Abstract
Controlling the chondrocytes phenotype remains a major issue for cartilage repair strategies. These cells are crucial for the biomechanical properties and cartilage integrity because they are responsible of the secretion of a specific matrix. But chondrocyte dedifferentiation is frequently observed in cartilage pathology as well as in tissue culture, making their study more difficult. Given that normal articular cartilage is hypoxic, chondrocytes have a specific and adapted response to low oxygen environment. While huge progress has been performed on deciphering intracellular hypoxia signalling the last few years, nothing was known about the particular case of the chondrocyte biology in response to hypoxia. Recent findings in this growing field showed crucial influence of the hypoxia signalling on chondrocytes physiology and raised new potential targets to repair cartilage and maintain tissue integrity. This review will thus focus on describing hypoxia-mediated chondrocyte function in the native articular cartilage.
Keywords: cartilage repair, chondrocytes, hypoxia
Introduction
Chondrocytes are the only resident cell type of the articular cartilage [with exception of a probable stem cell niche (Dowthwaite et al. 2004)]. Their essential known function consists in secreting a large and very specific extracellular matrix. Although the exact composition of the matrix is not entirely known, it is established that type II Collagen and Aggrecan are the most abundant matrix macromolecules, which confer the two main biomechanical properties to that tissue: tension resistance and ability to withstand compression forces respectively (Muir 1995; Buckwalter & Mankin 1998). Other collagens are also part of the cartilage matrix: collagen type 6 located in the pericellular matrix (Söder et al. 2002), collagen type 9 and 11, and, COMP (Hedbom et al. 1992), leucin-rich proteins PRELP (Bengtsson et al. 2002) and other related molecules.
Chondrocytes are very difficult to study. One reason is because normal human cells and tissue are very difficult to obtain. Another major obstacle comes from the chondrocyte instability when these cells are cultured in vitro (Von der Mark et al. 1977; Benya et al. 1978). Whereas they almost do not proliferate in cartilage, monolayer cultured primary chondrocytes start proliferating and de-differentiate with passage: they lose their round shape for a fibroblast-like shape and the main chondrocyte markers such as type II collagen (Col-2), Aggrecan or Sox9 rapidly drop (Glowacki et al. 1983). To date, Sox9 is considered as the key transcription factor controlling (in concert with L-Sox5 and Sox6) Col-2 and Aggrecan expression (Lefebvre et al. 1997, 1998). However, little is known about extracellular matrix gene regulation (Okazaki & Sandell 2004), and it is still unclear what controls the chondrocyte phenotype and what maintains a chondrocyte functional in adult cartilage. Answering these questions is thus crucial to understand patho-physiological situations where dedifferentiation occurs (such as OA) or to develop cartilage repair strategies based on autologous transplantation, where dedifferentiation also occurs.
Brief overview of what controls cartilage integrity
The list of extracellular signals controlling adult cartilage integrity has grown recently: Growth factors [anabolic factors such as TGF-beta, BMP… (Majumdar et al. 2001), or catabolic factors such as IL-1… (Kaiser et al. 2004)], were the most described. But crucial enzymes for cartilage degradation have been identified and also belong to these soluble factors such as metallo-proteinases [MMP-13 which degrades preferentially type II collagen (Knauper et al. 1996)] and aggrecanases [ADAMTS-4 and 5 which are the major cartilage degradation in OA (Tetlow et al. 2001; Glasson et al. 2005)]. It is accepted for long that the imbalance between these factors is the determinant of the cartilage integrity.
However, more recently, the role of other extracellular signals was shown: the 3D structure of the surrounding matrix [modulating chondrocyte cytoskeleton (Blain 2009)], and the matrix composition itself [modulating matrix assembly or the inflammatory response (Sjoberg et al. 2005; Halasz et al. 2007)]. Finally, it is now accepted that mechanical loading and hypoxia are two permanent stresses that impact dramatically the adult articular cartilage. Indeed, compression applied on cartilage is a potent regulator of matrix genes and chondrocyte physiology through mechano-transduction pathways. Exploration of hypoxia effect has only emerged recently with description of hypoxia intracellular signalling, although it was known for a long time that oxygen level was low in adult cartilage compared to other tissues (0.5–5% depending on depth) (Lund-Olesen 1970; Brighton & Heppenstall 1971). In fact, it now clear that hypoxia is a strong promoter of matrix deposition by the chondrocytes (Murphy & Sambanis 2001).
