (30.3) | 30.4 (28.8) | |
| t1/2 (h) | NA | 6.3 (19.8) | 6.6 (21.9) | |
| SN-38 (n=18–20)c | ||||
| Tmax (h)b | NA | 1.1 (1.0–4.2) | 1.5 (0.9–4.1) | |
| Tlast (h)c | NA | 24.0 | 24.0 | |
| Cmax (μg ml−1) | NA | 0.03 (51.4) | 0.04 (50.0) | 1.13 |
| AUC0–last (μg h ml−1) | NA | 0.31 (61.7) | 0.36 (52.8) | 1.20 |
Abbreviations: AUC0–∞=area under the plasma concentration–time curve (AUC) from time zero to infinity; AUC0–last=AUC from time zero to the last quantifiable sampling time point; C0D–7=cycle 0 (screening) day –7; C1D1=cycle 1 day 1; C2D1=cycle 2 day 1; CL=clearance; Cmax=maximum plasma concentration; CV=coefficient of variation; NA=not applicable; t1/2=terminal elimination half-life; Tlast=time when last sample collected; Tmax=time to Cmax.
Pharmacokinetic parameters estimated on C2D1 were corrected for carryover pre-dose concentrations where applicable.
Median and range values presented for the parameter Tmax.
Median value reported for Tlast.
For patients in both cohorts combined, the geometric mean ratios (sunitinib+irinotecan relative to sunitinib alone and sunitinib+irinotecan relative to irinotecan alone) of the pharmacokinetic parameter that related to maximum and total plasma exposure (i.e., Cmax, AUC0–last and AUC0–∞) were calculated. The geometric mean ratios (sunitinib+irinotecan relative to sunitinib alone) of Cmax and AUC0–last, for both cohorts combined were 0.82 and 0.88, respectively. Similarly, the geometric mean ratios for total drug were 0.90 and 0.95 for Cmax and AUC0–last, respectively, suggesting that the pharmacokinetics of sunitinib when coadministered with irinotecan did not appear to change as compared with when it was administered alone.
The higher geometric mean ratios and apparent lower plasma exposure of SU012662 could potentially have been related to low-capacity tight/target tissue binding sites, which would be only present after the first dose at very low concentrations with limited sampling. Therefore, the increase in the geometric mean ratios for SU012662 was potentially caused by the limited sampling scheme and not because of a decrease in the elimination of the metabolite when sunitinib was coadministered with irinotecan as compared with its administration alone.
The geometric mean ratios (sunitinib+irinotecan relative to irinotecan alone) of Cmax, AUC0–last and AUC0–∞ for both cohorts combined were 1.21, 1.12, and 1.13, respectively. Similarly, the geometric mean ratios of Cmax and AUC0–last for SN-38 were 1.13 and 1.20, respectively. Therefore, based on these data, coadministration of sunitinib with irinotecan did not appear to affect the pharmacokinetics of irinotecan or its active metabolite SN-38.
Discussion
The current standard of care for treatment of patients with advanced CRC is FOLFOX or FOLFIRI with bevacizumab. Based on the rationale for combining a tyrosine kinase inhibitor of VEGFRs with chemotherapy, the combination of sunitinib plus irinotecan might offer efficacy in this patient category. In our dose-finding study, evidence is presented for objective responses in patients treated with the higher dose of sunitinib 37.5 mg per day in combination with irinotecan. At this dose, one patient with NSCLC and two with tumours in the head-and-neck region achieved a partial response. A further two patients (one patient each with CRC and leiomyosarcoma) maintained stable disease for ⩾12 weeks. It may be that durable stable disease, rather than objective response, is the main efficacy benefit when sunitinib is added to chemotherapy. The lower-dose regimen (sunitinib 25 mg per day with irinotecan), however, did not result in clinically meaningful responses.
