Abstract
The female organs, which are regulated by steroid hormones, are targets of studies especially those related to senescence. However, although the female prostate is an organ influenced by hormones and susceptible to lesions, there is still little information about its histopathology. Thus, given the morphophysiological similarity between the prostate in women and female gerbils, the present study aimed to identify the spontaneous histopathological changes in this rodent to provide contributions to the understanding of lesions that also affect the human female prostate. The structural, ultrastructural, immunohistochemical, morphometric-stereological and serological aspects, as well as the quantification of the incidence, multiplicity and percentage of acini affected by different lesions were analyzed. Benign prostate lesions including hyperplasia, prostatitis, microcalculi and calculi; preneoplastic lesions like dysplasias; premalignant lesions, such as high grade prostatic intraepithelial neoplasia as well as malignant ones, specifically adenocarcinoma, were identified in the adult gland, but they were intensified during senescence, which is possibly due to the imbalance among steroid hormone levels. Although clinical attention focuses on other urogenital organs, the real condition of the histopathological injuries in the human female prostate should be considered. A serious preventive work regarding the female prostate could be applied in the gynaecological context in order to monitor the gland and avoid possible disturbances to women's health and consequently provide better quality of life.
Keywords: ageing, female prostate, histology, steroids
The ageing process is accompanied by a natural decrease in endocrine activity and a concomitant physiological decline that favours the development of histopathologies in the organism. According to Chahal and Drake (2007), glands suffer the effects of ageing and since most of their functions are interrelated, a reduced function of a particular one could affect the others.
The functioning of the female genital apparatus during the climacteric period is a subject of tremendous concern because of its hormonal dependence. However, these studies focus on changes in many organs, such as the breast, endometrium, ovaries and the uterus (Labrie 2006; Yeh 2007), whereas knowledge on the prostate during that period is scarce (Zaviačič 1999; Custodio et al. 2004, 2008).
The occurrence of the female prostate has been reported in several mammals, including humans (Zaviačič 1999) and rodents (Shehata 1980; Gross and Didio 1987) while its morphophysiological (Gross and Didio 1987; Zaviačič 1999; Santos et al. 2003; Custodio et al. 2004, 2008) histochemical-enzymatic and immunohistochemical aspects (Tepper et al. 1984; Wernet et al. 1992) show a similarity to the male prostate.
Researches related to this gland in women are restricted to postmortem collection, which reduces relevant knowledge and hinders the study of spontaneous injuries. But the use of rodents as experimental models enables understanding of the biology in this gland, corroborated by quantitative and physiological studies, as well as experiments on hormonal manipulation already published (Santos et al. 2003, 2006, 2008; Custodio et al. 2004, 2008; Santos & Taboga 2006). In that line, the Mongolian gerbil (Meriones unguiculatus) has become an important biological model for studying the female prostate because of its similarity to the human gland. Thus, the present study on the gerbil female prostate fills a gap in the knowledge of the pathological processes that spontaneously develop in this gland and may provide possible contributions to the understanding of injuries that affect this gland in women.
Material and methods
Animal and sample preparations
Forty-five female gerbils (Meriones unguiculatus, Gerbilinae: Muridae) were used for this analysis, with 15 animals used for each phase of postnatal development: young (1 month), adult (4 months), and senile (18 months). The animals were maintained under conventional conditions of temperature and humidity (25 °C, 40–70% relative humidity, 12-h light/12-h dark), with free access to chow and water. After being anesthetized by CO2 inhalation, the animals were decapitated. Blood samples from some of them were collected for serological analysis. The urethra plus adjacent tissues were dissected out using an Olympus SD-ILK stereoscopic microscope (Olympus Optical Co. LTD, Japan) to remove the adipose tissue and isolate the prostatic tissue plus the associated urethral segment. The separation of these components was performed by sectioning it at the base of bladder to obtain a block containing the entire urethra and prostate gland (UPG).
Animal care was performed according to the ethical guidelines of the Commission for Ethics in Animal Experimentation (CEEA) at the University of Campinas (UNICAMP), São Paulo, Brazil (process No 1213-1).
Serological analysis
After the animal's decapitation, blood was colleted and the serum was separated by centrifugation (3000 rpm) and stored at −20 °C for subsequent hormone assay. The determination of serum T levels was performed by luminescence immunoassay (mouse antitestosterone antibodies; Johnson & Johnson, Orthoclinical Diagnostics Division, Rochester, NY, USA) in an automatic analyzer: Vitros-ECi (Johnson & Johnson, Orthoclinical Diagnostics Division) for ultrasensitive chemiluminescence detection. The intraassay and interassay variations were 4.6 and 4.3% respectively. The tests are linear from 0 to 30 ng/ml (detection level). The sensitivity was 0.1–150 ng/ml for T and 0.1–3.814 pg/ml for E and for DHEA.
Structural analysis
The UPGs were fixed by immersion in Karnovsky's solution, or in 4% paraformaldehyde, during 24 h. After fixation, the tissues were dehydrated in ethanol gradient cleared in xylene, embedded in paraffin (Histosec, Merck, Darmstadt, Germany) or glycol methacrylate resin (Historesin embedding kit, Leica, Nussloch, Germany), and cut into 3 μm sections with a automatic rotatory microtome (Leica RM2155). Histological sections were stained with haematoxylin–eosin (H&E), Gömöri's reticulin, Feulgen reaction and AgNOR method. The specimens were analyzed with a Zeiss-Jenaval (Zeiss-Jenaval, Jena, Germany) or Olympus BX60 light microscope (Olympus, Hamburg, Germany), and the images were digitalized using the Image-Pro Plus version 4.5 for Windows software.
