Abstract
Although it is well known that cadmium (Cd) causes adverse effects on male rat reproductive organs, few studies have quantified alterations caused by its low doses. Quantification of these alterations, especially in the testis, was measured using morphometry. A single dose of cadmium chloride (1 or 1.2 mg/kg BW) was injected i.p. in adult rats, killed after 7 or 56 days. The lower dose caused slight alterations as measured by morphometrical analysis. The higher dose caused significant reduction in testis and epididymis weight, gonadossomatic index and length of seminiferous tubule (ST) after 7 and 56 days. Cadmium significantly reduced the ST diameter after 56 days. Decreased volume density of ST, after 7 and 56 days, was accompanied by an increase in interstitium volume density. The damage caused by the dose of 1.2 mg/kg can be clearly observed with light microscope. After 7 days, the tubule lumens were filled with degenerated germ cells and multinucleated spermatid aggregates. Vacuolization of the seminiferous epithelium was also observed. After 56 days, increased damage resulted in vacuolated ST, consisting only of Sertoli cells. Scanning electron microscopy examination of the testis showed that, in the group cadmium treated (1.2 mg/kg) and killed after 56 days, the interstitial tissue presents a compact and fibrous appearance with absence of fenestrae. The seminiferous epithelium height diminished and the absence of spermatozoa can be noted. The results show that a very small difference of Cd dose causes a sudden increase in testicular damage, apparently overpowering this tissue's natural defences.
Keywords: cadmium, light microscopy, morphometry, reproductive organs, scanning electron microscopy, testis
Introduction
Cadmium (Cd) is a heavy metal and a major environmental toxicant. The general population is exposed to Cd via contaminants found in drinking water and food, whereas occupational exposition to Cd usually takes place during mining and manufacturing of batteries and pigments that contain Cd. Industrial activities, such as smelting and refining of metals, and municipal waste incineration also release Cd into the atmosphere (Siu et al. 2009). Tobacco smoke is another important source of Cd exposure (Blanco et al. 2007). Cd toxicity is associated with severe damage in various organs, particularly the testes, in both humans and animals (Fouad et al. 2009). Rodent testes are especially sensitive to the toxic effects of Cd exposure. Cd impairs reproductive capacity by causing severe testicular degeneration, seminiferous tubule damage and necrosis in rats (Burukoğlu & Bayçu 2008). The adverse consequences of exposure to this heavy metal on the reproductive organs have been widely considered. After acute exposure, cadmium-induced damage can be found at interstitial and tubular levels. Permeability changes in the capillary endothelium (which cause oedema, haemorrhage or necrosis) seem to be implicated in the histopathological mechanism of these lesions (Blanco et al. 2007). The severity of intoxication depends on the route, dose and duration of exposure to the metal (El-Missiry & Shalaby 2000). An important factor involved in cadmium toxicity is metallothionein (MT). MT belongs to the family of cysteine-rich metal-binding proteins, which helps in Cd detoxification. In a previous study, Cd is shown as an effective inducer of MT synthesis, which in turn protects host tissue from Cd damage. However, it is clear that MT synthesis is induced with more difficulty in the testis than in the liver, for example, which may account for the higher susceptibility of the testis to Cd toxicity (Xu et al. 2005).
Although it is well known that Cd is associated with adverse effects on male reproductive organs, culminating in diminishing reproductive capacities, very few studies have quantified the morphological alterations caused by low doses of Cd exposure. Low doses were chosen for this research because preliminary testing using 1.4 mg/kg of CdCl2 (results not shown) resulted in extensive testicular damage, making morphometrical studies impossible. The current study was undertaken to evaluate histomorphometrically the threshold modifications of seminiferous tubules caused by single acute low doses of Cd.