Our dose-finding study indicated that in combination with irinotecan, the sunitinib dose of 37.5 mg per day was not well tolerated, with DLTs (including grade 4 neutropenia, grade 5 pneumococcal sepsis, and grade 3 fatigue) reported in four patients. Therefore, the MTD was determined to be sunitinib 25 mg per day, a dose level which was not associated with any DLTs. Indeed, the tolerability and safety results from this study in pretreated patients show that sunitinib 25 mg per day (days 1–14) with irinotecan 250 mg m−2 (day 1) in a 21-day cycle has a manageable safety profile. Most adverse events were mild–moderate at this dose, and haematological events, especially neutropenia and leukopenia, occurred at a lower frequency as compared with the higher dose.
Toxicities due to irinotecan normally include neutropenia, diarrhoea, and vomiting (Fuchs et al, 2003). In our study, these were among the most common all-causality adverse events, occurring in more than half of patients during cycles 1 or 2. Irinotecan/SN-38 toxicity is known to be affected by genetic and physiological variation in uridine-diphosphoglucuronosyl transferase 1A1 (UGT1A1) enzyme activity. Genotyping of UGT1A1 to permit potential reduction of drug dosing in patients with the UGT1A1*28 polymorphism is increasingly being carried out to avoid severe toxicities (Deeken et al, 2008; Funke et al, 2008; Rouits et al, 2008). Although 250 mg m−2 is a relatively low irinotecan dose, two patients in this study who developed grade 4 neutropenia within the first 2 weeks of cycle 1 were found to have decreased metabolism of SN-38 to its metabolite SN-38-glucuronide because of a UGT1A1*1/*28 and UGT1A1*28/*28 genotype, respectively.
The pharmacokinetic parameter values for sunitinib, SU012662, total drug (sunitinib + SU012662), irinotecan, and its active metabolite were consistent with data previously reported for sunitinib (Faivre et al, 2006; Britten et al, 2008), as well as for irinotecan (Rea et al, 2005; Camptosar, 2008). For patients in both cohorts combined, the geometric mean ratios (sunitinib+irinotecan relative to sunitinib alone, and sunitinib+irinotecan relative to irinotecan alone) of the pharmacokinetic parameters that related to maximum and total plasma exposure (i.e. Cmax, AUC0–last, and AUC0–∞, respectively) were calculated. It could be concluded that no significant drug–drug interaction was found between sunitinib and irinotecan.
In conclusion, the results of this study suggest that no clinically relevant pharmacokinetic interactions occur when sunitinib is administered on days 1–14 of a 21-day cycle with irinotecan given on day 1. The MTD was defined as sunitinib 25 mg per day (days 1–14) and irinotecan 250 mg m−2 on day 1; this combination was reasonably well tolerated, but did not show preliminary antitumour activity. Evidence of activity was observed at the higher 37.5 mg per day dose, but this exceeded the MTD. Therefore, this particular combination will not be pursued for further studies in unselected patient populations. UGT1A1 genotyping was not routinely performed in our study and may have been useful to identify patients with the UGT1A1*28 polymorphism who required irinotecan dose reduction. As a VEGFR tyrosine kinase inhibitor combined with chemotherapy did not appear to have synergistic antitumour activity in our study (as also observed in most phase III, randomised trials, in contrast to an anti-VEGF monoclonal antibody plus chemotherapy; Los et al, 2007), we do not recommend further phase II or III studies with this combination.
Acknowledgments
This study was supported by Pfizer, Inc. Medical writing support was provided by Jenni Macdougall of ACUMED (Tytherington, UK), and was funded by Pfizer Inc. We thank the contribution to the pharmacokinetics section of this paper by Jean Law, Pfizer Oncology, La Jolla, CA, USA.
