Abstract
The effects of experimental type 1 diabetes were investigated in the acinar epithelium of rat ventral prostate, focusing on the rates of cell proliferation and the frequency of apoptosis and p63-positive cells. Type 1 diabetes was induced in adult male Wistar rats by a single alloxan administration (42 mg/kg b.w.) and its effects were analysed for 1 week and 3 months after the establishment of the disease. A group of diabetic rats was treated daily with 5 IU of insulin during 1 week after diabetes had been diagnosed. Immunocytochemical methods for the localization of cell proliferation antigen (PCNA), androgen receptor (AR) and p63 protein were carried out, and apoptotic cells were identified by TUNEL essay. In diabetic rats, testosterone levels reduced drastically after 1 week and in a lower degree after 3 months. In short-term diabetic rats, cell proliferation decreased, and in medium-term, epithelial apoptotic rates increased. In both periods after the onset of diabetes, the frequency of p63-positive cells doubled. Insulin treatment was effective in preventing testosterone decrease, p63-positive cell increase and apoptotic rates, but did not interfere in cell proliferation. This investigation shows that, soon after diabetes onset, there are important modifications in cell proliferation within the acinar prostatic epithelium, and in longer term, there is a marked impact on kinetics of differentiation and cell death, which may initially be attributable to an androgenic fall, but is probably also because of other factors related to diabetes, as changes are considerably different from those resulting from castration.
Keywords: androgen receptor, apoptosis, cell proliferation, diabetes, p63, prostate
The prostate is the organ most affected by malignant lesions in men, and prostate cancer is the second most commonly diagnosed cause of death among men in many countries (Andreoni et al. 2001; Jemal et al. 2008; Ellison & Wilkins 2009). Prostate cancer arises from progressive transformation of acinar epithelium into prostate intra-epithelial neoplasias (PINs), justifying investigations into epithelial behaviour (De Marzo et al. 2007). The homeostatic maintenance of the prostatic epithelium requires a dynamic equilibrium between cell proliferation, differentiation and death (Isaacs 1987). Disturbances in some of these processes have a negative impact on epithelial kinetics and may be directly involved in the occurrence of intra-epithelial neoplasia and the subsequent development of malignancy (Cunha et al. 2004; De Marzo et al. 2007).
At least six cell types with distinct biological and phenotypic characteristics are found in the prostate secretory epithelium: stem cells, basal cells, transit-amplifying cells (TACs), intermediate cells, luminal or secretory cells and neuroendocrine cells (Isaacs 1987; Isaacs & Coffey 1989; Bonkhoff et al. 1994; De Marzo et al. 1998; Schalken & van Leenders 2003; Singh et al. 2006). TAC and intermediate cells correspond to intermediate stages, which exhibit an intense proliferative capacity, and the luminal secretory cells exist at terminally differentiated stage showing an increased secretory capacity (Isaacs & Coffey 1989; De Marzo et al. 1998; Singh et al. 2006). Stem cells are rare and correspond to less than 1% of the number of basal cells in the prostatic epithelium (Richarson 2004). In normal tissues, they are slow-cycle cells that rarely divide; however, deregulation of stem cell self-renewal is a likely requirement for the development of cancer (Lam & Reiter 2006). Several markers have been used for the identification of epithelial stem cells, but so far, none has proven completely reliable (Kelly & Yin 2008). The transcription factor p63, in particular the γ isoform, has been indicated as a good marker of this cell population in different stratified epithelia such as mammary, salivary and lachrymal glands (Yang et al. 1999; Kurita et al. 2004). This protein belongs to the family of p73 and p53 but, despite its structural similarity, it does not present tumour suppression activity (Kaghad et al. 1997; Yang et al. 1998). Signoretti et al. (2001) showed that the expression of p63 protein is required for the normal development of prostate cancer in rats, suggesting that p63-positive basal cells represent prostatic stem cells. This latter observation is in agreement with that observed for other tissues such as skin and the corneal limbus, where p63 protein is supposed to be expressed by the TAC (Pelegrini et al. 2001).
Androgens are probably the main trophic factor in the prostatic acinar epithelium being essential for cellular proliferation and differentiation. Therefore, their action is essential for both the induction of epithelial differentiation during embryonic development and postnatal maturation and the maintenance of secretory activity and normal differentiation (Cunha et al. 1992, 2004; Marker et al. 2003; Yan & Brown 2008; Yuan & Balk 2009). Thus, it is known that androgen deprivation, caused by castration, leads to massive cell death in the secretory luminal epithelium and regression of the gland (Kerr & Searle 1973; Isaacs 1984; Staak et al. 2003). Experimental type 1 diabetes impairs androgen biosynthesis by Leydig cells and reduces androgen uptake and retention in the prostate, thereby provoking regression of the gland (Tesone et al. 1976, 1980; Ikeda et al. 2000). Structural analyses have shown that diabetes-induced prostatic regression involves stromal remodelling, a decrease in the acinar profile and significant alterations in the acinar epithelium accomplished by atrophy (Cagnon et al. 2000; Carvalho et al. 2003; Ribeiro et al. 2006, 2009). An increase in PIN incidence has also been described in type 1 diabetes, and recent results suggest that alloxan-induced diabetes might promote malignancy in the medium-term (Ribeiro et al. 2006, 2008). Considering previous evidence showing significant morphological and physiological changes in the secretory acinar epithelium as a result of diabetes, this report evaluates the alterations in cell proliferation and apoptotic rates and the frequency of p63- and AR-positive cells, as well as the putative influence of insulin treatment on the acinar epithelium of short- and medium-term alloxan-induced diabetes.