Materials and methods
Animals
Male Wistar rats were obtained from the Animal Multi-Research Center for Biological Investigation (State University of Campinas, Campinas, SP, Brazil). The animals were housed three per cage, with a 12 h light–dark cycle. They were supplied with standard laboratory chow and water, ad libitum. The experimental protocol followed the Guide for Care and Use of Laboratory Animals and was approved by the Committee for Ethics in Animal Experimentation of UNICAMP.
Experimental design
Wistar rats, randomly assigned to three groups, were injected with saline or cadmium chloride (CdCl2) solution, in which the first group (n = 12) received a single i.p. injection of saline (vehicle of cadmium) and served as the control group. The animals of the second (n = 12) group received a single dose of 1 mg/kg of CdCl2 per body weight (BW), whereas the third group (n = 12) received a single dose of 1.2 mg/kg BW of CdCl2. Six animals of each group were killed after either 7 or 56 days. As cadmium is considered to be genotoxic (Giaginis et al. 2006), a 56-day interval was chosen considering the period necessary to complete a spermatogenic cycle (Russell et al. 1990). The 7-day interval was chosen to observe short-term modifications before a possible recovery process could have altered the degree of damage caused by the cadmium.
Tissue preparation
The animals under Ketamine (80 mg/BW) and Xylazine (5 mg/BW) anaesthesia were fixed by whole body perfusion. Briefly, after a saline wash to clear the vascular bed of the testis, they were perfused with glutaraldehyde 2.5% and paraformaldehyde 4% in sodium phosphate buffer 0.1 M (pH 7.2) for 25–30 min. Testis, epididymis, prostate, seminal vesicles and coagulating gland were removed, post fixed in the same solution overnight and then weighed. Historesin®-embedded testis fragments were sectioned at 3-μm thickness and stained with toluidine blue/1% sodium borate.
Biometry and morphometry
The weight of testicular parenchyma was obtained subtracting the mass occupied by the albuginea from the total testis weight, thus providing the net weight of the organ's functional portion. The gonadosomatic index (GSI) was expressed as percentage of the total body weight in relation to the testis weight, GSI = (testes weight/total body weight) × 100. The area of testicular tissue components was determined measuring the area occupied by seminiferous tubules and interstitium in fifteen fields per animal, using Image Pro Plus software associated to an Olympus BX-40 microscope at 400× magnification. The total area was used to obtain the volume density (proportion) of each component. For morphometric calculations, the specific gravity of testis tissue was considered to be 1.0 (Mori & Christensen 1980). The tubular diameter of seminiferous tubules was measured at 100× magnification with the Image Pro Plus program associated to an Olympus BX-40 microscope. Thirty tubular profiles that were round or nearly round were chosen randomly and measured for each animal. The total length of the seminiferous tubule, expressed in metres, was obtained by dividing seminiferous tubule volume by the squared radius of the tubule multiplied by the pi value. These values were expressed per testis and per gram of testis (Russell et al. 1990).
Scanning electron microscopy (SEM)
After whole body perfusion fixation, the specimen fragments were fixed in Karnovsky′s fixative and rinsed three times with 0.1 M sodium phosphate buffer, pH 7.2, then in increasing saccharose solutions (0.5, 1.5, 3%). The tissue was frozen in liquid nitrogen, fractured, post fixed in 1% osmium tetroxide, rinsed and dehydrated in an ascending ethanol series prior to critical point drying. The specimens were mounted on stubs, sputter-coated with gold and examined under the scanning electron microscope (Geol–JMS 560).
Statistical analysis
Comparison of the values of control and treated groups was carried out by the variance analysis, Statistical (anova), followed by Tukey's test. A value of P < 0.05 was considered statistically significant. For all values, the means ± standard error mean (SEM) was calculated.
Results
Biometry and morphometry
The biometric and morphometric data are presented in Tables 1 and 2. No significant difference was found between the body and liver weight of control and cadmium-treated rats in all of the groups studied. After 7 days, the higher dose of Cd caused a significant reduction in kidney weight. However, no alteration was observed for other treatments.
Table 1.