Immunocytochemistry analysis
Sections of 4% paraformaldehyde-fixed female prostates were subjected to immunocytochemistry for detection of Proliferating Cell Nuclear Antigen (PCNA). For immunohistochemical analysis, the sections were deparaffinized, rehydrated through graded alcohol, and antigen retrieval was performed in 10 mM citrate buffer pH 6.0, at 100 °C for 15 min. The blockade of endogenous peroxidases was obtained by covering the slides with H2O2 (3% in methanol) for 5 min. After pretreatment, the sections were incubated for 2 h at 37 °C with mouse anti-mouse PCNA antibody (1:1000 Santa Cruz Biotech, Santa Cruz, CA, USA) diluted in 1% bovine serum albumin in Tris-buffered saline (TBS). After the slides were washed in TBS and incubated for 40 min at 37 °C with NovoLink Polymer (Novocastra Laboratories, New Castle, UK). After more washing in TBS, the sections were visualized by diaminobenzidine (DAB) solution and then counterstained with routine haematoxylin.
Ultrastructural analysis
The UPG fragments were fixed by immersion in 3% glutaraldehyde plus 0.25% tannic acid solution in Milloning's buffer, pH 7.3, containing 0.54% glucose for 24 h. After washing with the same buffer, they were postfixed with 1% osmium tetroxide for 2 h, washed again, dehydrated in graded acetone series, and embedded in Araldite resin. Ultrathin sections (50–75 nm) were contrasted with 2% uranyl acetate followed by 2% lead citrate in sodium hydroxide solution. The samples were evaluated with a LEO-Zeiss 906 (Zeiss, Cambridge, UK) transmission electron microscope operated in 80 kV.
Morphometric analyses
Nuclei of epithelial cells, stained by AgNOR method, with the following numbers of nucleoli: zero (not detectable), one, two, and more than two were counted in 25 random fields selected by age. The nucleolus number obtained was divided by totally nucleus analyzed in the respective fields. The absolute values found were converted into percentages.
Quantification of the prostatic disorders
To perform this analysis, histological sections stained with H&E from 10 adult and senescent animals were randomly chosen. Young animals were not subjected to such measurements since they did not exhibit any kind of lesion and were considered as control young group. The lesions were classified according to Shappell et al. (2004) in addition to the Classification of Urinary System Tumors and Male Genital Organs from the World Health Organization –WHO (2004). Thus, lesions classified as benign were prostatic hyperplasia, microcalculi, prostate calculi and prostatitis; the premalignant ones were high-grade prostatic intraepithelial neoplasia (PIN) and malignancies were adenocarcinomas. The more intense disorders of tissue architecture were identified as dysplasia. Although PIN can be classified by the WHO (2004) as low or high grade, and both have been identified in the gland, only the latter was quantified.
The incidence (injury diagnosis/sample) of lesion in the gland was obtained by identifying the different lesions in relation to the total sample number, while the multiplicity (specific number of lesions/ sample) was calculated by the frequency each lesion was identified in the histological section in relation to the total number of examined animals. The percentage of lesions per acinus was determined by the number of acini that developed lesions in relation to the total number of acini in the histological section. The acinar profile was indicated by the number of acini identified in each histological section in relation to the total number of acini in the whole group sample.
Statistical analysis
All the statistical tests were performed with Statistica 6.0 software (StarSoft, Inc., Tulsa, OK, USA). The quantitative results are expressed as mean ± standard deviation, and the analysis of variance and Tukey honest significance difference (HDS) tests were applied, with P ≤ 0.05 was considered statistically significant.
Results
Evaluation of the hormonal serum levels
The adult age group presented higher serum levels of DHEA, T and E than the other ages, and during senescence the levels of DHEA and T showed significant reduction (Table 1). The rations between the levels of T and DHEA during all the ages of postnatal development were ten times higher that the ratios levels of T and E, however both hormonal rations were highlighted in adulthood (Table 1).
Table 1.
Serological dates of the young, adult and senile female Mongolian gerbil (n = 15/age)
| Serological data | |||
|---|---|---|---|
| Ages | |||
| Hormones | Young | Adult | Senile |
| Dehydroepiandrosterone (DHEA) (ng/ml)* | 2.16 ± 0.1a | 2.66 ± 0.3a | 1.52 ± 0.1b |
| Testosterone (T) (ng/ml)* | 0.60 ± 0.08a | 1.48 ± 0.5a | 0.18 ± 0.05b |
| Oestrogen (E) (pg/ml) | 22.50 ± 1.6 | 25.02 ± 2.9 | 18.56 ± 2.0 |
| T/E ratio | 0.027 ± 0.005 | 0.06 ± 0.1 | 0.01 ± 0.028 |
| T/DHEA ratio | 0.28 ± 0.78 | 0.56 ± 1.6 | 0.12 ± 0.35 |
Values represent mean ± SD and asterisks represent statistically significant differences between the ages (P ≤ 0.05). Superscript letters (a,b) represent statistically significant differences between the ages. Statistical analysis based on the anova and Tukey Tests. Ratio between the serum levels of T/DHEA and T/E in relation to the ages of postnatal development is showed (Mean ± SD).
Structural and ultrastructural analysis
The control young group showed a developed epithelial compartment inserted in vascularized and innervated fibromuscular stroma (Figure 1a). All animals of this experimental group had no histopathological disorders. Continuous reticular fibre arrangements of the stroma were observed in intimate contact with epithelium (Figure 2c). Although some injuries were identified in the adult gland, the histological documentation was restricted to the senescent gland due to the higher lesion incidence found during this age. The structural analyses of senescent prostate exhibited important morphological alterations (Figures 1b–h and 2a–g). The epithelium and stromal compartment showed a cellular and stromal hyperplasia (Figure 1b). Prostatic calculi (Figure 1d) and multiples microcalculi (Figure 1f) were observed disperse throughout the lumen. Some alveoli had lost their structural organization showing the glandular dysplasia (undocumented data). Atypical cells observed in proliferating epithelium too showed nucleus with more that one nucleolus being identified premalignant lesions, like the high grade PIN (Figure 1c, e, g, h). Inflammatory cells were showed in the epithelium and lumen (Figures 1e and 2d,e), which in the latter a prostatitis was identified. The anomalous cell proliferations with disarrangement of the reticular fibres was an important tool for distinguishing an adenocarcinoma of the gland (Figure 2b,f).
