Abstract
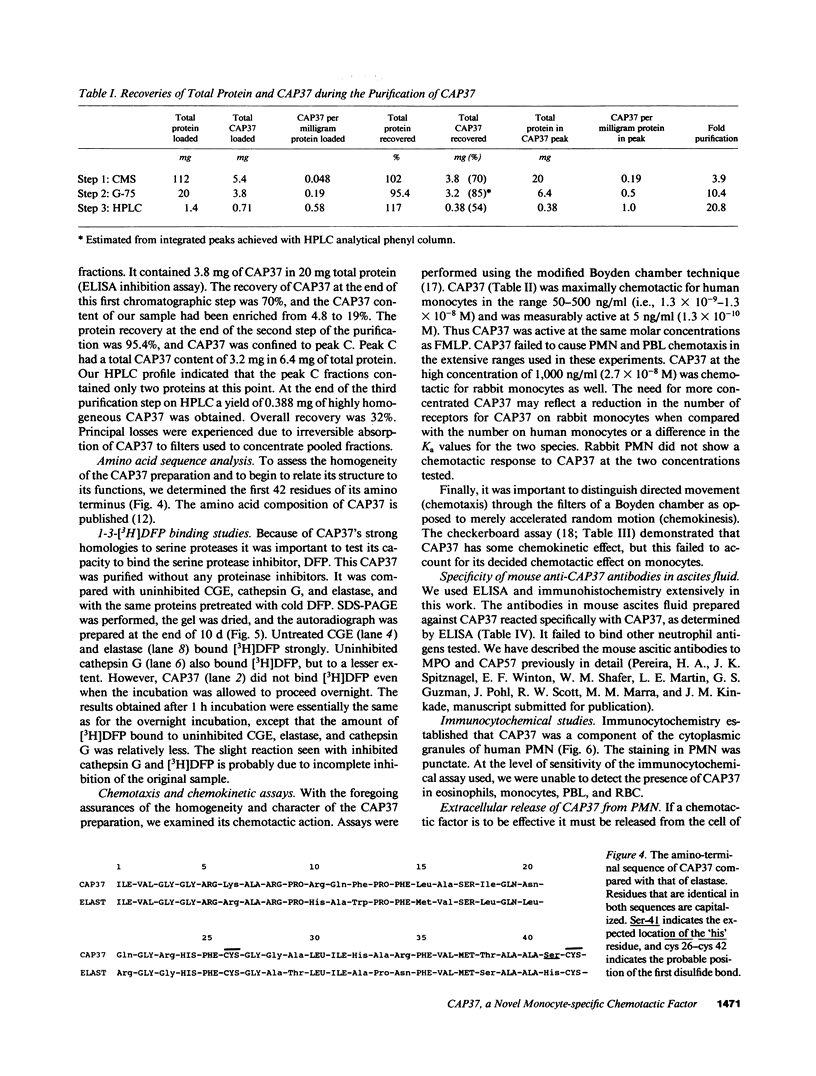
CAP37, an antimicrobial protein of human neutrophil granules, is a specific chemoattractant for monocytes. Purified to homogeneity by sequential chromatography over carboxymethyl Sephadex, G-75 Sephadex, and hydrophobic interaction HPLC, demonstratively endotoxin-free CAP37 was maximally chemotactic over a range of 1.3 X 10(-9)-10(-8) M. Thus it was active in the same molar concentrations as formyl-methionyl-leucyl-phenylalanine. CAP37 lacked chemotactic activity for neutrophils and lymphocytes. In checkerboard assays CAP37 had some chemokinetic activity as well. It was also chemotactic for rabbit mononuclear cells. Higher concentrations (2.7 X 10(-8) M) were required for activity with rabbit cells than with human. Sequence analysis of the first 42 NH2-terminal amino acid residues of CAP37 showed strong homologies with known serine proteases that mediate various functions in inflammation. However, a critical substitution of a serine for a histidine at position 41 suggested that CAP37 lacked serine protease action. This impression was supported by the failure of CAP37 to bind tritiated diisopropyl fluorophosphate. 89% of total CAP37 was released extracellularly from human neutrophils while they phagocytized Staphylococcus aureus. We propose that CAP37 released from neutrophils during phagocytosis and degranulation may mediate recruitment of monocytes in the second wave of inflammation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B. Exocytosis by neutrophils. Contemp Top Immunobiol. 1984;14:221–246. doi: 10.1007/978-1-4757-4862-8_8. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Yin F. H., Leder P. Cloning of the mast cell protease, RMCP II. Evidence for cell-specific expression and a multi-gene family. J Biol Chem. 1987 Apr 15;262(11):5377–5384. [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Bing D. H., Feldmann R. J., Fenton J. W., 2nd Structure-function relationships of thrombin based on the computer-generated three-dimensional model of the B chain of bovine thrombin. Ann N Y Acad Sci. 1986;485:104–119. doi: 10.1111/j.1749-6632.1986.tb34572.x. [DOI] [PubMed] [Google Scholar]
- Chambers W. H., Taylor J. R., Klesius P. H. Isolation of bovine polymorphonuclear leukocytes by density gradient centrifugation. Vet Immunol Immunopathol. 1983 Dec;5(2):197–202. doi: 10.1016/0165-2427(83)90020-x. [DOI] [PubMed] [Google Scholar]
- Farley D., Salvesen G., Travis J. Molecular cloning of human neutrophil elastase. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):3–7. [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granule deficiency. Annu Rev Med. 1985;36:263–274. doi: 10.1146/annurev.me.36.020185.001403. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granules: a fuse that ignites the inflammatory response. Clin Res. 1984 Sep;32(3):320–328. [PubMed] [Google Scholar]
- Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987 Mar;55(3):568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenfeld H. K., Weissman I. L. Cloning of a cDNA for a T cell-specific serine protease from a cytotoxic T lymphocyte. Science. 1986 May 16;232(4752):854–858. doi: 10.1126/science.2422755. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Honda M., Shimokawa Y., Hirashima M. Chemotactic factors associated with leukocyte emigration in immune tissue injury: their separation, characterization, and functional specificity. Int Rev Cytol. 1984;89:179–250. doi: 10.1016/s0074-7696(08)61304-2. [DOI] [PubMed] [Google Scholar]
- Johnson D. M., Gagnon J., Reid K. B. Amino acid sequence of human factor D of the complement system. Similarity in sequence between factor D and proteases of non-plasma origin. FEBS Lett. 1984 Jan 30;166(2):347–351. doi: 10.1016/0014-5793(84)80110-6. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Ueda K., Yamamoto T., Kambara T. The chemical mediation of delayed hypersensitivity skin reactions. IV. Activation of chemotactic factor precursor by a trypsin-like protease in guinea pig plasma. Am J Pathol. 1984 May;115(2):307–315. [PMC free article] [PubMed] [Google Scholar]