Body and organ weights (g) of adult rats treated with two low doses of cadmium (mean ± SEM)
| Control | Cd 1 mg/kg | Cd 1.2 mg/kg | ||||
|---|---|---|---|---|---|---|
| Parameters | 7 days | 56 days | 7 days | 56 days | 7 days | 56 days |
| Body | 387.83 ± 14.83 | 435.83 ± 15.93 | 383.17 ± 10.08 | 436.33 ± 10.09 | 366.83 ± 11.26 | 448.83 ± 21.67 |
| Kidney | 1.58 ± 0.07 | 1.64 ± 0.05 | 1.44 ± 0.05 | 1.67 ± 0.06 | 1.31 ± 0.05* | 1.62 ± 0.06 |
| Liver | 14.58 ± 0.3 | 14.49 ± 1.04 | 13.66 ± 0.56 | 14,84 ± 1.16 | 13.83 ± 0.74 | 16.80 ± 1.05 |
| Epididymis | 0.49 ± 0.02 | 0.51 ± 0.02 | 0.45 ± 0.02 | 0.53 ± 0.01 | 0.40 ± 0.01* | 0.35 ± 0.04* |
| Seminal vesicle | 0.97 ± 0.05 | 1.04 ± 0.06 | 0.96 ± 0.04 | 1.23 ± 0.08 | 0.63 ± 0.09* | 1.18 ± 0.08 |
| Coagulating gland | 0.20 ± 0.02 | 0.22 ± 0.01 | 0.19 ± 0.05 | 0.25 ± 0.02 | 0.14 ± 0.02 | 0.22 ± 0.02 |
| Ventral prostate | 0.35 ± 0.02 | 0.48 ± 0.03 | 0.33 ± 0.01 | 0.57 ± 0.03 | 0.29 ± 0.03 | 0.43 ± 0.02 |
Statistically significant differences by Tukey's test (P < 0.05). n = 6 animals.
Table 2.
Testis weight and testicular morphometric parameters of adult rats treated with two low doses of cadmium (mean ± SDM)
| Control | Cd 1 mg/kg | Cd 1.2 mg/kg | ||||
|---|---|---|---|---|---|---|
| Parameters | 7 days | 56 days | 7 days | 56 days | 7 days | 56 days |
| Testis (g) | 1.68 ± 0.04 | 1.60 ± 0.10 | 1.59 ± 0.07 | 1.69 ± 0.07 | 0.86 ± 0.08* | 0.91 ± 0.16* |
| GSI | 0.88 ± 0.04 | 0.73 ± 0.03 | 0.83 ± 0.04 | 0.78 ± 0.02 | 0.48 ± 0.05* | 0.40 ± 0.06* |
| Tubular diameter (μm) | 292.10 ± 6.30 | 298.22 ± 6.87 | 285.71 ± 2.83 | 295.90 ± 4.01 | 262.93 ± 13.24 | 250.92 ± 16.88* |
| TL/testis (m) | 20.70 ± 0.70 | 19.33 ± 1.95 | 19.96 ± 1.11 | 20.70 ± 0.87 | 10.94 ± 1.86* | 10.56 ± 0.91* |
| TL/g of testis (m) | 12.31 ± 0.51 | 11.94 ± 0.58 | 12.56 ± 0.25 | 12.23 ± 0.34 | 12.40 ± 1.06 | 13.52 ± 1.66 |
| Seminiferous tubule (%) | 85.39 ± 0.73 | 85.97 ± 0.55 | 84.15 ± 0.80 | 87.53 ± 0.36* | 71.98 ± 1.24* | 65.19 ± 1.61* |
| Interstitial tissue (%) | 14.61 ± 0.73 | 14.03 ± 0.55 | 15.85 ± 0.80 | 12.47 ± 0.36* | 28.02 ± 1.24* | 34.81 ± 1.61* |
GSI, gonadossomatic index; TL, tubular length.
Statistically significant differences by Tukey's test (P < 0.05). n = 6 animals.