Material and methods
Groups and treatments
Male adult Wistar rats (3 months old, 400–500 g) were purchased from Central Animal Breeding, São Paulo University, Ribeirão Preto, Brazil. Rats were kept at 25 °C on a 12 h light/dark cycle and had free access to food and water. They were kept in the Animal Breeding of the Department of Zoology and Botany of IBILCE-UNESP for 1 week before the beginning of the experiments. Experiments with animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health and were approved by the Institutional Committee for Ethics in Animal Experimentation (UNESP, Protocol no. 17/07-CEEA). Diabetes induction was carried out after 24 h of fasting by endovenous injection of 0.1 ml of physiological solution (NaCl 0.9%) with 42 mg/kg b.w. of alloxan (5,6 Dioxiuracil monohydrate; Sigma, St Louis, MO, USA), in the penian vein. Control rats (n = 12) were injected with physiological solution. After 30 min of alloxan treatment, groups were fed and blood glucose levels were daily measured in the tail tips using the glucose monitor Accu-Chek (Roche Diagnostics, Mannheim, Germany). Only rats presenting severe diabetes (glucose level up to 250 mg/dl in all tests, with loss of weight and increased urine debit) were utilized. Water and food intake were monitored during the experiment. Rats were killed 1 week (D1, n = 6) or 3 months (D2, n = 6) after the diagnosis of diabetes. Some short-term diabetic rats were daily treated with 5 IU of insulin (Humulin, Biobrás, MG, Brazil) (D1 + I, n = 6). All groups were killed by CO2 inhalation followed by decapitation.
Serum hormone analysis
Blood samples were collected immediately after decapitation. Serum was separated by centrifugation and stored at 20 °C for subsequent assays. Quantification of serum testosterone and oestradiol was performed using the Modular Analyzer for Immunoassay of Chemiluminescence ECI (Johnson and Johnson, Langhorne, PA, USA) (Weeks & Woodhead 1984). Five rats were used from each group, and the test was performed in triplicate. The intra- and inter-assay variations were 4.6% and 4.3% respectively. Serum insulin levels were not measured as it is widely accepted that alloxan administration drastically decreases insulin release from the pancreas and consequently, serum insulin levels after damage to the pancreatic beta cell, which, ultimately, lead to type 1 diabetes mellitus (Jansson & Sandler 1992; Soto et al. 2004; Lenzen 2008).
Tissue preparation
After the excision of the prostatic complex and the extraction of adipose tissue, ventral lobes were separated, weighed and transversally sectioned. Prostatic fragments were fixed by immersion in 4% formaldehyde in a phosphate buffer 0.1 M, pH 7.2 and embedded in paraffin for immunocytochemical analysis and TUNEL essay. Some of them were fixed in Karnovsky solution (2.5% formaldehyde, 2.5% glutaraldehyde in a phosphate buffer 0.2 M pH 7.2) for 24 h and embedded in plastic resin (Historesin Embedding Kit; Leica, Nussloch, Germany) or paraffin for histological purposes for 12–24 h.
The analysis of results was conducted in a Zeiss-Jenamed light microscope (Jena, Germany) coupled with a CCD camera and a semi-automatic image analysis system (Image-Pro Plus Media Cybernetics version 4.5 for Windows software, MD, USA).
Detection of apoptotic cells
Apoptotic cells were detected in situ using the DNA fragmentation assay associated to cell death based in TUNEL reaction (TdT-Fragel-Calbiochem; CN Biosciences, La Jolla, CA, USA), following the manufacturer's instructions. Briefly, after digestion with proteinase k (1:100 in 10 mM Tris pH 8.0) at room temperature for 23 min, slides were immersed in a solution of 3% H2O2 in methanol for 5 min to block endogenous peroxidases. In the next step, they were incubated with biotinilated TdT followed by enzyme deoxinucleotidyl terminal transferase (TdT), for 1 h at 37 °C. At the end of the reaction, the biotinilated nucleotides were detected by streptoavidin conjugated to peroxidase, and the reaction was revealed using diaminobenzidine (0.07% in distillate water). Slides were finally stained with haematoxylin. Negative controls were obtained by omitting the incubation with TdT enzyme.
Immunocytochemical reactions
Histological sections were submitted to antigen retrieval in citrate buffer pH 6.0, for 20 min and treated for a further 30 min in 3% H2O2 in methanol to block endogenous peroxidases. They were subsequently treated with a background sniper blocker to eliminate unspecific bindings (Biocare Medical, Concord, CA, USA), during 15 min. Sequentially, slides were incubated with the following primary antibodies (Santa Cruz Biotechnology, Palo Alto, CA, USA) diluted 1:100 in 1% BSA: rabbit anti-human androgen receptor (sc-816, overnight, at 4 °C), mouse anti-human PCNA (sc-56, 1 h, at 37 °C) and mouse anti-human p63 protein (sc-8431, 1 h, at 37 °C). AR detections were realized with biotinilated secondary antibody anti-rabbit, for 45 min, followed by ABC Staining System (sc2018; Santa Cruz Biotechnology, Santa Cruz, CA, USA), according to the manufacturer's instructions. Primary antibodies anti-PCNA and p63 protein were detected by Polymer conjugated to peroxidase (Novolink Polymer; Novocastra, Norwell, MA, USA), for 45 min. The reactions were revealed with diaminobenzidine (0.03% in TBS) and sections were stained with haematoxylin. Negative controls were obtained by omission of the primary antibody.
