Abstract
Mucoepidermoid carcinoma (MEC), the most common primary salivary malignancy, shows great variability in clinical behaviour, thus demanding investigation to identify of prognostic markers. Since Warburg's studies, unrestricted cell growth during tumorigenesis has been linked to altered metabolism, implying hypoxic stimulation of glycolysis and diminished contribution of mitochondrial oxidative phosphorylation to cellular ATP supply. Hypothesizing that the study of MEC metabolic status could lead to the discovery of prognostic markers, we investigated by immunohistochemistry the expression of glucose transporter 1 (Glut-1), mitochondrial antigen and peroxiredoxin I (Prx I) in samples of MEC from different histological grades. Our results showed that mitochondrial antigen and Prx I were expressed in the majority of the MEC cases independent of the histological grade. In contrast Glut-1 expression increased significantly as the tumours became more aggressive. These results suggested that oxidative phosphorylation may contribute to ATP supply in all stages of MEC progression, and that the relative contribution of glycolysis over mitochondria for cellular ATP supply increases during MEC progression, favouring growth under low oxygen concentration. In addition, the observed high Prx I protein levels could provide protection to tumour cells against reactive oxygen species generated as a consequence of mitochondrial function and hypoxia-reoxygenation cycling. Altogether our findings suggest that upregulation of Glut-1 and Prx I constitute successful adaptive strategies of MEC cells conferring a growth advantage over normal salivary gland cells in the unstable oxygenation tumour environment.
Keywords: glucose transporter protein 1, hypoxia, mucoepidermoid carcinoma, peroxiredoxins, salivary gland, tumour metabolism
Introduction
Mucoepidermoid carcinoma (MEC) is the most common primary salivary malignancy of both major and minor glands, characterized by mucous, intermediate and epidermoid cells (Gnepp et al. 2005). MEC presents great variability in clinical behaviour, which has conducted studies towards identification of prognostic markers. Clinical and histopathological features, as well as genetic alterations have all been studied as an attempt to predict biological outcome (Brandwein et al. 2001, Pires et al. 2004; Rapidis et al. 2007; Bell et al. 2008; Vargas et al. 2008). Currently, prognostic useful factors include MIB-1 index and the histopathological features such as the number of mitotic figures per 10 high-power fields, degree of anaplasia, necrosis incidence, neural involvement and the relative proportion of the cystic component in the entire tumour (Gnepp et al. 2005). These features constitute the basis for the World Health Organization classification of MEC, which distinguishes three grades of malignancy: low, intermediate and high (Gnepp et al. 2005). However, the reliability of this classification system may be compromised as a result of particular limitations, such as the sample size and cellularity of the tumour biopsy, and to subjective tasks, including determinations of the areas of highest mitotic activity and distinguishing of mitotic figures from similar chromatin changes. Studies on prognostic markers are still on progress.
Since Otto Waburg's studies, it has been recognized that cancer cells have a fundamental property of metabolic switching from oxidative phosphorylation to glycolysis as the predominant energy production pathway (Airley & Mobasheri 2007; Gillies & Gatenby 2007; Moreno-Sánchez et al. 2007; Denko 2008; Gatenby & Gillies 2008). This switching has been interpreted as an adaptation to intermittent hypoxia which occurs as a tumour outgrows its blood supply, aiming the balance of oxygen demand with its limited distribution (Airley & Mobasheri 2007; Gillies & Gatenby 2007; Moreno-Sánchez et al. 2007; Denko 2008; Gatenby & Gillies 2008). In fact, reaction-diffusion models have predicted that, among all substrates, oxygen is most limited as a result of its low solubility (Gillies & Gatenby 2007). Interestingly, the glycolytic phenotype persists even under normoxic conditions, characterizing aerobic glycolysis, i.e., the Warburg effect (Airley & Mobasheri 2007; Gillies & Gatenby 2007; Moreno-Sánchez et al. 2007; Denko 2008; Gatenby & Gillies 2008).