General overview of the HIF signalling pathway
In 1995, Semenza et al. discovered cells can sense surrounding oxygen level through Hypoxia–Inducible transcription Factors (initially named HIF1-α) whose expression and function are mainly post-translationally regulated by hydroxylation reactions (Semenza & Wang 1992; Semenza et al. 1994). Under high oxygen environment, these proteins have a very short half-life (<5 min). This is due to specific hydroxylated proline that are recognized by the Von Hippel-Lindau protein (pVHL-containing E3 ubiquitin ligase complex) and that targets them to degradation via the proteasome (Semenza 2000). Conversely, when oxygen level is low (5–1%), hydroxylation decreases and HIF-1α is prevented from a rapid degradation. Then HIF1-α heterodimerizes with the constitutively expressed HIF-1β (also called Aryl hydrocarbon Nuclear Translocator ARNT), translocates into the nucleus, and binds specific consensus sequences (-RCGTG-) on gene promoters (Figure 1).
Figure 1.
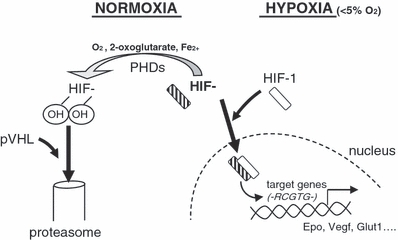
HIFs regulation and signalling. Hydroxylation of HIF-α is inhibited in low oxygen environment (less than 5%) because prolylhydroxylases (PHDs) activity is dependent on oxygen. Then non-hydroxylated HIF-α can heterodimerize with HIF1-β, and translocate into the nucleus, where it binds to consensus sequences.
More recently, HIFs family enlarged with the discovery of Hif-2α, whose product is highly similar to the other (∼50% homology) (Ema et al. 1997). A higher complexity came with Hif-3α identification because this gene was shown to produce at least six different proteins following alternative splicing (Maynard et al. 2003).
Hif-1α and -2α genes share the same organization, and thus give the same protein structure: a bHLH domain on the amino-terminal, an intermediate PAS domain (PER-ARNT-SIM) and a transactivation domain (TAD) (Sowter et al. 2003). The TAD contains one N-terminal TAD (NTAD) and one C-terminal domain (CTAD) (Yan et al. 2007). Unlike the others, HIF-3α lacks the TAD, suggesting it could act as a dominant negative of HIF-1α and -2α (Maynard et al. 2005).
But the ‘real’ oxygen sensors are the hydroxylases targeting the proline residues of HIFs (prolyl-hydroxylases, PHD-1 to 3), since they use oxygen as a co-factor and thus directly rely on oxygen level within the cell. In vitro experiments have shown that proline hydroxylation occurs on the NTAD (on the Pro402 and Pro564 of the human HIF-1α) in the oxygen degradation domain (ODD) (Jaakkola et al. 2001). Recently, it has been described that an asparagine residue located in the CTAD (on the Asn803 of the human HIF-1α) is also hydroxylated by an asparaginyl hydroxylase (Factor inhibiting HIF, FIH) inactivating HIF (Mahon et al. 2001; Lando et al. 2002). The recruitment of co-activator such as p300/CBP has been shown to be essential for its transcriptional activity (Lando et al. 2002).
Others post-translational modifications such as phosphorylation (by erk, AMP-activated Kinase) or acetylation (on the Lys532 of the human HIF-1α) were shown to increase its transcriptional activity (Richard et al. 1999) or to enhance its destabilization (Jeong et al. 2002) respectively but are still controversial.
HIF signalling applied to articular cartilage: role in chondrocyte differentiation and matrix deposition
Detection of the HIF protein in human articular cartilage is challenging, but some important findings describing the role of HIF-1α during mouse cartilage development have been performed. The first one came with Schipani et al. who demonstrated that the developmental growth plate was hypoxic: a cartilage specific depletion of HIF-1α was realized in mouse and showed an increase of chondrocyte death coupled with decreased expression of the CDK inhibitor p57, strongly suggesting HIF-1α was essential for chondrocyte growth arrest and survival (Schipani et al. 2001). Using epiphyseal chondrocytes from newborn mice lacking HIF-1α, it was proposed that VEGF is secreted within the growth plate, and thereby triggers survival signals (Cramer et al. 2004). Provot et al. recently detailed the role of HIF-1α during chondrogenesis in growth plate. Using a conditional knock-out, they showed early chondrogenesis (formation of cartilaginous primordiae) and joint formation were impaired (Provot et al. 2007). Because it was more recently discovered, HIF-2α is less documented, but it was shown to be elevated during chondrocyte differentiation accompanied by VEGF increase, suggesting a role in the metabolic shift in the growth plate (Stewart et al. 2006).