Quantitative and statistical analysis
The quantification of PCNA-, p63- and TUNEL-positive cells in the acinar epithelium of the intermediate region of the ventral prostate was performed by using 20 microscopic fields at 400×, randomly selected from two different histological sections per animal. Five rats were employed, resulting in 100 fields per group. The index of immunoreaction was calculated as the number of positive nuclei in epithelial cells divided by the total number of epithelial cell nuclei counted expressed as a percentage. Nuclei were counted in a total of approximately 2000 epithelial cells. The relative frequency of AR-positive epithelial cells (%) was estimated employing the system of point counting (Weibel 1963) based on the application of a multipurpose graticule M134. Thus, the AR frequency was calculated by counting the number of grid intersections falling on the positive cells.
The quantitative data were analysed by the parametric Student's t-test using statistica 6.0 (Statsoft Inc., Tulsa, OK, USA) and confronting the groups C1 vs. C2; C1 vs. D1; D1 vs. D1 + I and C2 vs. D2. Values of P < 0.05 were considered statistically significant.
Results
Biometrics and hormonal parameters
The biometric data, glucose and hormonal levels detected in the different groups of animals are shown in Table 1. Diabetic rats, in both experimental groups (D1, D2), exhibited blood glucose levels greater than 400 mg/dl. The insulin-treated group (D1 + I) showed 250 mg/dl of blood glucose levels, which was considered the limit for inclusion in diabetic group in this study. The relative weight of the prostate decreased 32% in D1, 27% in D1 + I and 40% in D2, when compared with their respective controls. Testosterone levels decreased 80% after 1 week and 68% after 3 months of diabetes. The oldest control (C2) also exhibited a sharp reduction in testosterone, showing less than half the levels noted in C1. Oestradiol serum levels were neither affected by diabetes in the short or medium-term, nor by insulin treatment. All these data, except for oestrogen serum levels, were statistically significant, as shown in Table 1.
Table 1.
Variation of biometric and physiological parameters (Average ± Standard Error) in 1 week diabetic (D1), insulin-treated diabetic (D1 + I) and 3 months diabetic (D2) rats compared with their respective controls (C1, C2)
| C1 | D1 | D1+I | C2 | D2 | |
|---|---|---|---|---|---|
| Body weight (g) | 523 ± 19.8 | 359 ± 21.9* | 465 ± 21.9‡ | 444 ± 12.9† | 227 ± 18.8* |
| Relative weight of prostate (×10³) | 1.3 ± 0.11 | 0.89 ± 0.09* | 0.95 ± 0.05‡ | 1.3 ± 0.08 | 0.78 ± 0.08* |
| Blood glucose (mg/dl) | 121 ± 8.01 | 497 ± 33.04* | 250 ± 48.5‡ | 89.3 ± 3.81 | 412 ± 15.17* |
| Serum testosterone (ng/dl) | 178.7 ± 37.8 | 34.21 ± 9.4* | 158.6 ± 29.35‡ | 82.2 ± 21.1† | 26.4 ± 6.34* |
| Serum oestradiol (mg/dl) | 31.85 ± 3.31 | 33.8 ± 7.95 | 28.7 ± 8.18 | 28.65 ± 0.82 | 30.7 ± 7.2 |
Statistically significant compared with control.
Statistically significant between ages.
Statistically significant compared with D1.
P < 0.05.
Ventral prostate morphology
There were no drastic changes in the prostatic histology in most of the D1 rats, especially in the intermediary region of the gland (Figure 1). In both control groups, the prostatic acinar epithelium was fully differentiated, containing abundant secretory columnar cells with a prominent Golgi complex area and few basal cells (Figure 1a,e). Short-term diabetes-induced pronounced wrinkling and epithelial disorganization besides reduction in the acinar lumen and stromal disorganization (Figures 1c,d). The prostate of the insulin-treated group was very similar to that of the control, exhibiting a columnar epithelium with a discrete reduction in epithelial height (Figure 1b). In the medium-term, diabetes promoted a drastic prostatic atrophy, showing a reduced acinar lumen and epithelial height that acquired a pavimentous aspect, making it difficult to distinguish between luminal cells and basal cells (Figure 1f).
Figure 1.