The metabolic switch is governed by hypoxia inducible factor (HIF)-1, which coordinately stimulates glycolysis and reduces mitochondrial function and biogenesis of these organelles (Denko 2008; Ortega et al. 2009). In fact, mitochondrial impairment, also through HIF-1- unrelated mechanisms, have been associated with enhanced glycolysis and cancer development, with a (still controversial) causal role first proposed by Warburg himself (Warburg 1956; Ristow 2006; Moreno-Sánchez et al. 2007; Denko 2008; Ortega et al. 2009). Besides energy production under low oxygen concentration, it has been suggested broadly that this switch affords the cancer cells additional growth advantages. For example acidification of the extracellular space favouring invasion; increased production of anabolic substrates and reducing equivalents (NADPH) by the pentose occurs phosphate pathway; and there is diminished generation of reactive oxygen species (ROS) by the mitochondria leading to apoptosis resistance (Gatenby & Gillies 2008). Therefore, glycolytic activity has been correlated with the degree of tumour malignancy, so that glycolysis is increased and oxidative phosphorylation is decreased in highly de-differentiated and fast-growing tumours when compared with slow-growing ones and normal cells (reviewed by Moreno-Sánchez et al. 2007).
The apparent paradox of cancer cells reliance on a far less efficient energy production process still remains. Oxidative phosphorylation generates almost 20-fold the ATP yield of glycolysis per mole of glucose. To compensate this, cancer cells take up much more glucose than the normal ones, which can be observed clinically through tumour imaging with fluorodeoxyglucose positron emission tomography (FDG-PET) and molecularly by the expression levels of glucose transporters, particularly Glut-1 (Airley & Mobasheri 2007; Gillies & Gatenby 2007; Moreno-Sánchez et al. 2007; Denko 2008; Gatenby & Gillies 2008). Overexpression of Glut-1 has been associated consistently with increased tumour aggressiveness and poor patient survival in most frequent human types of carcinomas (Airley & Mobasheri 2007; Busk et al. 2008; Ortega et al. 2009).
Searching for prognostic markers for salivary gland MEC, we studied Glut-1 expression as well as the level of mitochondrial antigen in samples of MEC presenting different histological grades. We also assessed peroxiredoxin I (Prx I) expression as it can be associated with increased mitochondrial function in view of its ability to decompose hydrogen peroxide (Wood et al. 2003; Kang et al. 2005; Fourquet et al. 2008), a secondary product proportionally increased with higher oxidative phosphorylation activity (Wallace 2005).
Materials and methods
Tissue samples
This study was approved by the Committee of Ethics of the University of Campinas, Brazil. It was performed in 26 human salivary MEC samples retrieved from the archives of the pathology department at the University of Campinas. Tissue samples were available as formalin-fixed and paraffin-embedded material. Haematoxylin and eosin stained sections were examined and tumours were scored and graded by three experienced pathologists according to the World Health Organization's grading system which is based on the relative proportion of the cystic component in the entire tumour, the number of mitotic figures per 10 high-power fields, degree of anaplasia, necrosis incidence and neural involvement.
Immunohistochemistry
Sections (3 μm) from the paraffin blocks were deparaffinized in xylene, rehydrated through descending ethanol series and were submitted to heat-induced antigen retrieval in water bath with citrate pH 6.0 buffer solution for 30 min. After that, sections were immersed in 0.3% hydrogen peroxide in methanol and incubated with primary antibody. The antibodies used were specific for human: Prx I, polyclonal (1:500) (Alexis Corp., Lausen, Switzerland), mitochondria antigen, monoclonal (MTCO2) (1:200) and Glut-1, polyclonal (1:400) (Abcam, Cambridge, MA, USA). Peroxidase-linked secondary antibody and diaminobenzidine tetrahydrochloride (DAB) (Peroxidase Envision kit; Dako Corp., Carpinteria, CA, USA) were used to detect specific binding. The sections were counterstained with haematoxylin, dehydrated and mounted. Digital photomicrography used a Zeiss Axioskop 2 plus microscope equipped with AxioCam digital camera and Axiovision application software (Carl Zeiss, Gottingen, Germany).
For quantitative evaluation, scores for the expression of each protein were assigned according to the percentage of stained tumour cells from 0 to 3 (0, no staining; 1, staining of up to 25% of tumour cells; 2, staining of 25–50% of cells; 3, staining of more than 50% of cells).
Statistical analyses of the correlation between the immunohistochemical findings for each protein (Glut-1, mitochondria antigen and Prx1) and tumour grading were performed using Fisher's Exact Test. Although we have previously reported Prx I expression in MEC cases (Costa et al. 2008), in this study we have evaluated its potential correlation with the grade of malignancy of this tumour.
Results
The expression levels of Prx I, mitochondria antigen and Glut-1 were evaluated using immunohistochemical analysis in samples of MEC presenting different histological grades.