Interestingly, experiments on mice also suggested that HIFs not only participate to the differentiation of chondrocytes but also to the function of chondrocytes. Indeed, it was shown that HIF-1α increases matrix deposition such as type II collagen (Pfander et al. 2003). Moreover, deletion of pVHL in chondrocytes (which results in HIF-1α and -2α overexpression) increases matrix deposition during growth-plate development (Pfander et al. 2004).
In vitro studies demonstrated that hypoxia promotes articular cartilage function by increasing expression of cartilage matrix genes in bovine and human articular chondrocytes (HACs) (Murphy & Sambanis 2001; Domm et al. 2002), with similar results in human meniscal cells (Adesida et al. 2006). Hypoxia was shown to increase type II collagen and Aggrecan and the key transcription factor Sox9 (Murphy & Polak 2004). Identification of the mechanism was performed using siRNA in HACs. By selective HIF-1α and HIF-2α depletion in HACs placed in prolonged hypoxia, we have shown that HIF-2α and not HIF-1α is critical for Sox9 induction and, as a consequence for Collagen 2 expression (Lafont et al. 2007).
Role of PHDs in cartilage
As PHDs are the true oxygen sensors of hypoxia, these enzymes may be critical in controlling the chondrocyte phenotype.
All the PHDs are expressed in maturing zone of the mouse growth plate (Terkhorn et al. 2007). We observed PHD2 was the most expressed compared to PHD3 and 1, in HACs in vitro (Lafont et al. 2008; Murphy et al. 2009). Interestingly, PHD2 was shown by Pouysségur et al. to be the main hydroxylase regulating HIF-1α (Berra et al. 2003). Selective PHD activity for HIF-1α or HIF-2α has been already shown by Ratcliffe and Gleadle (Appelhoff et al. 2004), suggesting that PHD could have a tissue specific selective activity.
Because we have shown HIF-2α and not HIF-1α was involved in the control of the human chondrocyte phenotype (Lafont et al. 2007), it is now tempting to find out which PHD selectively controls HIF-2α expression in HACs. Inhibiting one specific PHD in cartilage could thus lead, through the specific HIF-2α increase, to a better control of the chondrocyte phenotype without HIF-1α overexpression [which promotes angiogenic phenotype, favouring tumourigenesis (Fang et al. 2001), or induces catabolic cytokines such as Interleukin-1 (Zhang et al. 2006)]. In vivo approaches targeting HIFs proteins have already been performed: inhibition of HIF-1α expression has been obtained in mouse knee after intra-articular injection of the anti-angiogenic compound 2-methoxyestradiol (through inhibition of HIFs) (Gelse et al. 2008). As a result, authors described a progression of OA (osteophytes, signs of degeneration, loss of superficial matrix), showing that in vivo inhibition of HIFs signalling impairs cartilage integrity and validating the approach targeting HIF in vivo to control cartilage integrity.
Transcriptional regulation of chondrocytes genes by the HIFs
Study of the transcriptional regulation of chondrocyte genes by HIFs has just been started a few years ago. Sox9 is the first one to studied because its promoter contains several putative HIF Responsive Elements (HRE). To do so, mouse stromal cells (ST2) were transfected with a Sox9 promoter construct containing 6.8 kb. Authors showed an up-regulation of the promoter activity under hypoxia. When putative HRE sequences (located within the first 500 bp) were mutated, such an up-regulation was abolished especially with two of them (Robins et al. 2005). These results have been confirmed more recently in micromass culture. Amarilio et al. showed by chromatin immunoprecipitation, a recruitment of HIF-1α on the Sox9 promoter precisely on the same HRE (Amarilio et al. 2007). Whether HIF-2α directly binds to Sox9 promoter in humans remains unknown.
Very little is known about transcriptional mechanisms by which chondrocytes markers are regulated. Because articular cartilage is chronically hypoxic, HIFs proteins are permanently highly expressed within the chondrocytes, which must affect their transcriptional response to others stimulus. As a consequence, studying transcriptional regulation of chondrocytes genes by HIFs is the next crucial step.