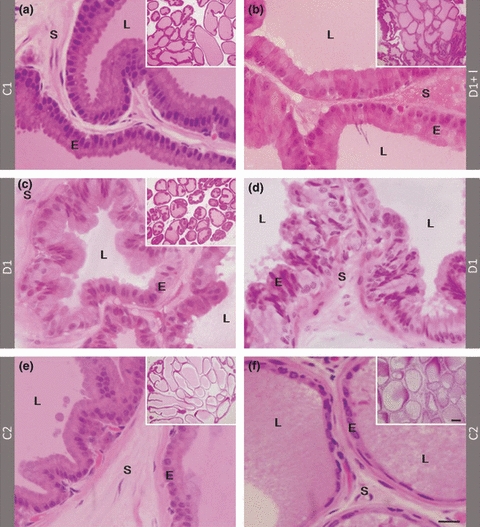
Histological sections in historresin stained with H&E showing general aspect (small images) and details of acinar epithelium (large images) of the ventral prostate in control, C1 (a) and C2 (e), 1 week diabetic insulin-treated (b), 1 week diabetic without treatment (c and d) and 3 months diabetic rats (f). In control groups, the acinar epithelium shows columnar luminal cells and large luminal area (a, e). After 1 week of diabetes, there is a slight reduction in epithelial height (c) and intense acinar disorganization in some glandular regions (d). In medium-term diabetic group, epithelial cells appear cubic or flattened, indicating the intense epithelial atrophy (f). Arrows: areas of Golgi complex. E: Prostatic acinar epithelium; L: acinar lumen; S: Stroma. Scale bars are the same for all small and large images in the figure: 100 and 20 μm respectively.
Immunocytochemical and cell death analysis of acinar epithelium
The frequency of PCNA-positive cells in the acinar epithelium of the prostate decreased dramatically with ageing, as noted by immunocytochemistry (Figures 2a–e and 4b). The frequency of PCNA-positive cells also showed a significant reduction, notably after1 week of diabetes, regardless of insulin treatment (Figures 2a–c and 4b). On the other hand, 3 months after diabetes, the frequency of PCNA-positive cells did not change significantly compared with their controls (Figures 2e and 4b).
Figure 2.
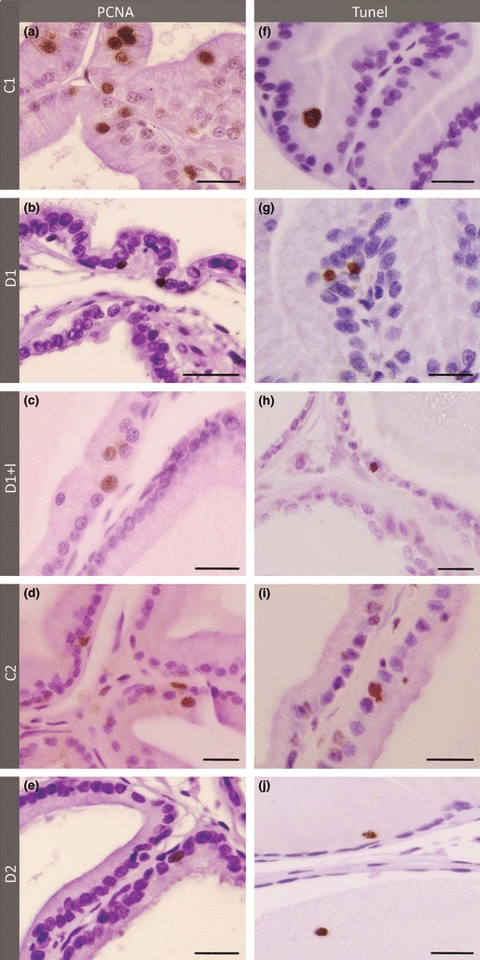
PCNA Immunocytochemistry (a–e) and TUNEL method (f–j) in the ventral prostate of 1 week control (a and f), 1 week diabetic (b and g), 1 week diabetic insulin-treated (c and h), 3 months control (d and i) and 3 months diabetic rats (e and j). Note that proliferation in prostate epithelium decreases significantly in older group (d) and also in diabetic groups, especially in short-term (b). The levels of apoptosis increased in prostate after diabetes (g and j), mainly in long-term rats (j). Insulin treatment completely reverses apoptotic levels but not cell proliferation in prostatic epithelium. Scale bar: 20 μm.
Figure 4.
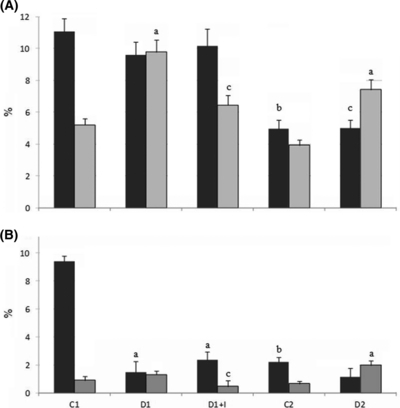
(A) Relative frequency (%) of immunoreactions for androgen receptor (black bars) and p63-positive cells (grey bars). (B) Relative frequency (%) of immunoreactions for cell proliferation (black bars) and apoptotic cells (grey bars). (a) Significantly different from respective control, (b) Significantly different between ages, (c) Significantly different from short-term diabetic group (D1). P < 0.05.
A tendency to increase was observed in apoptosis levels in the acinar epithelium of D1 (Figure 2f,g). These levels tripled after 3 months of diabetes (Figures 2i,j and 4b). Interestingly, levels of apoptotic cells were lower in the insulin-treated group compared with those of the control, although this difference was not statistically significant (Figures 2h and 4b).