At the Cd dose of 1.0 mg/kg, no alteration was observed in testicular and epididymal weights and in GSI; however, at the 1.2 mg/kg dose, significant reduction occurred after 7 and 56 days. There was only a significant decrease in the higher dose group in the seminal vesicle, after 7 days. However, the ventral prostate, seminal vesicle and coagulating gland weights were not altered in all the groups studied.
Cd significantly reduced the seminiferous tubule (ST) diameter in the higher dose after 56 days. However, in the other groups no alteration was detected. The low dose of Cd caused no alteration in the total length of ST per testis and per gram of testis in relation to the control group. At the higher dose, the total length of ST per testis diminished significantly after 7 and 56 days, although the value per gram of testis was not altered.
The volume density of ST in the group submitted to the lower Cd dose increased, whereas the volume density of the interstitium decreased, both significantly, after 56 days. However, in the animals submitted to the higher dose of Cd (7 days), the volume density of ST decreased and the volume density of interstitium increased significantly, and after 56 days this alteration was more prominent.
Light and scanning electron microscopy
Observations with light microscopy (Figure 1) showed no marked changes in testicular tissue of animals submitted to the dose of 1 mg/kg in relation to controls, as demonstrated by morphometry. Thus, normal spermatogenesis was observed with the usual interaction of germ cells and Sertoli cells. Moreover, in the interstitium and Leydig cells no alterations were observed. However, at the dose of 1.2 mg/kg, progressive damage was observed after 7 and 56 days. After 7 days, the tubule lumens were filled with degenerated germ cells and multinucleated spermatid aggregates with apoptotic nuclei. Vacuolization of the seminiferous epithelium was also observed. After 56 days, increased damage resulted in vacuolated seminiferous tubules, consisting only of Sertoli cells.
Figure 1.
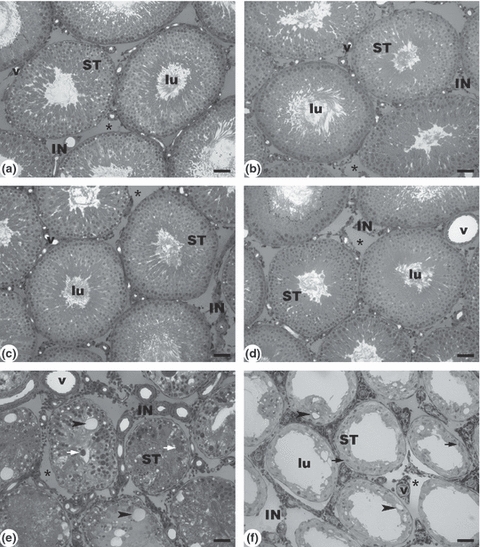
Light microscopy of a control rat testis and under the effect of cadmium. The seminiferous tubules (ST) are well preserved in the controls (a: 7 days and b: 56 days) and in rats treated with cadmium 1 mg/kg BW (c: 7 days and d: 56 days). Rats treated with cadmium 1.2 mg/kg BW (e) exhibit degenerated seminiferous tubules with multinucleated spermatid aggregates with apoptotic nuclei, after 7 days. Note the reduced tubular diameter, absence of lumen (lu) and the thick, fibrous interstitium (IN). After 56 days (f), the progressive damage resulted in seminiferous tubules lined only by Sertoli cells. Multinucleated spermatid aggregates with apoptotic nuclei and Sertoli cells are indicated by white and black arrows respectively. Blood vessels (v). Fluid space (Star). Vacuolization (Arrow head). Scale bars: 50 μm.
The SEM examination of this tissue (Figure 2) showed the interstitial tissue of control animals as having an intricate three-dimensional network. The interstitial tissue forms a delicate lattice with large fenestrae and well spaced cells, surrounding each seminiferous tubule. The seminiferous epithelium was intact and the seminiferous tubules showed the lumen filled with sperm flagella. A distinct space for fluid was observed between the interstitial tissue and the seminiferous tubules. In the group treated with 1.2 mg/kg cadmium and killed after 56 days, the interstitial tissue presents a compact, fibrous appearance with absence of fenestrae, but with a larger number of cells. In the seminiferous tubule, the epithelium height diminished and the absence of spermatozoa in the lumen can be noted.