The clinicopathological features of the 26 patients as well as the histological grade of the tumours are listed in Table 1. Staining indexes of Prx I, mitochondria antigen and Glut-1 are also summarized in Table 1.
Table 1.
Clinicopathological and immunophenotypic features of Mucoepidermoid carcinoma (MEC) cases
| Patient no | Age (year) | Sex | Location | Histological grade | Prx I | Mito antigen | Glut-1 |
|---|---|---|---|---|---|---|---|
| 1 | 49 | M | Parotid | Low | 3 | 3 | 0 |
| 2 | NA | NA | NA | Low | 2 | 2 | 0 |
| 3 | 25 | M | Parotid | Low | 2 | 2 | 0 |
| 4 | 55 | F | Palate | Low | 3 | 3 | 0 |
| 5 | NA | NA | Parotid | Low | 3 | 3 | 1 |
| 6 | 13 | M | Parotid | Low | 2 | 2 | 0 |
| 7 | NA | NA | NA | Low | 3 | NA | NA |
| 8 | 43 | F | Palate | Low | 1 | 1 | 0 |
| 9 | 23 | F | Palate | Low | 2 | 2 | 0 |
| 10 | 23 | F | Parotid | Low | 3 | 3 | 0 |
| 11 | 13 | M | Parotid | Low | 3 | 3 | 0 |
| 12 | NA | NA | NA | Low | 3 | 3 | 1 |
| 13 | 53 | M | NA | Low | 0 | 0 | 0 |
| 14 | 57 | M | Retromolar region | Low | 2 | 2 | 2 |
| 15 | 65 | F | Buccal mucosa | Low | 0 | 0 | 0 |
| 16 | 25 | M | Parotid | Intermediate | 3 | 2 | 2 |
| 17 | 45 | F | Buccal mucosa | Intermediate | 3 | 3 | 1 |
| 18 | 27 | F | Palate | Intermediate | 3 | 3 | 1 |
| 19 | 33 | M | Parotid | Intermediate | 3 | 3 | 0 |
| 20 | NA | NA | NA | Intermediate | 3 | NA | NA |
| 21 | 62 | F | Submandibular | Intermediate | 2 | 2 | 1 |
| 22 | 57 | M | Palate | High | 2 | 2 | 1 |
| 23 | 65 | F | Submandibular | High | 3 | 2 | 2 |
| 24 | 71 | M | Parotid | High | 1 | 2 | 3 |
| 25 | 51 | M | Parotid | High | 0 | 0 | 3 |
| 26 | NA | NA | NA | High | 3 | 3 | 2 |
Glut-1, Glucose transporter protein 1; NA, not available.
Staining scores: 0, no staining; 1, staining of up to 25% of tumor cells; 2, staining of 25–50% of cells; 3, staining of more than 50% of cells.
Malignant cells were intensely and commonly positive for Prx I and mitochondrial antigen (Figure 1), and they were expressed in the majority of the MEC cases independently of the histological grade, (Figure 1), (Table 1). Therefore, neither expression of Prx I nor that of mitochondrial antigen correlated with MEC histological grade (P = 0.6798 and P = 0.9271, respectively, Fisher's exact test).
Figure 1.
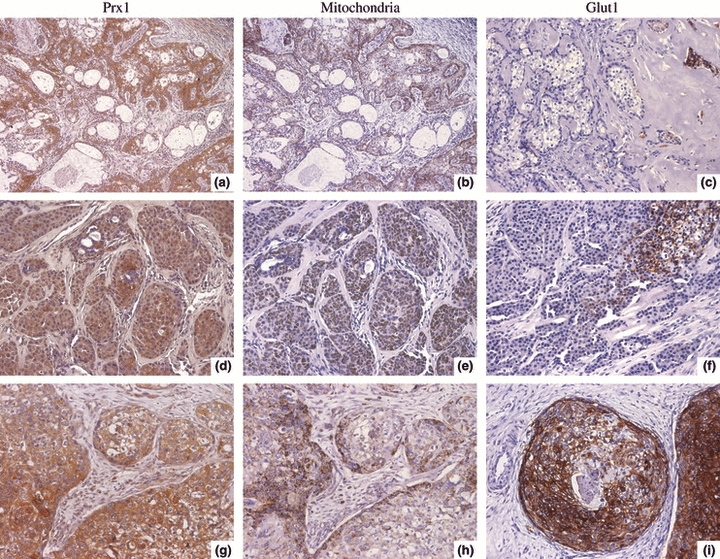
Immunohistochemical expression of Prx I, mitochondrial antigen and glucose transporter protein 1 (Glut-1) in Mucoepidermoid carcinoma (MEC) presenting different histological grades. (a–c) Low-grade MEC. (a, b) Positive Prx I and mitochondrial antigen staining. (c) No reactivity to Glut-1, except in the scanty tumour cells scattered within hyalinized area (arrow). (d–f) Intermediate grade MEC. (d, e) Malignant cell blocks exhibit reactivity to Prx I and mitochondria antigen. (f) Membranous Glut-1 expression in a focal area. (g–i) High-grade MEC. (g, h) Positive Prx I and mitochondrial antigen staining. (i) Malignant cell blocks frequently exhibit intense membranous Glut-1 reactivity. (Original magnifications ×400, except a, b ×200).
