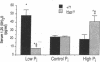Abstract
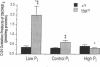
Hyp mice exhibit increased renal catabolism of vitamin D metabolites by the C-24 oxidation pathway (1988. J. Clin. Invest. 81:461-465). To examine the regulatory influence of dietary phosphate on the renal vitamin D catabolic pathway in Hyp mice, we measured C-24 oxidation of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in renal mitochondria isolated from Hyp mice and normal littermates fed diets containing 0.03% (low-Pi), 1% (control-Pi), and 1.6% (high-Pi) phosphate. In normal mice the low-Pi diet led to a rise in serum 1,25(OH)2D (22.2 +/- 1.8 to 48.1 +/- 6.8 pg/ml, P less than 0.05) and no change in C-24 oxidation products (0.053 +/- 0.006 to 0.066 +/- 0.008 pmol/mg protein per min) when compared with the control diet. In Hyp mice the low-Pi diet elicited a fall in serum 1,25(OH)2D (21.9 +/- 1.2 to 8.0 +/- 0.2 pg/ml, P less than 0.05) and a dramatic increase in C-24 oxidation products (0.120 +/- 0.017 to 0.526 +/- 0.053 pmol/mg protein per min, P less than 0.05) when compared with the control diet. The high-Pi diet did not significantly alter serum levels of 1,25(OH)2D or C-24 oxidation products in normal mice. Hyp mice on the high-Pi diet experienced a rise in serum 1,25(OH)2D (21.9 +/- 1.2 to 40.4 +/- 7.3, P less than 0.05) and a fall in C-24 oxidation products (0.120 +/- 0.017 to 0.043 +/- 0.007 pmol/mg protein per min, P less than 0.05). The present results demonstrate that the defect in C-24 oxidation of 1,25(OH)2D3 in Hyp mice is exacerbated by phosphate depletion and corrected by phosphate supplementation. The data suggest that the disorder in vitamin D metabolism in the mutant strain is secondary to the perturbation in phosphate homeostasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baxter L. A., DeLuca H. F. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem. 1976 May 25;251(10):3158–3161. [PubMed] [Google Scholar]
- Cunningham J., Gomes H., Seino Y., Chase L. R. Abnormal 24-hydroxylation of 25-hydroxyvitamin D in the X-linked hypophosphatemic mouse. Endocrinology. 1983 Feb;112(2):633–638. doi: 10.1210/endo-112-2-633. [DOI] [PubMed] [Google Scholar]
- Drezner M. K. The role of abnormal vitamin D metabolism in X-linked hypophosphatemic rickets and osteomalacia. Adv Exp Med Biol. 1984;178:399–404. doi: 10.1007/978-1-4684-4808-5_48. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Southard J. L., Scriver C. R., Glorieux F. H. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R. Regulation of the metabolism of vitamin D. Physiol Rev. 1980 Apr;60(2):551–613. doi: 10.1152/physrev.1980.60.2.551. [DOI] [PubMed] [Google Scholar]
- Glorieux F. H., Marie P. J., Pettifor J. M., Delvin E. E. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980 Oct 30;303(18):1023–1031. doi: 10.1056/NEJM198010303031802. [DOI] [PubMed] [Google Scholar]
- Gray R. W. Control of plasma 1,25-(OH)2-vitamin D concentrations by calcium and phosphorus in the rat: effects of hypophysectomy. Calcif Tissue Int. 1981;33(5):485–488. doi: 10.1007/BF02409478. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Napoli J. L. Dietary phosphate deprivation increases 1,25-dihyroxyvitamin D3 synthesis in rat kidney in vitro. J Biol Chem. 1983 Jan 25;258(2):1152–1155. [PubMed] [Google Scholar]
- Harrell R. M., Lyles K. W., Harrelson J. M., Friedman N. E., Drezner M. K. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia. Induction and maintenance with phosphorus and calcitriol. J Clin Invest. 1985 Jun;75(6):1858–1868. doi: 10.1172/JCI111900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. A new pathway of 25-hydroxyvitamin D3 metabolism. Methods Enzymol. 1986;123:141–154. doi: 10.1016/s0076-6879(86)23017-7. [DOI] [PubMed] [Google Scholar]