Figure 2.
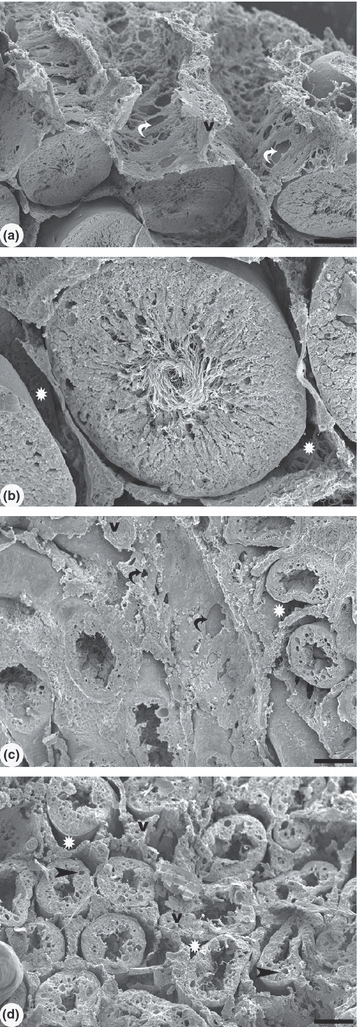
Scanning electron microscopy of control rat testis and under the effect of cadmium after 56 days. (a) Testicular parenchyma shows a delicate lattice with large fenestrae of interstitial tissue. The seminiferous tubules are well preserved in the control group. A large space for fluid was observed between the interstitial tissue and the seminiferous tubules. (b) Detail of the seminiferous tubule with intact epithelium and the presence of spermatozoa in the lumen. (c) In cadmium (1.2 mg/kg BW) treated group, the interstitium shows a dense fibrous aspect with few fenestrae and reduced fluid space (white star). (d) The reduced epithelium height and vacuolization is observed in the seminiferous tubules. Note the absence of spermatozoa in the lumen. The fluid space was diminished or not clearly observed. Blood vessels (v) have thicker walls. White and black curved arrows: fenestrae. Scale bars: a, c, d: 100 μm; b: 10 μm.
Discussion
The present results showed that the toxic effects of Cd in the male reproductive system were dose-dependent. The difference between the lower and higher doses is very small, but the morphological and morphometrical results are clearly very different. This suggests that the defences of the testis against Cd contamination are efficient up to a very precise level, possibly depending on the level of MT present. It is widely accepted that the Cd bound to MT is non-toxic, thus MT protects tissue against Cd toxicity in this way. The balance between Cd-MT and free Cd in the tissue has been shown to be crucially important for toxicity (Xu et al. 2005). Moreover, Siu et al. (2009) and Xu et al. (2005) affirm that Cd treatment induces metallothionein production, but this appears to be limited to a certain level in the testis. Toxic effects probably result as the amount of MT becomes insufficient to bind with the Cd present, resulting in oxidative stress and disruption of spermatogenesis (Xu et al. 2005). The level of defence appears to vary for each animal, as the effects were not uniform for the two groups.
The previous studies (Gupta et al. 2003; El-Demerdash et al. 2004) have reported weight reductions of accessory sex organs after Cd administration. In this study, after the higher dose of cadmium, the seminal vesicle reduced its weight, when measured on the seventh day, but after 56 days the usual weight was restored. Probably, the natural defences of this organ required more than 7 days to recover its usual weight after Cd toxicity, as was observed after the longer period. However, prostate and coagulating gland showed unaltered weight after either dose.