How oxygen tension affects the chondrocyte biology
Despite several studies have been performed to understand how hypoxia could affect chondrocyte specific gene expression, none of them tried to describe the overall effect on the chondrocyte transcriptome. To answer this basic question and characterize the gene response of HACs to hypoxia, a large scale assay has been recently performed in our group using a microarray analysis (Lafont et al. 2008). A list of 101 chondrocyte specific and hypoxia responsive genes has been identified by comparing HACs in 20% oxygen with 1% oxygen. They were identified as down-regulated by passage (dedifferentiation) and up-regulated (redifferentiation) by hypoxia and thus are considered as possible chondrocytes markers/regulators.
The first finding of this large scale study reveals that all the already established chondrocytes markers (sox9, collagen 2 and aggrecan, all the chondrocyte-specific collagens etc.…) are positively regulated by hypoxia, reinforcing the positive effect of low oxygen on chondrocytes.
Beside these genes, other hypoxia-responsive genes were found of potential functional interest for the chondrocyte phenotype: (i) matrix maturing enzymes such as hydroxylases or proteoglycan synthases (participating to the matrix turnover); (ii) inflammatory mediators; (iii) transcription factors including DEC1, Dlx5 [associated with the chondrocyte differentiation (Hsu et al. 2006) but never reported in HACs] or Mef2c [recently identified as involved in the craniofacial development (Arnold et al. 2007; Verzi et al. 2007)]; (iv) growth factors including the angiogenic VEGF and antiangiogenic ChMI or TGF-beta family members; and (v) receptors such as FGFR-3 or the Collagen receptor DDR1.
A very recent study has shown that HIF-2α, by preventing apoptosis and limiting ROS production, is also of importance in chondrocyte autophagy regulation (Bohensky et al. 2009). Human tissue analysis showed that decreased expression of HIF-2α is associated with autophagy in OA tissue and ageing cartilage, suggesting a positive role for HIF-2α in maintaining cartilage integrity. These results again demonstrate the importance of the hypoxic environment for the chondrocyte biology, having a larger effect on the chondrocyte phenotype than originally thought. Because hypoxia triggers essential signals, affecting many aspects of the chondrocyte biology and involved in the chondrocyte phenotype maintenance, hypoxia can be considered as a potent anabolic factor on dedifferentiated chondrocytes.
Hypoxia and pathologies of cartilage
Analyses of diseased joints, such as RA joints, showed new blood vessels, which is now recognized as an aspect of the progression of the disease (Etherington et al. 2002). High proportion of vessels in RA joint synovium associated with neovascular markers is also detected. Measurement of oxygen in synovial fluid in different pathologies such as RA or OA revealed many differences (Lund-Olesen 1970, Sivakumar et al. 2008), suggesting that oxygen level is essential for normal healing and repair in the joint. Recent data showed HIF-2α was expressed with VEGF in synovial tissue. However it is still unclear how oxygen tension affects development of joint pathologies (Sivakumar et al. 2008).
Conclusion and perspectives
Despite its simple cell composition (mainly one resident cell type), cartilage should be considered in its whole complexity: an interface structure between soft tissue and bone tissue, harbouring interactions with different cell type (monocytes, synoviocytes, osteocytes…), being in a permanent stress (hypoxia, pH, mechanical stimulation). We suggested that hypoxia, through the hypoxia inducible transcription factor HIF-2α, is triggering a chondro-anabolic stimulus, enhancing the matrix deposition of dedifferentiated chondrocytes, and thus probably contributing to articular cartilage integrity (Figure 2). It raises the possibility of improving therapies through manipulation of HIF-2α via the inhibition of a specific prolylhydroxylase.
Figure 2.
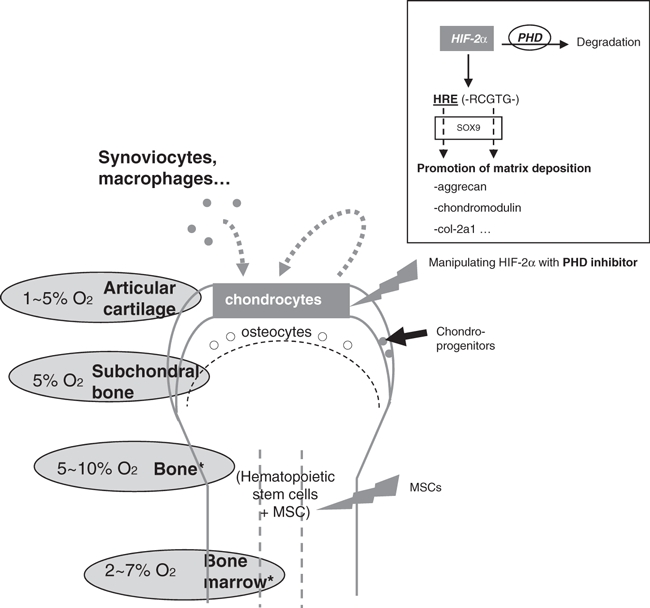
Importance of HIF-2α in cartilage repair strategies. Levels of oxygen are low in the surrounding tissues of joints (Lund-Olesen 1970; Ishikawa & Ito 1988). In articular chondrocytes, it promotes matrix deposition. Manipulating the oxygen-dependent transcription factor HIF-2α could be of help in cartilage repair strategies.