Immunocytochemistry demonstrated that in young rats (C1), most of the cells in the prostatic acinar epithelium were AR-positive (Figure 3a), showing a significant decrease in the older group (C2) (Figures 3d,e and 4a). Diabetes does not interfere with the frequency of AR-positive cells when compared with their respective controls, but the intensity of reaction for this receptor in the cell nuclei is lower 1 week after diabetes (Figures 3b and 4a).
Figure 3.
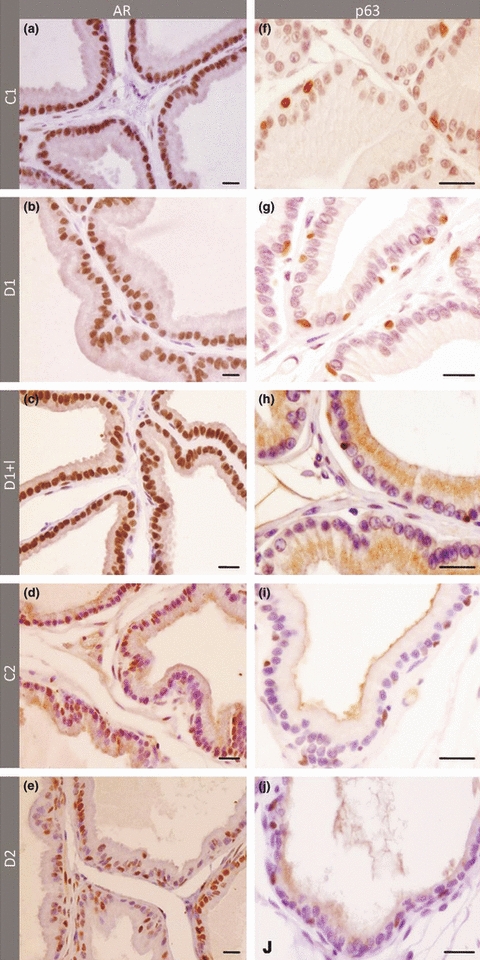
Immunocytochemistry for androgen receptor AR (a–e) and p63 (f–j) in the ventral prostate of 1 week control (a and f), 1 week diabetic (b and g), 1 week diabetic insulin-treated (c and h), 3 months control (d and i) and 3 months diabetic rats (e and j). The frequency of AR significantly decreased because of ageing (d), but not because of diabetes (b and e), although a lower intensity of immunoreaction could be observed in short-term diabetic group (b). The number of p63-positive cells is significantly greater in diabetic rats (g and j) and insulin treatment reduced its expression levels close to those observed in control group (h). Scale Bar: 20 μm.
Intense p63 protein immunolabelling was detected in the nuclei of basal cells in the prostate acinar epithelium from control groups (Figures 3f–j and 4a). The frequency of p63-positive cells doubled after 1 week and 3 months of diabetes, but did not change significantly in the diabetic group treated with insulin.
Discussion
This study evaluated the influence of short- and medium-term alloxan-induced diabetes and the possible implications of circulating androgen and insulin on proliferation and cell death in acinar prostatic epithelium. A summary of our data is shown in Table 2. Marked differences were found in the epithelial kinetics of the control groups at different ages as older rats showed fewer AR-positive cells and lower cell proliferation rates. Cell proliferation was determined here by immunocytochemistry for PCNA protein and is highly expressed in the nuclei during the S-phase of cell cycle (Bravo & Macdonald-Bravo 1987; Kelman 1997; Gulbahar et al. 2005). The decrease in cell proliferation in older rats paralleled the 50% reduction in testosterone levels. This indicates that ageing itself affects androgen availability and consequently the mechanisms of cell proliferation in the prostatic epithelium and confirms the auto-regulation of AR in the ventral prostate (Prins & Birch 1993; Prins et al. 1996).
Table 2.
Summary of results on frequency of positive cells after immunocytochemistry for androgen receptor (AR), PCNA, p63 and TUNEL essay. The decrease or increase in cell frequency is shown, respectively, by arrow down and arrow up
| Age (C1/C2) | Short-term diabetes (C1/D1) | Medium-term diabetes (C2/D2) | Insulin treatment (D1/D1 + I) | |
|---|---|---|---|---|
| AR | ↓ | 0 | 0 | 0 |
| PCNA | ↓ | ↓ | 0 | ↓ |
| p63 | 0 | ↑ | ↑ | 0 |
| TUNEL | 0 | 0 | ↑ | 0 |
Zero indicates no variation.
In the short-term, diabetes led to a significant reduction in androgen levels, together with an eight-fold reduction in cell proliferation levels in the acinar epithelium. A lower decrease in testosterone was observed in medium-term diabetes, as these levels are already low in older rats, and no significant variations in cell proliferation rates were found. The positive correlation between testosterone levels and epithelial proliferation during ageing and in diabetes was expected, as androgens are the major growth factor for prostate cells. Under normal physiological conditions, the proliferation in the acinar epithelium is indirectly controlled via androgenic stimulation of stromal cells, which secrete growth factors such as FGF-7, FGF-10 and IGF-I. These andromedins activate the proliferation of TAC and are also essential for the survival of luminal cells (Cunha et al. 2004; Uzgare et al. 2004). On the other hand, the direct action of AR in epithelial cells results in the differentiation of luminal epithelial cells and regulates the transcription of protein products that are required for prostate function, such as prostate specific antigen (Sugimura et al. 1986; Cunha et al. 1998; Gao & Isaacs 1998; Yuan & Balk 2009). Alloxan-induced diabetes and testosterone decrease did not interfere in the frequency of AR-positive cells in the acinar epithelium, but in the short-term, there was a notable lower intensity of immunolabelling per nuclei and, in the medium-term, marked epithelial atrophy and lumen reduction. These findings indicate that in the short-term diabetes, andromedin secretion is probably affected impairing cell proliferation, whereas in the medium-term, it affected secretory activity. Thus, although diabetes did not interfere in the frequency of AR-positive cells as analysed by immunocytochemistry, the signalling pathway involved in the stimulation of epithelial cell differentiation and secretion is probably affected.