Figure 1.
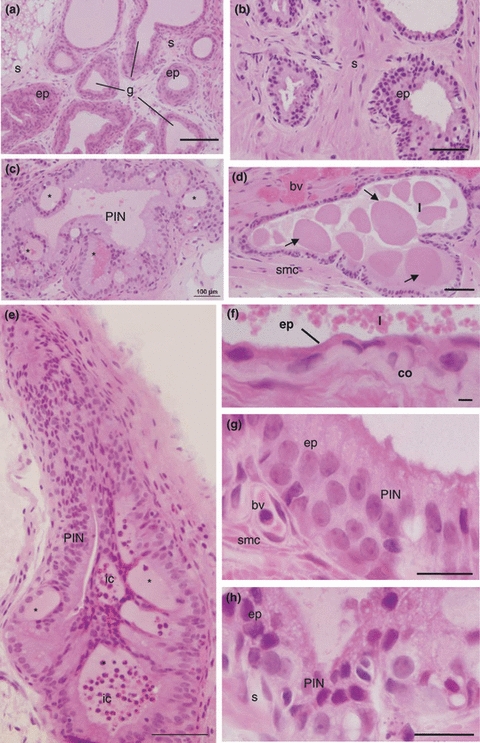
(a) Structural aspects of the control young female prostate. General view of the gland (g) with epithelium compartment (ep) inserted in the fibromuscular stroma (s) H&E. Bar 200 μm. (b–h) Histopathological aspects of the female prostate disorders in old gerbil stained by H&E. (b) Gland general aspect with epithelial hyperplasia. Stratified secretory epithelium (ep) inserted in dense stroma (s). Bar 100 μm. (c) General view of an acinus with intraepithelial neoplasia (PIN) containing several intraepithelial arcs (*). Bars 200 μm. (d) Alveolus with dysplasia and prostatic calculi (arrows) scattered although lumen (l) (smc smooth muscular cells, bv blood vessel). Bars 200 μm. (e) General view of an acinus with intraepithelial neoplasia (PIN) containing several intraepithelial arcs (*). To this lesion is associated inflammatory cells (ic). Bars 100 μm. (f) Detail of the gland showing the atrophic epithelium with extremely flat cells and microcalculi disperse throughout the lumen (l) (co collagen). Bar 50 μm. (g–h) Epithelial compartment (ep) proliferated showing prostatic intraepithelial neoplasia (PIN) and atypical cells. Bar 10 μm.
Figure 2.
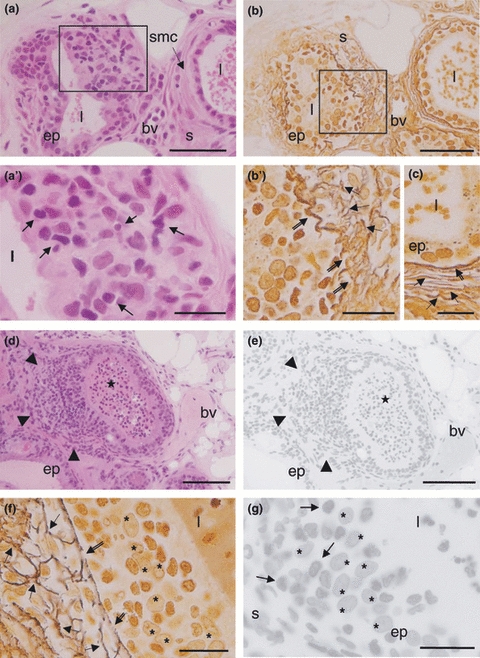
Histopathological aspects of the female prostate in old gerbil. (a) Gland alveolus showing morphologic disarrangement and cellular proliferation (left) distinguishing the adenocarcinoma contrasting with normal alveolus (right) (ep epithelium, l lumen, s stroma, smc smooth muscular cells, bv blood vessel). H&E. Bar 100 μm. (a’) Detail of anomalous cell proliferation with inflammatory cells (arrows). Bar 20 μm. (b) Disarrangement of the reticular fibre (left) distinguishing the adenocarcinoma contrasting with normal one (right) (ep epithelium, l lumen, s stroma, bv blood vessel). Gömöri's stain. Bar 100 μm. (b’) Detail of the fragmentation reticular fibres network in the basal lamina (double arrows) and stroma (arrows). Bar 20 μm. (c) In the normal acini, continuous basal lamina (double arrows) and organized reticular fibres network (arrows) is observed (l lumen, ep epithelium). Gömöri's stain. Bar 20 μm. (d) Anomalous epithelium cells proliferated with local invasion to stroma (arrowheads) associated with inflammatory cells distinguishing the adenocarcinoma of the gland (asterisk prostatitis, ep epithelium, bv blood vessel). H&E. Bar100 μm. (e) Adenocarcinoma of the gland stained by Feulgen reaction. (arrowheads epithelium cells proliferated, asterisk prostatitis). Bar 100 μm. (f) Detail of the base's epithelium, stained by Gömöri's stain, where can observe disrupted subepithelial (double arrows) reticular fibres and in the stroma (arrows) (asterisk epithelium cells). Bar 20 μm. (g) Detail of adenocarcinoma stained by Feulgen reaction showing different intensity of reaction in the chromatin. Note that dark inflammatory cells (arrows) and clear epithelial tumour cells (asterisk) (ep epithelium, s stroma). Bar 20 μm.