Studies of the consequences of Cd contamination have demonstrated that the testis is more sensitive to Cd than other important organs. In addition, low doses (with no detectable effects on general health) can interfere with testis function (Blanco et al. 2007). These observations agree with our results showing that the weight and morphology of the testis were clearly affected only by the higher dose of Cd, whereas the physical appearance and body weights of the animals remained unaltered. Despite the reduction of kidney weight after 7 days, this toxic effect was not observed after 56 days, so it was a temporary change overcome by the natural defences of the animal.
The previous studies of acute Cd exposure have reported diminished testicular weight in relation to the Cd dosage. These studies attributed this effect to the necrotic and degenerative cadmium-induced changes (Blanco et al. 2007). With histological analysis, the absence of tubular lumen, germ cell loss and presence of multinuclear giant cells were observed. These findings for the higher dose of Cd were in accordance with the previous studies (Biswas et al. 2001; Yang et al. 2006; Blanco et al. 2007). When massive cellular loss from seminiferous epithelium occurs, a sharp decline in testicular morphometric parameters can be verified (França & Russell 1998). Indeed, this decline has also been observed in the current work. A positive relationship usually exists between the tubular diameter and the spermatogenic activity of the testis (Sinha-Hikim et al. 1989; França & Russell 1998). Our data showed a marked reduction of seminiferous tubular diameter after the higher dose of Cd, along with the conspicuous decrease of the tubular volume density, which means that Cd caused a significant reduction in the relative seminiferous tubule length. Taking into account the fact that the weight of the testis was also reduced, it can be deduced that the total length of seminiferous tubule clearly diminished as a consequence of cadmium exposure. Supporting these results, França and Russell (1998) state that the total seminiferous tubule length is related to three structural parameters: testis size, tubular diameter and seminiferous tubule volume density. An increase in testicular intertubular compartment volume density occurred after Cd treatment; probably because of lymphatic and/or interstitial oedema. According to Lirdi et al. (2008), this oedema is a direct consequence of disruption of the endothelial layer, allowing fluids from the blood to flow into the interstitium.
This study demonstrates the importance of morphometrical methods, as very sensitive instruments to evaluate threshold changes, which could not be confirmed merely by the observation of testis morphology. The sensitivity of this method permitted the detection of differences such as the fact that few of the small modifications verified for the higher dose were found for the lower dose, and that there were differences in the tissue alterations according to a shorter or longer time lapse when the effect on spermatogonia can be felt.
Clark (1976) describes the three-dimensional organization of normal Sprague–Dawley testicular interstitial tissue. All parts of the interstitium are directly continuous and all seminiferous tubules are totally surrounded by interstitial tissue. However, the interstitium and the tubules are completely separated without cellular connections. Thus, a fluid space is formed and the fenestrae in the interstitium allow the fluid around adjacent tubules to communicate freely. No attention has been paid to the configuration of the tissue as a whole in cadmium treated animals. The SEM observation in this study showed cadmium-induced alterations in the interstitial tissue architecture that were not observed with light microscopy and morphometry analysis. In this study, the interstitial organization was similar to that in the control animals, which corroborates the data of Clark (1976). After cadmium treatment, the interstitium had a thick, compact, fibrous aspect with few fenestrae. The few fenestrae could affect the fluid movement and the communication between the tubules. An increase in the cellular component, possibly fibroblasts, was observed, as well as thicker walled capillaries. The presence of vacuoles in Sertoli cells and the almost complete absence of spermatozoa demonstrate the impairment of spermatogenesis. According to Creasy (2001), vacuolization is the most common morphological response of Sertoli cells to various injuries. Subsequent to vacuolization, germ cell degeneration, disorganization, or exfoliation is generally seen, as observed in this experiment. Injury to the Sertoli cell has potentially serious consequences because of its pivotal role in supporting spermatogenesis. Although cell death rarely occurs, the metabolic and regulatory pathways of Sertoli cells are easily disturbed (Creasy 2001).