By contrast, reactivity for Glut-1, frequently localized in the cell membrane, was observed in a grade-dependent fashion: it was mostly not detected in tumour cells of low-grade MEC samples, but higher grade tumours significantly expressed rising levels of membranous Glut-1 (P = 0.0023, Fisher's exact test) (Figure 1), (Table 1). The pattern of Glut-1 staining was heterogeneous, always restricted to focal areas of the whole tumour, differently from those of Prx I and mitochondrial antigen, frequently with homogenous distribution across the tumour sections (Figure 1). Interestingly, in the low-grade MEC samples where Glut-1 expression was detected (three from 15, Table 1), it occurred in the scanty tumour cells scattered within hyalinized areas (Figure 1c). Intravascular erythrocytes, were used as positive controls for Glut-1. For Prx I and mitochondrial antigen, the positive controls were the duct cells of normal salivary gland adjacent to the tumours.
Discussion
In this study, we demonstrated a statistical relationship between Glut-1 expression and MEC grade of malignancy, which suggests that this glucose transporter protein could be considered an additional prognostic marker for this tumour.
Based on Darwinian principles, our results suggest that Glut-1 expressing cells of some intermediate and of all high-grade MEC have acquired the bioenergetic phenotype of increased glycolysis as an adaptive response to environmental selection forces, most likely to hypoxia, which conferred to them growth advantage over normal cells. In fact, it has been accepted that immunohistochemical staining for hypoxia-inducible proteins, such as Glut-1, can be used to see biological hypoxia (Gillies & Gatenby 2007). In addition, the environmental acidosis provoked by increased glycolysis, could have favoured tumour cell invasion through destruction of neighbouring cells, degradation of the extracellular matrix and promotion of angiogenesis (Gatenby & Gillies 2008). Probably, low-grade tumours have not experienced this kind of environmental pressure, at least not to the extent of the higher grade ones, so their cells could still rely predominantly on oxidative phophorylation for energy supply. This suggestion was also consistent with our previous report (Costa et al. 2008) in which microvessel density (MVD) was evaluated in the same MEC samples. In addition, MEC samples classified as high grade showed considerably less MVD compared with those of lower grades, supporting our speculation hypoxic stimulation of the glycolytic metabolism. In agreement about, imaging studies have shown that with this uncoupling of blood flow and metabolism is frequently found in large aggressive tumours, including head and neck, lung, high-grade gliomas, breast and liver, and following therapy (reviewed by Miles & Williams 2008). Despite these considerations which corroborate hypoxia-induced Glut-1 expression however, we can not exclude completely the possibility of glycolysis upregulation in the presence of oxygen, characterizing aerobic glycolysis (Warburg effect).
The constitutive upregulation of glycolysis has been associated with cycling hypoxia, a phenomenon derived from the architectural and functional abnormalities of the tumour vasculature (Gatenby & Gillies 2004). Oxic-hypoxic cycles may occur in tumours with periodicities of minutes, hours or days (Gatenby & Gillies 2004). Fluctuations of red blood cell flux are responsible for rapid cycling hypoxia – few cycles per hour, periodicities over days involve vascular remodelling or cycles of neoangiogenesis and regression through hypoxia-induced expression of vascular endothelial growth factor (VEGF) (Gatenby & Gillies 2004; Dewhirst et al. 2008). Therefore, the hypoxic environment of tumours is heterogeneous, both spatially and temporally, i.e., cells that are hypoxic at one time, can be no longer hypoxic some minutes later (Airley & Mobasheri 2007; Dewhirst et al. 2008). This could explain the focal and heterogeneous pattern of Glut-1 staining observed in our MEC samples. Probably, the unique regions where hypoxia is sustained are the paucicellular hyalinized areas, characterized by very low cellularity and prominent fibrosis, which are described to be secondary to extensive infarction. We suggest that the scanty tumour cells within these poorly vascularized areas, observed in some low-grade MEC, are obligated to rely on glycolysis, which would explain Glut-1 expression in these cells.