The immunohistochemical analysis reveal that the normal epithelium of the female prostate presented few cells immunoreactive to PCNA reaction (Figure 3). While in glands that showed some disorders, such as hyperplasia, the number of cells positive PCNA was remarkable.
Figure 3.
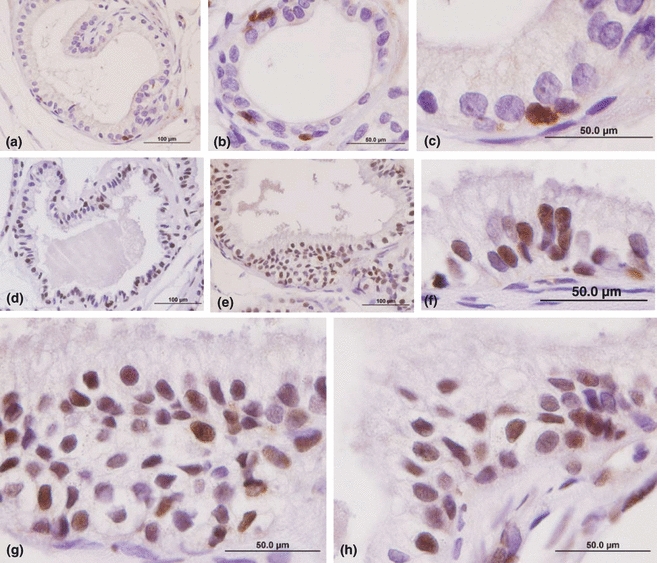
PCNA immunocytochemistry counterstained by haematoxylin. (a–c) Epithelial compartment of control female prostate free of the histopathology disorders. Demarcation of nuclear proliferation is very low in normal epithelium (immunoreactive nucleus is brown). Bars: (a) 200 μm, (b) 100μm, (c) 50 μm. (d–h) Hyperplasic secretory epithelial compartment with large number of positive PCNA cells. Bars: (d,e) 200 μm, (f–h) 50 μm.
The ultrastructural analyses of the senile prostate (Figure 4a–d) showed a typical atrophic epithelial cells (Figure 4a) but its endomembrane system remained integrity with secretory organelles few developed. The formation of the intraepithelial arches (Figure 4b) and microlumens (Figure 4c) showed the disarrangement in the glandular ultrastructure. A dense stroma characterized by thick collagen layer was found in the subepithelial region (Figure 4a) and also between stromal cells such as fibroblasts and smooth muscle cells (Figure 4a). A continuous basement membrane limited the epithelial compartment in benign lesion, (inset of Figure 4a), while the disruption in this structure (Figure 4d) probably allowed epithelial cells to spread into the stromal region, which characterized malignant lesions of the senile prostates.
Figure 4.
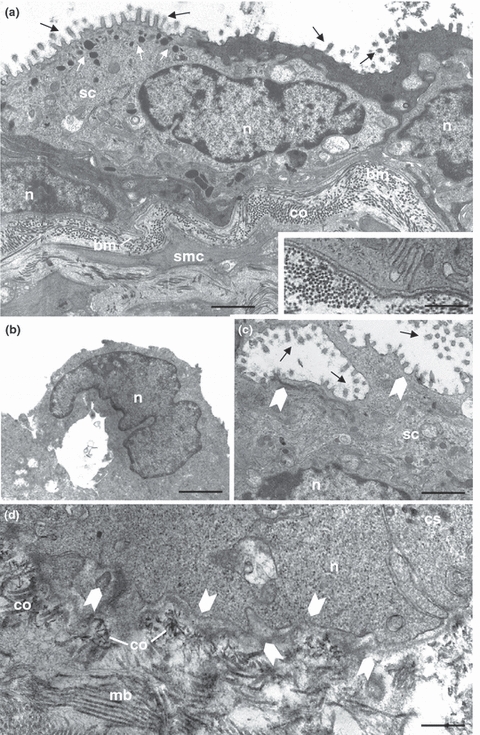
Ultrastructural aspects of the senile female prostate exhibiting disorders histopathology. (a) Squamous epithelium constitutive by secretory cells (sc) of heterogeneous phenotype and voluminous nucleus (n), with lipid droplets (white arrows) dispersed although cytoplasm and microvillous (black arrows) in its apical superficies. Dense stroma containing abundant collagen fibres (co) and smooth muscular cells (smc) which are below of the continuous basement membrane (bm). Bar 5.5 μm. Inset: Detail of the basal lamina showing the ultrastructural integrity. Bar 1.0 μm. (b) Detail of the intraepithelial arcs in early stage (n nucleus). Bar 1.86 μm. (d) Microlumens (full arrows) characteristics of the PIN high grade with cribriform pattern (sc secretory cell, n nucleus, dark arrows microvilous). Bar 0.7 μm. (e) Shown are fenestrations (full arrows) in the basement membrane (bm) allowing the contact between epithelial and stromal compartments designating a possible malignant disorder (n nucleus, co collagen). Bar 0.7 μm.
Analysis of nucleoli
The nucleoli of secretory epithelial cells in young prostates varied significantly, and in adulthood their frequency had dropped by almost 50%. The adult epithelial cells showed a significant rise in the number of nuclei without detectable nucleoli, which, moreover, were absent in senescence, the phase during which nuclei with a single nucleolus were significantly decreased. Those nuclei with two nucleoli were most abundant in adult cells, reducing significantly in senescent prostatic epithelium. The number of nuclei showing more than two nucleoli was reduced among adults, but rose significantly during senescence (Table 2).
Table 2.