References
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM (2003) SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2: 471–478 [PubMed] [Google Scholar]
- Britten CD, Kabbinavar F, Hecht JR, Bello CL, Li J, Baum C, Slamon D (2008) A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol 61: 515–524 [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26: 1810–1816 [DOI] [PubMed] [Google Scholar]
- Camptosar (2008) http://media.pfizer.com/files/products/uspi_camptosar.pdf
- Deeken JF, Slack R, Marshall JL (2008) Irinotecan and uridine diphosphate glucuronosyltransferase 1A1 pharmacogenetics: to test or not to test, that is the question. Cancer 113: 1502–1510 [DOI] [PubMed] [Google Scholar]
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368: 1329–1338 [DOI] [PubMed] [Google Scholar]
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24: 25–35 [DOI] [PubMed] [Google Scholar]
- Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S, Harmon C, Ruiz-Garcia A, Lechuga MJ, Cheng AL (2009) Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol 10: 794–800 [DOI] [PubMed] [Google Scholar]
- FDA (2003) Guidance to Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products - General Considerations. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf
- Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughsey T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL (1999) Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17: 1516–1525 [DOI] [PubMed] [Google Scholar]
- Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR (2003) Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21: 807–814 [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Niitani H, Suzuki A, Motomiya M, Hasegawa K, Nishiwaki Y, Kuriyama T, Ariyoshi Y, Negoro S, Masuda N (1992) A phase II study of CPT-11, a new derivative of camptothecin, for previously untreated non-small-cell lung cancer. J Clin Oncol 10: 16–20 [DOI] [PubMed] [Google Scholar]
- Funke S, Brenner H, Chang-Claude J (2008) Pharmacogenetics in colorectal cancer: a systematic review. Pharmacogenomics 9: 1079–1099 [DOI] [PubMed] [Google Scholar]
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson III AB (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25: 1539–1544 [DOI] [PubMed] [Google Scholar]
- Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R (2007) Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 13: 1367–1373 [DOI] [PubMed] [Google Scholar]
- Gravalos C, Cassinello J, Fernandez-Ranada I, Holgado E (2007) Role of tyrosine kinase inhibitors in the treatment of advanced colorectal cancer. Clin Colorectal Cancer 6: 691–699 [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 20: 3502–3508 [DOI] [PubMed] [Google Scholar]
- Hutson TE, Figlin RA, Kuhn JG, Motzer RJ (2008) Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist 13: 1084–1096 [DOI] [PubMed] [Google Scholar]
- Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, Park SH, Hwang JH, Rha SY, Kweon GR, Lee SJ, Jo KW, Shong M (2006) An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab 91: 4070–4076 [DOI] [PubMed] [Google Scholar]
- Kulke M, Lenz HJ, Meropol N, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li J, Baum C, Fuchs C (2008) Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 26: 3403–3410 [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V, Deray G (2009) Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs 20: 81–82 [DOI] [PubMed] [Google Scholar]
- Los M, Roodhart JM, Voest EE (2007) Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist 12: 443–450 [DOI] [PubMed] [Google Scholar]
- Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Negoro S, Nishioka M, Nakagawa K, Takada M (1992) CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 10: 1225–1229 [DOI] [PubMed] [Google Scholar]
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9: 327–337 [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124 [DOI] [PubMed] [Google Scholar]
- Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O'Farrell AM, Cherrington JM, Pryer NK (2003) SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 20: 757–766 [DOI] [PubMed] [Google Scholar]
- O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM (2003) SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101: 3597–3605 [DOI] [PubMed] [Google Scholar]
- Rea DW, Nortier JW, Ten Bokkel Huinink WW, Falk S, Richel DJ, Maughan T, Groenewegen G, Smit JM, Steven N, Bakker JM, Semiond D, Kerr DJ, Punt CJ (2005) A phase I/II and pharmacokinetic study of irinotecan in combination with capecitabine as first-line therapy for advanced colorectal cancer. Ann Oncol 16: 1123–1132 [DOI] [PubMed] [Google Scholar]
- Rouits E, Charasson V, Petain A, Boisdron-Celle M, Delord JP, Fonck M, Laurand A, Poirier AL, Morel A, Chatelut E, Robert J, Gamelin E (2008) Pharmacokinetic and pharmacogenetic determinants of the activity and toxicity of irinotecan in metastatic colorectal cancer patients. Br J Cancer 99: 1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, Bergsland EK, Haller DG, Lockhart AC, Rocha Lima CM, Huang X, DePrimo SE, Chow-Maneval E, Chao RC, Lenz HJ (2007) Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol 25: 4793–4799 [DOI] [PubMed] [Google Scholar]
- Socinski MA, Novello S, Brahmer JR, Rosell R, Sanchez JM, Belani CP, Govindan R, Atkins JN, Gillenwater HH, Pallares C, Tye L, Selaru P, Chao RC, Scagliotti GV (2008) Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 26: 650–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]