Based on the same concept of the reoxygenation injury following myocardial infarction or cerebral ischaemia, tumour cells are predisposed to damage as a result of cycling hypoxia (Li & Jackson 2002; Dewhirst et al. 2008). This damage is associated with cellular exposure to ROS, generated at high levels following reperfusion, which leads to an abrupt oxygen tension increase in the previously hypoxic cells (Li & Jackson 2002; Dewhirst et al. 2008). The remarkable expression of Prx I observed in MEC could provide antioxidant protection to the cells in the unstable oxygenation tumour environment. In agreement with this, it was demonstrated recently that Prx I is upregulated by in vitro simulation of hypoxia/reoxygenation (Kim et al. 2007).
H2O2 has been reported to be responsible for the stabilization of the regulating HIF1 subunit, HIF1α, under aerobic conditions (Dewhirst et al. 2008). Outstandingly, it was observed that when catalase is overexpressed, stabilization of this subunit disappears. Considering that Prx I has a higher affinity to H2O2 compared with catalase and is more abundant (Wood et al. 2003, Kang et al. 2005), we suggest that, by degrading H2O2, Prx I would impair stabilization of HIF1 α and the subsequent formation of the heterodimer with HIF1β, thereby diminishing the transcriptional activation of HIF1-target genes. However, this transcription factor is also influenced by hypoxia and nitrosative stress besides H2O2 (Dewhirst et al. 2008). Given that the amplitude of HIF1 activity is an outcome of the balance among all these environmental forces acting simultaneously, we propose that high-grade MEC, where almost certainly hypoxia prevailed, were more likely to show HIF1 activation, as evidenced by Glut-1 expression.
Our results showed that mitochondrial antigen expression, which we used as an indicator of oxidative metabolism, although observed in the majority of the MEC samples, was not correlated, either positively or negatively, to MEC grade. By contrast, Glut-1 expression increased as the tumours became more aggressive. It has long been shown that the support of glycolysis to the cell's ATP may vary from 10% in normal tissues to over 50%, depending on the tumour, with the remainder being generated by mitochondrial oxidative phosphorylation (Warburg 1956; Moreno-Sánchez et al. 2007). Using immunohistochemistry, we were not able to establish such a functional proportion between these bioenergetic pathways. Furthermore, this technique did not allow evaluation of the mitochondrial performance. Despite this, our results indicated that (i) oxidative phosphorylation may contribute to ATP supply in all stages of MEC progression, (ii) low-grade MECs possibly meet their energy demands predominantly via oxidative phosphorylation and (iii) the relative contribution of glycolysis over mitochondria for cellular ATP supply increases during MEC progression.
As expected, Prx I expression followed the pattern of mitochondrial antigen, consistent with its induction by H2O2 derived from the respiratory chain activity (Demasi et al. 2001). As H2O2 may be involved in carcinogenesis, by causing DNA damage, and also through activation of proliferation and hypoxia signalling pathways, Prx I could initially be considered a tumour suppressor, avoiding mutation and modulating the signalling pathways which are commonly deregulated in malignancies (Immenschuh & Baumgart-Vogt 2005; Kang et al. 2005; Rhee et al. 2005; Neumann & Fang 2007). However, once the malignant phenotype has been established, Prx I could exert tumour supportive functions, based on the protection of malignant cells against oxidative stress-induced apoptosis, enhancing cellular resistance to ionizing radiation and to chemotherapeutic agents (Chen et al. 2002; Kang et al. 2005; Zhang et al. 2005; Neumann & Fang 2007; Kim et al. 2008). At the end, Prx I functional versatility may account for its lack of significance as a prognostic marker of MEC.