In conclusion, our findings showed the progressive morphological and morphometrical alterations caused by low doses of Cd because of their direct effect on rat testis. Our results highlight the direct relationship of dose and time lapse to morphological alterations. Moreover, a small difference in dose causes very different degrees of damage, apparently overcoming the natural defences of this tissue.
Acknowledgments
FSP is supported by a scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP: proc. 2006/06142-8) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Biswas NM, Gupta RS, Chattopadhyay A, Choudhury GR, Sarkar M. Effect of atenolol on cadmium-induced testicular toxicity in male rats. Reprod. Toxicol. 2001;15:699–704. doi: 10.1016/s0890-6238(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Blanco A, Moyano R, Vivo J, et al. Quantitative changes in the testicular structure in mice exposed to low doses of cadmium. Environ. Toxicol. Pharmacol. 2007;23:96–101. doi: 10.1016/j.etap.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Burukoğlu D, Bayçu C. Protective effects of zinc on testes of cadmium-treated rats. Bull. Environ. Contam. Toxicol. 2008;81:521–524. doi: 10.1007/s00128-007-9211-x. [DOI] [PubMed] [Google Scholar]
- Clark RV. Three-dimensional organization of testicular interstitial tissue and lymphatic space in the rat. Anat. Rec. 1976;184:203–225. doi: 10.1002/ar.1091840207. [DOI] [PubMed] [Google Scholar]
- Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol. Pathol. 2001;29:64–76. doi: 10.1080/019262301301418865. [DOI] [PubMed] [Google Scholar]
- El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem. Toxicol. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- El-Missiry MA, Shalaby F. Role of β-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. J. Biochem. Mol. Toxicol. 2000;14:238–243. doi: 10.1002/1099-0461(2000)14:5<238::AID-JBT2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Ali AA. Protective effect of hemin against cadmium-induced testicular damage in rats. Toxicology. 2009;257:153–160. doi: 10.1016/j.tox.2008.12.022. [DOI] [PubMed] [Google Scholar]
- França LR, Russell LD. The testes of domestic animals. In: Regadera J, Martinez-Garcia R, editors. Male Reproduction. A Multidisciplinary Overview. Madrid: Churchill Livingstone; 1998. pp. 197–219. [Google Scholar]
- Giaginis C, Gatzidou E, Theocharis S. DNA repair systems as targets of cadmium toxicity. Toxicol. Appl. Pharmacol. 2006;213:282–290. doi: 10.1016/j.taap.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Sharma R, Chaudhary R, Yadav RY, Khan TI. Effect of textile waste water on the spermatogenesis of male albino rats. J. Appl. Toxicol. 2003;23:171–175. doi: 10.1002/jat.862. [DOI] [PubMed] [Google Scholar]
- Lirdi LC, Stumpp T, Sasso-Cerri E, Miraglia SM. Amifostine protective effect on Cisplatin-treated rat testis. Anat. Rec. 2008;291:797–808. doi: 10.1002/ar.20693. [DOI] [PubMed] [Google Scholar]
- Mori H, Christensen AK. Morphometric analysis of Leydig cells in the normal rat testis. J. Cell. Biol. 1980;84:340–354. doi: 10.1083/jcb.84.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha-Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. [Google Scholar]
- Sinha-Hikim AP, Bartke A, Russell L. Morphometric studies on hamster testis in gonadally active and inactive states: light microscopic findings. Biol. Reprod. 1989;39:1225–1237. doi: 10.1095/biolreprod39.5.1225. [DOI] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009;238:440–449. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Sun H, Wang S, Song L, Chang H-C, Wang X. The roles of metallothionein on cadmium-induced testes damages in Sprague–Dawley rats. Environ. Toxicol. Pharmacol. 2005;20:83–87. doi: 10.1016/j.etap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Yang HS, Han DK, Kim JR, Sim JC. Effects of α-Tocopheral on cadmium-induced toxicity in rat testis and spermatogenesis. J. Korean Med. Sci. 2006;21:445–455. doi: 10.3346/jkms.2006.21.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]