- Jones G. Chromatographic separation of 24(R),25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3-26,23-lactone using a cyano-bonded phase packing. J Chromatogr. 1983 Aug 12;276(1):69–75. [PubMed] [Google Scholar]
- Jones G., Yip A., Tenenhause H. S. Side-chain oxidation of vitamin D3 in mouse kidney mitochondria: effect of the Hyp mutation and 1,25-dihydroxyvitamin D3 treatment. Biochem Cell Biol. 1987 Oct;65(10):853–859. doi: 10.1139/o87-111. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Gray R. W., Meyer M. H. Abnormal vitamin D metabolism in the X-linked hypophosphatemic mouse. Endocrinology. 1980 Nov;107(5):1577–1581. doi: 10.1210/endo-107-5-1577. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Drezner M. K., Lobaugh B. Abnormal parathyroid hormone stimulation of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the hypophosphatemic mouse. Evidence for a generalized defect of vitamin D metabolism. J Clin Invest. 1986 Jan;77(1):181–187. doi: 10.1172/JCI112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Murphy M. M., Morris R. C., Jr Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986 Jan;77(1):7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S. Abnormal renal mitochondrial 25-hydroxyvitamin D3-1-hydroxylase activity in the vitamin D and calcium deficient X-linked Hyp mouse. Endocrinology. 1983 Aug;113(2):816–818. doi: 10.1210/endo-113-2-816. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Fast D. K., Scriver C. R., Koltay M. Intestinal transport of phosphate anion is not impaired in the Hyp (hypophosphatemic) mouse. Biochem Biophys Res Commun. 1981 May 29;100(2):537–543. doi: 10.1016/s0006-291x(81)80210-0. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S. Investigation of the mechanism for abnormal renal 25-hydroxyvitamin D3-1-hydroxylase activity in the X-linked Hyp mouse. Endocrinology. 1984 Aug;115(2):634–639. doi: 10.1210/endo-115-2-634. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Jones G. Effect of the X-linked Hyp mutation and vitamin D status on induction of renal 25-hydroxyvitamin D3-24-hydroxylase. Endocrinology. 1987 Feb;120(2):609–616. doi: 10.1210/endo-120-2-609. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S. Metabolism of 25-hydroxyvitamin D3 in renal slices from the X-linked hypophosphatemic (Hyp) mouse: abnormal response to fall in serum calcium. Cell Calcium. 1984 Feb;5(1):43–55. doi: 10.1016/0143-4160(84)90153-2. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. Effect of 1,25-dihydroxyvitamin D3 on phosphate homeostasis in the X-linked hypophosphatemic (Hyp) mouse. Endocrinology. 1981 Aug;109(2):658–660. doi: 10.1210/endo-109-2-658. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Yip A., Jones G. Increased renal catabolism of 1,25-dihydroxyvitamin D3 in murine X-linked hypophosphatemic rickets. J Clin Invest. 1988 Feb;81(2):461–465. doi: 10.1172/JCI113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth R., Fraser D. Kinetic behavior of 25-hydroxyvitamin D-1-hydroxylase and -24-hydroxylase in rat kidney mitochondria. J Biol Chem. 1979 Dec 25;254(24):12455–12460. [PubMed] [Google Scholar]
- Yamaoka K., Seino Y., Satomura K., Tanaka Y., Yabuuchi H., Haussler M. R. Abnormal relationship between serum phosphate concentration and renal 25-hydroxycholecalciferol-1-alpha-hydroxylase activity in X-linked hypophosphatemic mice. Miner Electrolyte Metab. 1986;12(3):194–198. [PubMed] [Google Scholar]