Percentage distribution of the nucleolus number by nuclei in secretory epithelial cells of the female prostate in young, adult and senescent ages
| % of the nuclei containing each number of nucleoli | |||||
|---|---|---|---|---|---|
| Number of nucleoli in each nucleus | |||||
| Ages | 0* (not detectable) | 1* | 2* | More than 2* | Average number of nucleoli in group* |
| Young | 3.41 ± 1.45a | 5.85 ± 1.52a | 7.63 ± 1.82a | 83.09 ± 3.68a | 38.79 ± 3.35a |
| Adult | 9.11 ± 1.98b | 9.74 ± 2.67a | 12.56 ± 2.75a | 68.57 ± 5.34b | 20.96 ± 1.32b |
| Senile | 0.00 ± 0.00a | 2.10 ± 1.27b | 0.83 ± 0.58b | 97.05 ± 1.69c | 31.32 ± 2.23a |
Values represent mean ± SD and asterisks represent statistically significant differences between the ages (P ≤ 0.05). Superscript letters (a,b,c) represent statistically significant differences between the ages. Statistical analysis based on the anova and Tukey Tests.
Quantification of the prostatic disorders
During the ageing process, there was an increase in the multiplicity of all lesions, except for prostatic calculi. For dysplasias, multiplicity was found to be 10 times higher in senescent prostates than in adult ones. Prostatic hyperplasia doubled its multiplicity, while microcalculi, PIN and adenocarcinoma were three times more frequent. The percentage of lesion per acinus showed a significant augmentation during ageing, while this increase was not significant in hyperplasia, prostatitis, microcalculi, PIN and adenocarcinoma. An elevation in the acinar profile was observed between adulthood and senescence (Table 3), but this change was not statistically significant.
Table 3.
Quantification of the prostatic disorders: lesion incidence and multiplicity, lesion percentage per acinus and acinar profile inter-ages
| Female prostate lesions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Benignant | Premalignant | Malignant | ||||||
| Alveolus profile | Dysplasias* | Hyperplasias | Prostatitis | Prostatic micro calculi | Calculi | High-grade PIN | Adeno-carcinoma | |
| Adult | ||||||||
| %I (%) | 20 | 70 | 10 | 30 | 30 | 30 | 10 | |
| %M* | 12.0 ± 1.81 | 0.2 ± 0.13a | 2.1 ± 0.87 | 0.3 ± 0.3 | 0.5 ± 0.30 | 0.6 ± 0.33 | 0.6 ± 1.07 | 0.1 ± 1.0 |
| % Lesions* | 2.67 ± 1.79a | 16.7 ± 4.63 | 1.30 ± 1.30 | 3.25 ± 1.81 | 4.16 ± 2.13 | 5.11 ± 3.10 | 1.0 ± 1.0 | |
| Senile | ||||||||
| %I (%) | 90 | 100 | 20 | 30 | 30 | 20 | 20 | |
| %M* | 17.5 ± 2.57 | 1.9 ± 0.54b | 4.2 ± 0.98 | 0.4 ± 0.30 | 1.4 ± 0.85 | 0.5 ± 0.26 | 1.6 ± 1.07 | 0.3 ± 0.21 |
| % Lesions* | 10.28 ± 1.90b | 23.38 ± 3.94 | 2.08 ± 1.42 | 9.27 ± 6.41 | 3.47 ± 2.02 | 6.51 ± 4.37 | 1.74 ± 1.16 | |
Values represent mean ± SD and asterisks represent statistically significant differences between the ages (P ≤ 0.05). Superscript letters (a,b,c) represent inter-ages significant differences. Statistical analysis based on the anova and Tukey Tests.
Discussion
According to Santos and Taboga (2006), besides the biological implications related to this gland, the main focus in the female prostate emanates from its capacity to develop severe lesions during senescence. However, Zaviačič (1999) previously reported that many pathological diagnoses have been imprecisely issued by referring to these diseases as urinary tract disorders and not as prostatic ones, due to the acceptance of the vestigial concept and disbelief in the importance of this gland to women's health. Studies on the gerbil prostate indicated that, apart from the early functionality presented by the female gland in relation to the male one (Custodio et al. 2004), discrete morphological changes were identified from adulthood (Custodio et al. 2008). In males, on the other hand, this occurs only in senescence (Pegorin de Campos et al. 2006).
The major proliferative disorder evaluated in the present study was epithelial and stromal hyperplasia. This process led to the glandular increase, evidenced by the acini expansion and in consequence of this fact was observed some epithelial metaplasia regions. Folson and O’Brien (1943) reported that this disorder is very frequent in women but little recognized and treated, and it should be included among the lower urinary tract symptoms, similar to those observed in men. This disorder causes obstruction and urinary retention as well as a greater injury of the urogenital system, as has been proven at autopsy (Zaviačič 1999). As well as in man, the prostatic hyperplasia is characterized by progressive glandular and stromal hyperplasia around the urethra, causing urodynamic obstructions (Untergasser et al. 2005). Marcelli and Cunningham (1999) reported that the cellular dynamic caused by increased number of cells, related to high proliferation and low apoptosis, is one of the consequences of lumen enlargement in the prostate.
Starting from adulthood the incidence, multiplicity and percentage of prostatic hyperplasia per acini were expressive. However, impairment occurred in senescence, during which all the females presented this lesion, thus strongly relating it to age. The same is observed in the male prostate and advanced age is one of the risk factors for the development of this disorder (Carson & Rittmaster 2003; Untergasser et al. 2005). Calculi, also diagnosed in the prostate, were classified according to their size. Small structures, with crystalloid aspect, scattered in the luminal acini were identified as microcalculi, while the real prostatic calculi had larger dimensions and occupied most acini. The incidence of both injuries showed no changes with ageing, but the multiplicity of microcalculi doubled and calculi showed a slight decrease. The calcification of the corpora amylacea or the precipitation of prostatic secretion may be the responsible for the formation of these structures that contribute to the symptoms of lower urinary tract diseases (Klimas et al. 1985). Corpora amylacea and prostatic calculi contain salts of magnesium and potassium as well as calcium phosphate, calcium carbonate and calcium oxalate that usually are found in male benign prostatic hyperplasia (Geramoutsos et al. 2004). However, in the female gerbil, these concomitant lesions were less frequent. These structures can obstruct ducts and acini leading to inflammatory reaction that can cause abscesses.