Although locally the correction may be weak, it has been acknowledged that globally, there is a strong correlation between glucose metabolism (FDG uptake) and transport capacity (Glut-1 expression) (Busk et al. 2008). Our results suggest potentially that Glut-1 overexpression could be potentially useful to predict poor prognosis in patients with MEC; however, further studies are necessary to prove that it is a prognosticator independent of grade. If so, FDG-PET could be helpful as a non-invasive indicator of MEC progression and response to treatment.
References
- Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- Bell D, Luna MA, Weber RS, Kaye FJ, El-Naggar AK. CRTC1/MAML2 fusion transcript in Warthin's tumor and mucoepidermoid carcinoma: evidence for a common genetic association. Genes Chromosomes Cancer. 2008;47:309–314. doi: 10.1002/gcc.20534. [DOI] [PubMed] [Google Scholar]
- Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am. J. Surg. Pathol. 2001;25:835–845. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- Busk M, Horsman MR, Kristjansen PE, van der Kogel AJ, Bussink J, Overgaard J. Aerobic glycolysis in cancers: implications for the usability of oxygen-responsive genes and fluorodeoxyglucose-PET as markers of tissue hypoxia. Int. J. Cancer. 2008;122:2726–2734. doi: 10.1002/ijc.23449. [DOI] [PubMed] [Google Scholar]
- Chen WC, McBride WH, Iwamoto KS, et al. Induction of radioprotective peroxiredoxin-I by ionizing irradiation. J. Neurosci. Res. 2002;70:794–798. doi: 10.1002/jnr.10435. [DOI] [PubMed] [Google Scholar]
- Costa AF, Demasi AP, Bonfitto VL, et al. Angiogenesis in salivary carcinomas with and without myoepithelial differentiation. Virchows Arch. 2008;453:359–367. doi: 10.1007/s00428-008-0664-z. [DOI] [PubMed] [Google Scholar]
- Demasi AP, Pereira GA, Netto LE. Cytosolic thioredoxin peroxidase I is essential for the antioxidant defense of yeast with dysfunctional mitochondria. FEBS Lett. 2001;509:430–434. doi: 10.1016/s0014-5793(01)03215-x. [DOI] [PubMed] [Google Scholar]
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourquet S, Huang ME, D’Autreaux B, Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid. Redox Signal. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J. Bioenerg. Biomembr. 2007;39:251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- Gnepp DR, Brandwein-Gensler MS, El-Naggar AK, Nagao T. Mucoepidermoid carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology & Genetics. Head and Neck Tumous. 1st edn. Lyon: IARC Press; 2005. pp. 219–220. [Google Scholar]
- Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid. Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim TJ, Lee KY. A novel function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett. 2008;582:1913–1918. doi: 10.1016/j.febslet.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Miles KA, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging. 2008;8:81–86. doi: 10.1102/1470-7330.2008.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- Neumann CA, Fang Q. Are peroxiredoxins tumor suppressors? Curr. Opin. Pharmacol. 2007;7:375–380. doi: 10.1016/j.coph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ortega AD, Sánchez-Aragó M, Giner-Sánchez D, Sánchez-Cenizo L, Willers I, Cuezva JM. Glucose avidity of carcinomas. Cancer Lett. 2009;276:125–135. doi: 10.1016/j.canlet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Pires FR, de Almeida OP, de Araújo VC, Kowalski LP. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch. Otolaryngol. Head Neck Surg. 2004;130:174–180. doi: 10.1001/archotol.130.2.174. [DOI] [PubMed] [Google Scholar]
- Rapidis AD, Givalos N, Gakiopoulou H, et al. Mucoepidermoid carcinoma of the salivary glands. Review of the literature and clinicopathological analysis of 18 patients. Oral Oncol. 2007;43:130–136. doi: 10.1016/j.oraloncology.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ristow M. Oxidative metabolism in cancer growth. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- Vargas PA, Cheng Y, Barrett AW, Craig GT, Speight PM. Expression of Mcm-2, Ki-67 and geminin in benign and malignant salivary gland tumours. J. Oral Pathol. Med. 2008;37:309–318. doi: 10.1111/j.1600-0714.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- Wood ZA, Schröder E, Robin HJ, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Zhang B, Su Y, Ai G, Wang Y, Wang T, Wang F. Involvement of peroxiredoxin I in protecting cells from radiation-induced death. J. Radiat. Res. (Tokyo) 2005;46:305–312. doi: 10.1269/jrr.46.305. [DOI] [PubMed] [Google Scholar]