As explained in this review, articular cartilage is in fact integrating permanently and simultaneously several signals. Beside its intrinsic effects through HIF-2α, hypoxia probably affects dramatically other signalling pathways that constantly influence chondrocytes. Thus, future work will certainly have to revisit the effects of some of growth factors by integrating the ‘hypoxia’ factor in cartilage.
Acknowledgments
We thank Dr Chris L. Murphy for his support and helpful discussions during preparation of this manuscript. This study was supported by grant no. BBSB0126X from the Biotechnology and Biological Sciences Research Council (BBSRC), and by the Arthritis Research Campaign (ARC), British Society for Matrix Biology (BSMB).
References
- Adesida AB, Grady LM, Kahn WS, Hardingham TE. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res. Ther. 2006;8:R61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Arnold MA, Kim Y, Czubryt MP. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Bengtsson E, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 2002;277:15061–15068. doi: 10.1074/jbc.M108285200. [DOI] [PubMed] [Google Scholar]
- Benya PD, Padilla SR, Nimmi ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain EJ. Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int. J. Exp. Pathol. 2009;90:1–15. doi: 10.1111/j.1365-2613.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohensky J, Terkhorn SP, Srinivas V, Shapiro IM. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60:1406–1415. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton CT, Heppenstall RB. Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis. An in vitro and in vivo study in rats and rabbits. J. Bone Joint Surg. Am. 1971;53:719–728. [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cramer T, Schipani E, Johnson RS, Swoboda B, Pfander D. Expression of VEGF isoforms by epiphyseal chondrocytes during low-oxygen tension is HIF-1 alpha dependent. Osteoarthritis Cartilage. 2004;12:433–439. doi: 10.1016/j.joca.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Domm C, Schunke M, Christesen K, Kurz B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage. 2002;10:13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Ema M, Taya S, Yokotami N, Sogowa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl Acad. Sci. USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington PJ, Winlove P, Taylor P, Paleolog E, Miotla JM. VEGF release is associated with reduced oxygen tensions in experimental inflammatory arthritis. Clin. Exp. Rheumatol. 2002;20:799–805. [PubMed] [Google Scholar]
- Fang J, Yan L, Shing Y, Moses MA. HIF-1alpha-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001;61:5731–5735. [PubMed] [Google Scholar]
- Gelse K, Pfander D, Obier S, et al. Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints. Arthritis Res. Ther. 2008;10:R111. doi: 10.1186/ar2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Glowacki J, Trepman E, Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc. Soc. Exp. Biol. Med. 1983;172:93–98. doi: 10.3181/00379727-172-41533. [DOI] [PubMed] [Google Scholar]
- Halasz K, Kassner A, Mörgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- Hedbom E, Antonsson P, Hejerpe A. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 1992;267:6132–6136. [PubMed] [Google Scholar]
- Hsu SH, Noamani B, Albernethy DE, Zhu H, Levi G, Bendall AJ. Dlx5- and Dlx6-mediated chondrogenesis: differential domain requirements for a conserved function. Mech. Dev. 2006;123:819–830. doi: 10.1016/j.mod.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Ito T. Kinetics of hemopoietic stem cells in a hypoxic culture. Eur. J. Haematol. 1988;40:126–129. doi: 10.1111/j.1600-0609.1988.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Haag J, Söder S, Bau B, Aigner T. Bone morphogenetic protein and transforming growth factor beta inhibitory Smads 6 and 7 are expressed in human adult normal and osteoarthritic cartilage in vivo and are differentially regulated in vitro by interleukin-1beta. Arthritis Rheum. 2004;50:3535–3540. doi: 10.1002/art.20750. [DOI] [PubMed] [Google Scholar]