An increase in the incidence and multiplicity of inflammatory infiltrates also occurred along with ageing. This benign lesion was identified in the lumen as well as in the interstices around the acini and was sometimes associated with adenocarcinoma. Zaviačič (1999) reported that, for a long period, these human female prostate infections were known as female urethral syndrome and were treated as urethral diseases. After the importance of this gland became known, the infection, which is similar to the male one, was called prostatitis and thus, appropriate therapeutic strategies began. Currently, it is well established that the most common infection of the female urinary tract, cystitis, originates in the prostate on account of communication among the prostate, the anterior wall of the urethra and the vagina. Thus, when inflammation of this organ occurs (prostatitis or Skenitis), the infection can spread throughout the female reproductive tract, constituting the well known urethro-prostato-cystitis.
Another lesion observed in the female prostate was characterized by an anomalous proliferation of the epithelial cells which were PCNA positive. Although this disorder was observed in few animals, it was impaired with ageing since its multiplicity tripled compared to adulthood. Such altered cell proliferation is defined as a premalignant lesion (Lippman & Hong 2002) known as prostatic intraepithelial neoplasia (PIN) and classified as low or high grade (WHO 2004) due to its complex architecture and morphological cell abnormality. However, according to the WHO (2004), it is difficult to distinguish the low grade PIN from normal epithelium and hyperplasia. Moreover, in clinical reports, this type of cell proliferation can progress, but it may not signify a potential lesion to the gland. Thus, despite low-grade PIN having been identified in this study, only the high grade PIN was analyzed. These lesions are heterogeneous and consist of large cells with prominent nucleoli. According to the cellular arrangement, it is possible to describe four different morphological patterns of PIN including flat, tufting, micropapillary and cribriform (Brawer 1992; Shappell et al. 2004; WHO 2004). This latter morphological pattern was a common finding in our study and showed characteristics similar to those described by Brawer (1992), where intraepithelial arcs are organized to form micro-lumens in acini. According to Mostofi et al. (1992), this arrangement is an important lesion of premalignancy that can be confused with prostate cancer.
According to cellular characteristics, adenocarcinoma was also detected in female prostate, and its incidence doubled with ageing. Moreover, this histopathology may be more intense, since its multiplicity was three times higher than in adulthood. This result, despite contradicting what was reported by Zaviačič (1999), reinforces his hypothesis. According to this author, the low incidence of female prostate cancer is probably due to both delayed recognition of its glandular functionality and inaccurate diagnosis since other female genital tissues, such as the urethra, are involved in disorders. In addition, as previously reported, immunohistochemical studies found that this tissue is the origin of many urogenital cancers (Huffman 1952; Dodson et al. 1994; Ali et al. 1995; Sloboda et al. 1998; Islam et al. 2001; Kato et al. 2005). Because the morphological characteristics of the female prostate, neoplastic cells may easily spread to other urogenital organs. Furthermore, given the characteristics of malignant cells such as migration, uncontrolled proliferation and loss of cell differentiation, the prostate may experience unregulated malignant growth (Reynolds & Kyprianou 2006). The similarities between the human (Zaviačič 1999) and gerbil gland (Santos et al. 2003, 2006; Custodio et al. 2004, 2008; Santos & Taboga 2006) suggest that both consist of a set of glands and ducts, positioned laterally to the urethra and inserted into the fibromuscular stroma. However, despite the existence of stromal constituents, the female gland does not present an effective protection as occurs in male prostate, whether in humans or rodents, where the dense layer of smooth muscle cells and surrounding collagen form a prostate capsule (McNeal 1983; Pegorin de Campos et al. 2006). Regardless of the severity of lesions, the ultrastructural analysis of epithelial secretory and stromal cells of the female gland showed no impairment of their activity since the endomembrane system was intact and functional.
While an important increase in the number of nuclei with more than two nucleoli was noted in the epithelial cells of the senescent female prostate, at the three ages of male postnatal development these cells showed no nucleolus or only a nucleolar corpuscle (Pegorin de Campos et al. 2006). However, these secretory cells of the female gland showed reductions in area, perimeter and nuclear form factor in old age (Custodio et al. 2008). According to Taboga et al. (2003), analysis of the nuclear form factor constitutes part of standard evaluation of prostatic lesions. Nevertheless, this factor was not analyzed in the present study due to the variety of lesions identified, which could hinder execution of this analysis. On the other hand, it cannot be hypothesized, based on the reduced nuclear measurements, that there was a decrease in the transcriptional activity during lesion development or even postnatal development in general. On the other hand, the ultrastructural features combined with the quantitative analysis of the nucleolar percentage per nucleus demonstrated significant cellular activity. In light of this result, it can be verified that the DNA content in the nucleoli remains determinant in the degree of cell proliferation (Trére 2000); and the use of nucleolar phenotype is an indicator of metabolic activation, as found in previous cancer studies (Derenzini et al. 2000).