Insulin is also an important stimulator of cell proliferation and is usually employed in vitro for a better growth of prostate cell lineages (Straus 1981; Cheatham & Kahn 1995; Antonioli 2003). Insulin treatment avoided testosterone reduction, but it was not able to avoid the reduction of cell proliferation in the acinar epithelium, indicating that other factors beyond insulin and androgen should be considered in the regulation of this process. As the insulin treatment employed here did not re-establish normal glycaemia, it is reasonable to presume that tissue alterations related to hyperglycaemia must be implicated. Another putative factor that could be involved is TGFβ1, which has been shown to act as a negative regulator of epithelial cell proliferation under conditions of co-culture of normal prostatic cells (Blanchere et al. 2001), and which increases in diabetes (Lamers et al. 2007).
Apoptosis, combined with decreased cell proliferation, is one of the main mechanisms leading to prostate regression in situations of androgen deprivation. Ten days after castration, the rat ventral prostate regresses completely, with the loss of 80% of androgen-dependent epithelial cells (Kyprianou & Isaacs 1988). In this situation, high levels of apoptosis occur 3–4 days after castration, but they normalize after prolonged periods, as the surviving cells are androgen-independent (Kyprianou & Isaacs 1988; Staak et al. 2003). Our data showed that apoptotic levels were not very elevated 1 week after diabetes. However, the marked reduction of serum testosterone and the decrease of about 32% in prostate weight as well as the increase in relative frequency of epithelial basal cells are strong indicators of massive loss of luminal epithelial cells. Thus, it is possible that the peak of apoptosis occurred before the seventh day as has been observed in castration, and therefore, the cellular debris has already been digested and is undetectable. Previous reports with non-obese diabetic mice have pointed to increased rates of apoptosis in the prostatic acinar epithelium after 20 days of diabetes (Favaro et al. 2008). Consistent with these results, the epithelial apoptotic rates tripled 3 months after alloxan-induced diabetes. Considering that androgen levels were similar in both diabetic groups, we assume that diabetes has a prolonged effect on the apoptosis of prostatic epithelial cells. Additionally, this effect is not exclusively dependent on androgen as, after 3 months of androgen reduction, most androgen-dependent cells might have died. The injury of the immune system together with the ageing process, which is associated to a greater accumulation of lesions in DNA, could possibly be responsible for the higher apoptotic levels in medium-term diabetes. It is also probable that the increase in products of advanced glycation (AGE) and the consequent increase in oxidative stress, because of hyperglycaemia are involved in increased apoptosis. Such alterations have been reported in other organs affected by diabetes, and a direct correlation between an increase in AGE and oxidative stress and the death of retinal pericytes has been shown (Chen et al. 2006). Interestingly, the normalization of androgen levels after insulin treatment resulted in apoptotic rates lower than control, suggesting a protective effect of insulin against the apoptosis of prostatic epithelial cells. Similar data have been found in experiments with retinal cells in vitro, showing that insulin prevents cell death in a dose-dependent pathway (Kobayashi & Puro 2007).
Short- or medium-term diabetes doubled the frequency of p63-positive cells in the rat ventral prostate, and insulin treatment prevented it. Considering previous reports, it is clear that the p63 protein, which is restricted to basal compartment, has an important role in regulating the development and differentiation of epithelial cells (Senoo et al. 2007), in addition to controlling cell proliferation, apoptosis and senescence (Yang et al. 1998; Senoo et al. 2007). Basal stem cells, which are able to survive in an environment with low levels of androgens are responsible, but not essential, for the reconstitution of the prostate epithelium after the administration of testosterone in castrated animals (Bonkhoff 1996; Bonkhoff & Remberger 1996; Kurita et al. 2004; Grisanzio & Signoretti 2008). Studies in other epithelia have also linked the expression of p63 protein with diabetes, and higher levels of p63 were reported by Galkowska et al. (2003) in keratinocytes obtained from diabetic patients. The increase in frequency of p63-positive cells in the prostate of diabetic rats is probably explained by the massive death of luminal cells, as observed after castration. However, the possibility that alterations in the hormonal/paracrine environment may have stimulated the activation of stem cells or implemented the proliferation of TACs cannot be excluded. Anyway, the preservation of basal cells in diabetes explains the maintenance of the structural integrity of the prostate, as these cells and p63 protein are essential in the ductal structure.