- Knauper V, Lopez-Otin C, Smith B, et al. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- Lafont JE, Talma S, Hopfgarten C, Murphy CL. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 2008;283:4778–4786. doi: 10.1074/jbc.M707729200. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harvey VR, Goodfellow PN, Decrombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Li P, Decrombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970;13:769–776. doi: 10.1002/art.1780130606. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- Maynard MA, Qi H, Ching J, et al. Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J. Biol. Chem. 2003;278:11032–11040. doi: 10.1074/jbc.M208681200. [DOI] [PubMed] [Google Scholar]
- Maynard MA, Evans AJ, Hosomi T, Hara S, Jewett MA, Ohh M. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB J. 2005;19:1396–1406. doi: 10.1096/fj.05-3788com. [DOI] [PubMed] [Google Scholar]
- Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995;17:1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- Murphy CL, Polak JM. Control of human articular chondrocyte differentiation by reduced oxygen tension. J. Cell. Physiol. 2004;199:451–459. doi: 10.1002/jcp.10481. [DOI] [PubMed] [Google Scholar]
- Murphy CL, Sambanis A. Effect of oxygen tension and alginate encapsulation on restoration of the differentiated phenotype of passaged chondrocytes. Tissue Eng. 2001;7:791–803. doi: 10.1089/107632701753337735. [DOI] [PubMed] [Google Scholar]
- Murphy CL, Thoms BL, Vaghjiani RJ, Lafont J. Hypoxia. HIF-mediated articular chondrocyte function: prospects for cartilage repair. Arthritis Res. Ther. 2009;11:213. doi: 10.1186/ar2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Sandell LJ. Extracellular matrix gene regulation. Clin. Orthop. Relat. Res. 2004;(427 Suppl.):S123–S128. doi: 10.1097/01.blo.0000144478.51284.f3. ??? [DOI] [PubMed] [Google Scholar]
- Pfander D, Cramer T, Schipani E, Johnson RS. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J. Cell Sci. 2003;116(Pt 9):1819–1826. doi: 10.1242/jcs.00385. [DOI] [PubMed] [Google Scholar]
- Pfander D, Kobayashi T, Knight MC, et al. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development. 2004;131:2497–2508. doi: 10.1242/dev.01138. [DOI] [PubMed] [Google Scholar]
- Provot S, Zinyk D, Gunes Y, et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DE, Berra E, Gothié E, Roux D, Pouysségur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Robins JC, Akeno N, Mukherjee A, et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Schipani E, Ryan HE, Didrikson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Sivakumar B, Akhavani MA, Winlove CP, Taylor P, Paleolog E, Kang N. Synovial hypoxia as a cause of tendon rupture in rheumatoid arthritis. J. Hand Surg. Am. 2008;33:49–58. doi: 10.1016/j.jhsa.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Sjoberg A, Onnerfjord P, Mörgelin M, Heinegard D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 2005;280:32301–32308. doi: 10.1074/jbc.M504828200. [DOI] [PubMed] [Google Scholar]
- Söder S, Hambach L, Lisser R, Kirchner T, Aigner T. Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2002;10:464–470. doi: 10.1053/joca.2002.0512. [DOI] [PubMed] [Google Scholar]
- Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- Stewart AJ, Houston B, Farquharson C. Elevated expression of hypoxia inducible factor-2alpha in terminally differentiating growth plate chondrocytes. J. Cell. Physiol. 2006;206:435–440. doi: 10.1002/jcp.20481. [DOI] [PubMed] [Google Scholar]
- Terkhorn SP, Bohensky J, Shapiro IM, Koyama E, Srinivas V. Expression of HIF prolyl hydroxylase isozymes in growth plate chondrocytes: relationship between maturation and apoptotic sensitivity. J. Cell. Physiol. 2007;210:257–265. doi: 10.1002/jcp.20873. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Verzi MP, Agarwal P, Brown C, McCulley DJ, Schwartz JJ, Black BL. The transcription factor MEF2C is required for craniofacial development. Dev. Cell. 2007;12:645–652. doi: 10.1016/j.devcel.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Mark K, Gauss V, Von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- Yan Q, Bartz S, Mao M, Kaelin WG., Jr. The hypoxia-inducible factor 2α N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol. Cell. Biol. 2007;27:2092–2102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Petrovic JM, Callagham D, et al. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J. Neuroimmunol. 2006;174:63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]