- Lacy M. J., Voss E. W., Jr A modified method to induce immune polyclonal ascites fluid in BALB/c mice using Sp2/0-Ag14 cells. J Immunol Methods. 1986 Mar 13;87(2):169–177. doi: 10.1016/0022-1759(86)90527-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Intracellular and extracellular degranulation of human polymorphonuclear azurophil and specific granules induced by immune complexes. Infect Immun. 1974 Dec;10(6):1241–1249. doi: 10.1128/iai.10.6.1241-1249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe C. G., Finlay B. B., Paranchych W., Paetkau V. H., Bleackley R. C. Novel serine proteases encoded by two cytotoxic T lymphocyte-specific genes. Science. 1986 May 16;232(4752):858–861. doi: 10.1126/science.3518058. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Neurath H. Proteolytic enzymes, past and present. Fed Proc. 1985 Nov;44(14):2907–2913. [PubMed] [Google Scholar]
- Niemann M. A., Bhown A. S., Bennett J. C., Volanakis J. E. Amino acid sequence of human D of the alternative complement pathway. Biochemistry. 1984 May 22;23(11):2482–2486. doi: 10.1021/bi00306a025. [DOI] [PubMed] [Google Scholar]
- Okano K., Aoki Y., Sakurai T., Kajitani M., Kanai S., Shimazu T., Shimizu H., Naruto M. Molecular cloning of complementary DNA for human medullasin: an inflammatory serine protease in bone marrow cells. J Biochem. 1987 Jul;102(1):13–16. doi: 10.1093/oxfordjournals.jbchem.a122024. [DOI] [PubMed] [Google Scholar]
- PAGE A. R., GOOD R. A. A clinical and experimental study of the function of neutrophils in the inflammatory response. Am J Pathol. 1958 Jul-Aug;34(4):645–669. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. A., Martin L. E., Spitznagel J. K. Quantitation of a cationic antimicrobial granule protein of human polymorphonuclear leukocytes by ELISA. J Immunol Methods. 1989 Feb 8;117(1):115–120. doi: 10.1016/0022-1759(89)90125-7. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Kinkade J. M., Jr, Parmley R. T. High resolution of heterogeneity among human neutrophil granules: physical, biochemical, and ultrastructural properties of isolated fractions. Blood. 1986 Aug;68(2):541–555. [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Disorders of phagocyte function. Annu Rev Immunol. 1987;5:127–150. doi: 10.1146/annurev.iy.05.040187.001015. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Farley D., Shuman J., Przybyla A., Reilly C., Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987 Apr 21;26(8):2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granulocytes. Infect Immun. 1986 Sep;53(3):651–655. doi: 10.1128/iai.53.3.651-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Pereira H. A., Martin L. E., Guzman G. S., Shafer W. M. A monoclonal antibody that inhibits the antimicrobial action of a 57 KD cationic protein of human polymorphonuclear leukocytes. J Immunol. 1987 Aug 15;139(4):1291–1296. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Olsson I. Cellular and subcellular localization of the bactericidal/permeability-increasing protein of neutrophils. Blood. 1987 Feb;69(2):652–659. [PubMed] [Google Scholar]
- Woodbury R. G., Katunuma N., Kobayashi K., Titani K., Neurath H., Anderson W. F., Matthews B. W. Covalent structure of a group-specific protease from rat small intestine. Appendix: crystallographic data for a group specific protease from rat intestine. Biochemistry. 1978 Mar 7;17(5):811–819. doi: 10.1021/bi00598a010. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. A functional differentiation of human neutrophil granules: generation of C5a by a specific (secondary) granule product and inactivation of C5a by azurophil (primary) granule products. J Immunol. 1977 Sep;119(3):1068–1076. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]