In conclusion, the epithelial response to alloxan-induced diabetes differed in the short- and medium-term not only because the epithelial kinetic varies according to age, but also because the processes affected are distinct. In the short-term, diabetes reduces cell proliferation, whereas in the medium-term, it increases apoptotic levels. This study shows that diabetes led to a fast reduction in testosterone, affecting the proliferative behaviour of the epithelium and inducing a drastic prostate atrophy. Insulin treatment attenuates these effects, but it is not sufficient to prevent them completely, particularly with regard to cell proliferation. This finding suggests the joint action of insulin, testosterone and other factors associated to diabetes, such as high glucose levels may be the cause of epithelial alterations induced by this disease. Furthermore, this investigation provide evidences that the prostatic epithelial atrophy observed in diabetic individuals is accompanied by marked changes in the kinetic of differentiation of epithelial cells, which may have implications for prostatic homeostasis and the incidence of diseases of epithelial origin.
Acknowledgments
This work was supported by National Counsel of Technological and Scientific Development CNPq-PIBIC scholarship and Foundation for Scientific Research of Sao Paulo State-FAPESP(Grant 06/07008-3). The authors would like to thank Mr. Luiz Roberto Faleiros Jr. and Ms. Samantha Yuri Maeda for the technical support.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
References
- Andreoni GI, Veneziano DB, Giannotti-Filho O, Marigo C, Mirra AP, Fonseca LAM. Cancer incidence in eighteen cities of the State of São Paulo, Brazil Incidência de câncer em dezoito cidades do Estado de São Paulo. Rev. Saúde Pública. 2001;35:362–367. doi: 10.1590/s0034-89102001000400005. [DOI] [PubMed] [Google Scholar]
- Antonioli E. 77f Modulação hormonal do comportamento das células musculares lisas prostáticas in vitro e in vivoDissertação (Mestrado) Campinas: Instituto de Biologia- Universidade Estadual de Campinas; 2003. [Google Scholar]
- Blanchere M, Mestayer C, Saunier E, Broshuis M. Transforming growth factor B in the human prostate: its role in stromal-epithelial interactions in non-cancerous cell culture. Prostate. 2001;46:311–318. doi: 10.1002/1097-0045(20010301)46:4<311::aid-pros1038>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H. Role of the basal cells in premalignant changes of the human prostate: a stem cell concept for the development of prostate cancer. Eur. Urol. 1996;30:201–205. doi: 10.1159/000474170. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J. Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnon VHA, Camargo AM, Rosa RM, Fabiani CR, Padovani CR, Martinez FE. Ultrastructure study of the ventral lobe of the prostate of mice with streptozotocin induced diabetes (C57bl/6j) Tissue Cell. 2000;32:275–283. doi: 10.1054/tice.2000.0123. [DOI] [PubMed] [Google Scholar]
- Carvalho CA, Camargo AM, Cagnon VHA, Padovani CR. Effects of experimental diabetes on the structure and ultrastructure of the coagulating gland of C57BL/6J and NOD mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;270:129–136. doi: 10.1002/ar.a.10014. [DOI] [PubMed] [Google Scholar]
- Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocrinol. Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- Chen B, Jiang D, Tang L. Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci. 2006;79:1040–1048. doi: 10.1016/j.lfs.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J. Androl. 1992;13:465–475. [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Hayward SW. Reciprocal mesenchymal-epithelial interaction in development of the male urogenital tract. In: Korach KS, editor. Reproductive and Developmental Toxicology. New York: Marcel Dekker; 1998. pp. 509–530. [Google Scholar]
- Cunha RG, Cooke PS, Kurita T. Role of estromal-epithelial interactions in hormonal responses. Arch. Histol. Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. J. Urol. 1998;160:2381–2392. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urol. Oncol. 2007;25:398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ellison LF, Wilkins K. Cancer prevalence in the Canadian population. Health Rep. 2009;20:7–19. [PubMed] [Google Scholar]
- Favaro WJ, Padovani CR, Cagnon VHA. Ultrastructural and proliferative features of the ventral lobe of the prostate in non-obese diabetic mice (NOD) following androgen and estrogen replacement associated to insulin therapy. Tissue Cell. 2008;41:119–132. doi: 10.1016/j.tice.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Galkowska H, Olszewsk WL, Wojewodzka U, Mijal J, Filipiuk E. Expression of apoptosis- and cell cycle-related proteins in epidermis of venous leg and diabetic foot ulcers. Surgery. 2003;134:213–220. doi: 10.1067/msy.2003.223. [DOI] [PubMed] [Google Scholar]
- Gao J, Isaacs JT. Development of an androgen receptor-null model for identifying the initiation site for androgen stimulation of proliferation and suppression of programmed (apoptotic) death of PC-82 human prostate cancer cells. Cancer Res. 1998;58:3299–3306. [PubMed] [Google Scholar]
- Grisanzio C, Signoretti S. p63 in Prostate Biology and Pathology. J. Cell. Biochem. 2008;103:1354–1368. doi: 10.1002/jcb.21555. [DOI] [PubMed] [Google Scholar]
- Gulbahar MY, Yuksel H, Guvenc T, Okut H. Assessment of proliferative activity by AgNOR and PCNA in prostatic tissues of lambs implanted with zeranol. Reprod. Domest. Anim. 2005;40:468–474. doi: 10.1111/j.1439-0531.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wada Y, Foster HE, Jr, Wang Z, Weiss RM, Latifpour J. Experimental diabetes-induced regression of the rat prostate is associated with an increased expression of transforming growth factor-β. J. Urol. 2000;164:180–185. [PubMed] [Google Scholar]
- Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. Development and characteristics of the available animal model systems for the study of prostatic cancer. Prog. Clin. Biol. Res. 1987;239:513–576. [PubMed] [Google Scholar]
- Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- Jansson L, Sandler S. Alloxan, but not streptozotocin, increases blood perfusion of pancreativ islet in rats. Am. J. Physiol. 1992;263:E57–E63. doi: 10.1152/ajpendo.1992.263.1.E57. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Kelly K, Yin JJ. Prostate cancer and metastasis initiating stem cells. Cell Res. 2008;18:528–537. doi: 10.1038/cr.2008.50. [DOI] [PubMed] [Google Scholar]
- Kelman Zvi. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch. 1973;13:87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Puro DG. Loss of insulin-mediated vasoprotection: early effect of diabetes on pericyte-containing microvessels of the retina. Invest. Ophthalmol. Vis. Sci. 2007;48:2350–2355. doi: 10.1167/iovs.06-1357. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4064. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Activation of programmed cell death in the rate ventral prostate after castration. Endocrinology. 1988;122:552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Lam JS, Reiter RE. Stem cells in prostate an prostate cancer development. Urol. Oncol. 2006;24:131–140. doi: 10.1016/j.urolonc.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Lamers ML, Gimenes FA, Nogueira FN, Nicolau J, Gama P, Santos MF. Chronic hyperglycaemia increases TGFbeta2 signaling and the expression of extracellular matrix proteins in the rat parotid gland. Matrix Biol. 2007;26:572–582. doi: 10.1016/j.matbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal cellular and molecular control of prostatic development. Dev. Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Immunocytochemical analysis of androgen receptor along the ducts of the separate rat prostate lobes after androgen withdrawal and replacement. Endocrinology. 1993;132:169–178. doi: 10.1210/endo.132.1.8419121. [DOI] [PubMed] [Google Scholar]
- Prins GS, Jung MH, Vellanoweth RL, Chatterjee B, Roy AK. Age-dependent expression of the androgen receptor gene in the prostate and its implication in glandular differentiation and hyperplasia. Dev. Genet. 1996;18:99–106. doi: 10.1002/(SICI)1520-6408(1996)18:2<99::AID-DVG2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ribeiro DL, Candido EM, Caldeira E, Manzaro AJ, Taboga SR, Cagnon VHA. Prostatic stromal microenvironment and experimental diabetes. Eur. J. Histochem. 2006;5:51–60. [PubMed] [Google Scholar]
- Ribeiro DL, Marques SF, Alberti S, et al. Malignant lesions in the ventral prostate of alloxan-induced diabetic rats. Int. J. Exp. Pathol. 2008;89:276–283. doi: 10.1111/j.1365-2613.2008.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DL, Taboga SR, Góes RM. Diabetes induces stromal remodelling and increase in chondroitin sulfate proteoglycans of the rat ventral prostate. Int. J. Exp. Pathol. 2009:400–411. doi: 10.1111/j.1365-2613.2009.00657.x. doi: 10.1111/j.1365-2613.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarson G. The banking of embryonic stem cells: the legal and ethical framework in the UK. Law Hum. Genome Rev. 2004;20:147–160. [PubMed] [Google Scholar]
- Schalken JA, van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11–20. doi: 10.1016/s0090-4295(03)00758-1. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, et al. p63 is a prostate basal cell marker and is required for prostate development. Am. J. Pathol. 2001;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr. Relat. Cancer. 2006;13:653–666. doi: 10.1677/erc.1.00797. [DOI] [PubMed] [Google Scholar]
- Soto C, Mena R, Luna J, et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;75:2167–2180. doi: 10.1016/j.lfs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Staak A, Kassis AP, Olshen A, et al. Quantitation of apoptotic activity following castration in human prostatic tissue invivo. Prostate. 2003;54:212–219. doi: 10.1002/pros.10179. [DOI] [PubMed] [Google Scholar]
- Straus DS. Effets of insulin on cellular growth and proliferation. Life Sci. 1981;29:2131–2139. doi: 10.1016/0024-3205(81)90482-3. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Tesone M, de Souza Valle LB, Foglia VG, Charreau EH. Endocrine function of the testis in streptozotocin diabetic rats. Acta Physiol. Lat. Am. 1976;26:387–394. [PubMed] [Google Scholar]
- Tesone M, Oliveira-Filho RM, Valle LB, et al. Androgen receptors in the diabetic rat. Diabetologia. 1980;18:385–390. doi: 10.1007/BF00276819. [DOI] [PubMed] [Google Scholar]
- Uzgare AR, Xu Y, Isaacs JT. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. J. Cell. Biochem. 2004;91:196–205. doi: 10.1002/jcb.10764. [DOI] [PubMed] [Google Scholar]
- Weeks I, Woodhead JS. Chemiluminescence immunoassays. J. Clin. Immunoassays. 1984;7:82–89. [Google Scholar]
- Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab. Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- Yan J, Brown TR. Cell proliferation and expression of cell cycle regulatory proteins that control the g1/s transition are age dependent and lobe specific in the brown Norway rat model of prostatic hyperplasia. Endocrinology. 2008;149:193–207. doi: 10.1210/en.2007-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, Mckeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]