The histopathological impairment in the gerbil female prostate during the ageing process was characterized by increased incidence, multiplicity and percentage of lesions per acinus in most of lesions diagnosed in the gland. This result is probably related to the hormonal levels found in those animals. Studies in women (Miller 2001) confirmed a reduction in the steroid levels during senescence in association with many pathological disorders that are not diagnosed in women of reproductive age. However, analysis of this experimental model showed that the histopathology was detected from the adult age which coincides with the elevation of the hormonal levels and the increase of the ration between T/E and T/DHEA levels. On the other hand, any type of lesion was identified in early life when, despite the low steroid levels. Risbridger et al. (2003) using E administration in hypogonadal mice and a model of aromatase knockout mice (ArKO) to study, respectively, the actions of E and T in the prostate noted that the action of these hormones individually caused only proliferative processes without malignant alterations. A balance between these steroids is critical to both normal prostatic function and the development of histopathologies in this gland. In this work, the relationship between T/E and T/DHEA levels decreased over the ageing process. Probably, this reduction in the steroid levels is associated not only with impaired ovarian function that is a hallmark of this phase, but also with reduced DHEA levels. This steroid precursor is converted into androgen and/ or E in the peripheral tissues (Labrie et al. 2005), which may also occur in the prostate. Thus, an individual action of these steroids cannot be the main cause of such changes. On the contrary, a combined hormonal action may have a potential function in this gland as indicated by studies showing the decreased ratio between T/E and T/DHEA levels and its relation to prostatic diseases.
The present investigation definitively shows, in the female prostate, lesions which have long been identified in male gland (McNeal 1983; Brawer 1992; Mostofi et al. 1992) and which present a higher incidence in senescence. Moreover, it defines the quantitative reality of these prostatic histopathologies through the analysis of incidence, multiplicity and percentage of lesion per acini. The knowledge of the pathological processes that affect the prostate in both sexes requires a combination of multidisciplinary expertise, especially in relation to cancer (Hsing et al. 2002). This approach includes epidemiological, urological, pathological, biochemical, endocrinology genetic and molecular data as well as the primary condition of extreme importance: the total acceptance of the prostate as a gland of the female genital apparatus which is predisposed to develop different type of lesions. Despite the surprising reality of the female prostate presented herein, all the clinical attentions, until now, have been focused on other urogenital organs. Information on the biology of this female gland, provided by experimental models and combined with basic preventions performed during routine urological examination, could be introduced into the clinical context.
Acknowledgments
This study is part of the thesis presented by AMGC to the Institute of Biology, UNICAMP, in partial fulfillment of the requirement for a PhD degree. The authors thank Mr. Luiz Roberto Falleiros Júnior and MSc. Rosana Silistino de Souza for their technical assistance, as well as all other researchers at the Microscopy and Microanalysis Laboratory. The authors also thank Mr. James Welsh for English-language revision of this study. Special thanks to Dra. Daniele Lisboa Ribeiro for suggesting in the final form of the manuscript and English-language revision and advice. The Brazilian National Research and Development Council - CNPq provided a research fellowship to S.R.T. (Process nr.301111/05-7) and a doctoral fellowship to A.M.G.C. (Process nr. 141722/2003-7). This work was supported by the National Council of Scientific and Technological Development (CNPq) and the São Paulo State Research Foundation (FAPESP).
References
- Ali SZ, Smilari TF, Gal D, Lovecchio JL, Teichberg S. Primary adenoid cystic carcinoma of Skene′s glands. Gynecol. Oncol. 1995;57:257–261. doi: 10.1006/gyno.1995.1137. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Remberger K. Morphogenetic concepts of normal and abnormal growth in the human prostate. Virchows Arch. 1998;433:195–202. doi: 10.1007/s004280050236. [DOI] [PubMed] [Google Scholar]
- Brawer MK. Prostatic intraepithelial neoplasia: a premalignant lesion. Symposium: the pathology of prostate cancer – prt 1: classic aspects of prostate cancer pathology. Hum. Pathol. 1992;23:242–248. doi: 10.1016/0046-8177(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Carson C, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61:2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Chahal HS, Drake WM. The endocrine system and ageing. J. Pathol. 2007;21:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- Custodio AMG, Góes RM, Taboga SR. Acid phosphatase activity in gerbil prostate: comparative study in male and female during postnatal development. Cell Biol. Int. 2004;28:335–344. doi: 10.1016/j.cellbi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Custodio AMG, Santos FCA, Campos SGP, Vilamaior PSL, Góes RM, Taboga SR. Aging effects of the on Mongolin gerbil female prostate: structural, ultrastructural quantitative and hormonal evaluations. Anat. Rec. 2008;291:463–474. doi: 10.1002/ar.20637. [DOI] [PubMed] [Google Scholar]
- Derenzini M, Trere D, Pession A, Govoni M, Sirri V, Chieco P. Nucleolar size indicates the rapidity of cell proliferation in câncer tissues. J. Pathol. 2000;191:181–186. doi: 10.1002/(SICI)1096-9896(200006)191:2<181::AID-PATH607>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Dodson MK, Cliby WA, Keeney GL, Peterson MF, Podratz KC. Skene′s gland adenocarcinoma with increased serum level of prostate-specific antigen. Gynecol. Oncol. 1994;55:304–307. doi: 10.1006/gyno.1994.1294. [DOI] [PubMed] [Google Scholar]
- Folson AL, O’Brien HÁ. The female urethra. The connecting link between the urologist and the gynecologist. JAMA. 1943;128:408–414. [Google Scholar]
- Geramoutsos I, Gyftopoulos K, Perimenis P, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur. Urol. 2004;45:333–337. doi: 10.1016/j.eururo.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–235. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- Huffman JW. Clinical significance of the paraurethral ducts and glands. Arch. Surg. 1952;62:615–625. doi: 10.1001/archsurg.1951.01250030625002. [DOI] [PubMed] [Google Scholar]
- Islam AHMM, Kato H, Hayama M, Kobayashi S, Ota H, Nishizawa O. Adenocarcinoma of female paraurethral duct showing neuroendocrine differentiation. Urology. 2001;58:1058. doi: 10.1016/s0090-4295(01)01437-6. [DOI] [PubMed] [Google Scholar]
- Kato H, Kobayashi S, Islam AM, Nishizawa O. Female para-urethral adenocarcinoma: histological and immunohistochemical study. Int. J. Urol. 2005;12:117–119. doi: 10.1111/j.1442-2042.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- Klimas R, Bennet B, Gardner WA., Jr Prostatic caculi: a review. Prostate. 1985;7:91–99. doi: 10.1002/pros.2990070110. [DOI] [PubMed] [Google Scholar]
- Labrie F. Dehydroepiandrosterone, androgens and the mammary gland. Gynecol. Endocrinol. 2006;22:118–130. doi: 10.1080/09513590600624440. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Bélanger A, et al. Is dehydroepiandrosterone a hormone? J. Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Hong WK. Cancer prevention by delay. Commentary re: J. A. O'Shaughnessy et al., treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin. Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- Marcelli M, Cunningham GR. Hormonal signaling in prostatic hyperplasia and neoplasia. J. Clin. Endocrinol. Metab. 1999;84:3463–3468. doi: 10.1210/jcem.84.10.6083. [DOI] [PubMed] [Google Scholar]
- McNeal JE. The prostate gland: morphology and pathobiology. Monogr. Urol. 1983;4:3–37. [Google Scholar]
- Miller KK. Androgen deficiency in women. J. Clin. Endocrinol. Metab. 2001;86:2395–2401. doi: 10.1210/jcem.86.6.7610. [DOI] [PubMed] [Google Scholar]
- Mostofi FK, Sesterhenn IA, Davis CJ., Jr Prostatic carcinoma: problems in the interpretation of prostatic biopsies. Symposium: the pathology of prostate cancer – prt 1: classic aspects of prostate cancer pathology. Hum. Pathol. 1992;23:223–241. doi: 10.1016/0046-8177(92)90103-a. [DOI] [PubMed] [Google Scholar]
- Pegorin de Campos SG, Zanetoni C, Góes RM, Taboga SR. Biological behavior of the gerbil ventral prostate in three phases of postnatal development. Anat. Rec. 2006;288:723–733. doi: 10.1002/ar.a.20347. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Kyprianou N. Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting. Br. J. Pharmacol. 2006;147:144–152. doi: 10.1038/sj.bjp.0706635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbridger GP, Bianco JJ, Ellem SJ, McPherson SJ. Oestrogens and prostate cancer. Endocr. Relat. Cancer. 2003;10:187–191. doi: 10.1677/erc.0.0100187. [DOI] [PubMed] [Google Scholar]
- Santos FCA, Taboga SR. Female prostate: a review about the biological repercussions of this gland in humans and rodents. Anim. Reprod. 2006;3:3–18. [Google Scholar]
- Santos FCA, Carvalho HF, Góes RM, Taboga SR. Structure, histochemistry and ultrastructure of the epithelium and stroma in the gerbil (Meriones unguiculatus) female prostate. Tissue Cell. 2003;35:447–457. doi: 10.1016/s0040-8166(03)00071-5. [DOI] [PubMed] [Google Scholar]
- Santos FCA, Leite RP, Custodio AMG, et al. Testosterone stimulates growth and secretory activity of the adult female prostate of the gerbil (Meriones unguiculatus) Biol. Reprod. 2006;75:370–379. doi: 10.1095/biolreprod.106.051789. [DOI] [PubMed] [Google Scholar]
- Santos FCA, Custodio AMG, Campos SGP, Vilamaior PSL, Góes RM, Taboga SR. Antiestrogen therapies affect tissue homeostasis of the gerbil (Meriones unguiculatus) female prostate and ovaries. Biol. Reprod. 2008;79:674–685. doi: 10.1095/biolreprod.108.068759. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;15:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Shehata R. Female prostate and urethral glands in the home rat, Rattus norvegicus. Acta Anat. 1980;107:286–288. doi: 10.1159/000145252. [DOI] [PubMed] [Google Scholar]
- Sloboda J, Zaviačič M, Jakubovský J, Hammar E, Johnsen J. Metastasizing adenocarcinoma of the female prostate (Skene′s paraurethrl galnds). Histological and immunohistochemical prostate markers studies and first ultraestructural observation. Pathol. Res. Pract. 1998;194:129–136. doi: 10.1016/S0344-0338(98)80080-0. [DOI] [PubMed] [Google Scholar]
- Taboga SR, Santos AB, Gonzatti AGR, Vidal BC, Mello MLS. Nuclear phenotypes and morphometry of human secretory prostate cells: a comparative study of benign and malignant lesions in Brazilian patients. Caryologia. 2003;56:313–220. [Google Scholar]
- Tepper SL, Jagirdar J, Heath D, Geller SA. Homology between the female parauretrhal (Skenes';s) glands and the prostate. Arch. Pathol. Lab. Med. 1984;108:423–425. [PubMed] [Google Scholar]
- Trére D. AgNOR staining and quantification. Mícron. 2000;31:127–131. doi: 10.1016/s0968-4328(99)00069-4. [DOI] [PubMed] [Google Scholar]
- Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp. Gerontol. 2005;40:121–128. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Wernet N, Albrecht M, Sesterhenn I, et al. The “female prostate”: location, morphology, immunohistochemical characteristics and significance. Eur. Urol. 1992;22:64–69. doi: 10.1159/000474724. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Classification of Tumors. Pathology and Genetics. Tumours of the Urinary System and Male Genital Organs. France: IARC Press Lyon; 2004. [Google Scholar]
- Yeh IT. Postmenopausal hormone replacement therapy: endometrial and breast effects. Adv. Anat. Pathol. 2007;14:17–24. doi: 10.1097/PAP.0b013e31802ef00f. [DOI] [PubMed] [Google Scholar]
- Zaviačič M. The Female Prostate: From Vestigial Skene's Parauretral Glands and Ducts to Woman's Functional Prostate. Bratislava: Slovack Academic Press; 1999. [Google Scholar]


