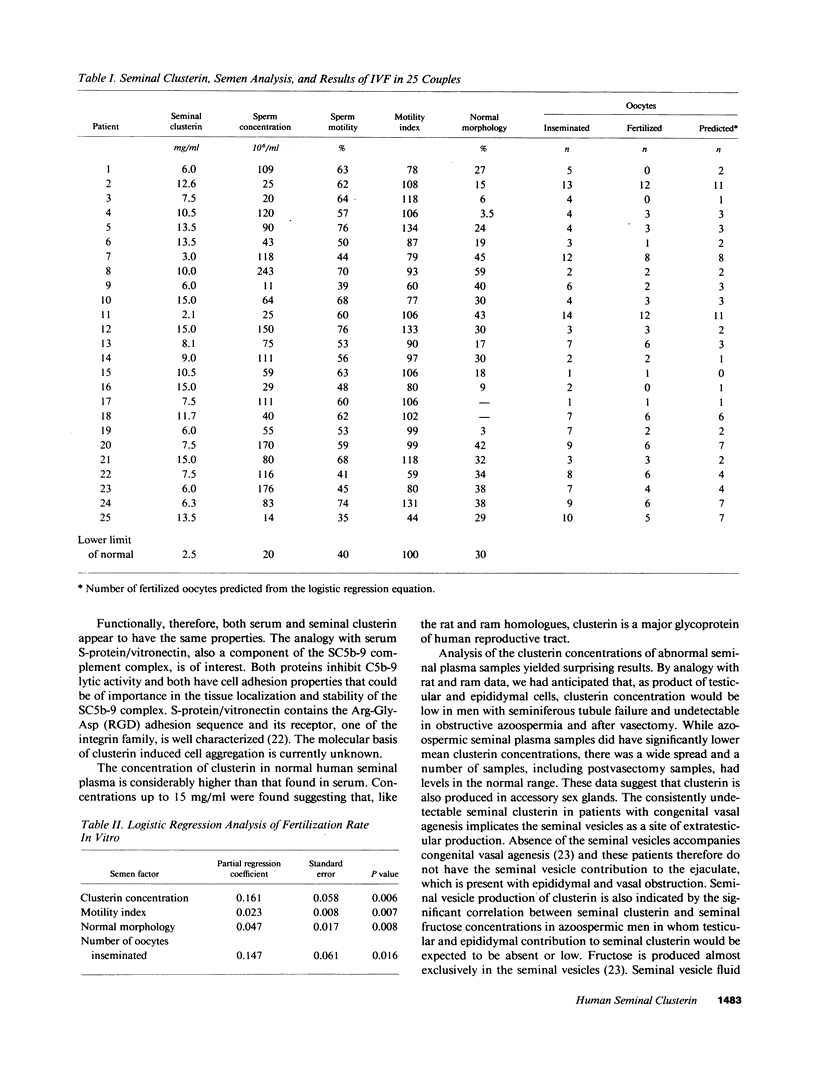
Abstract
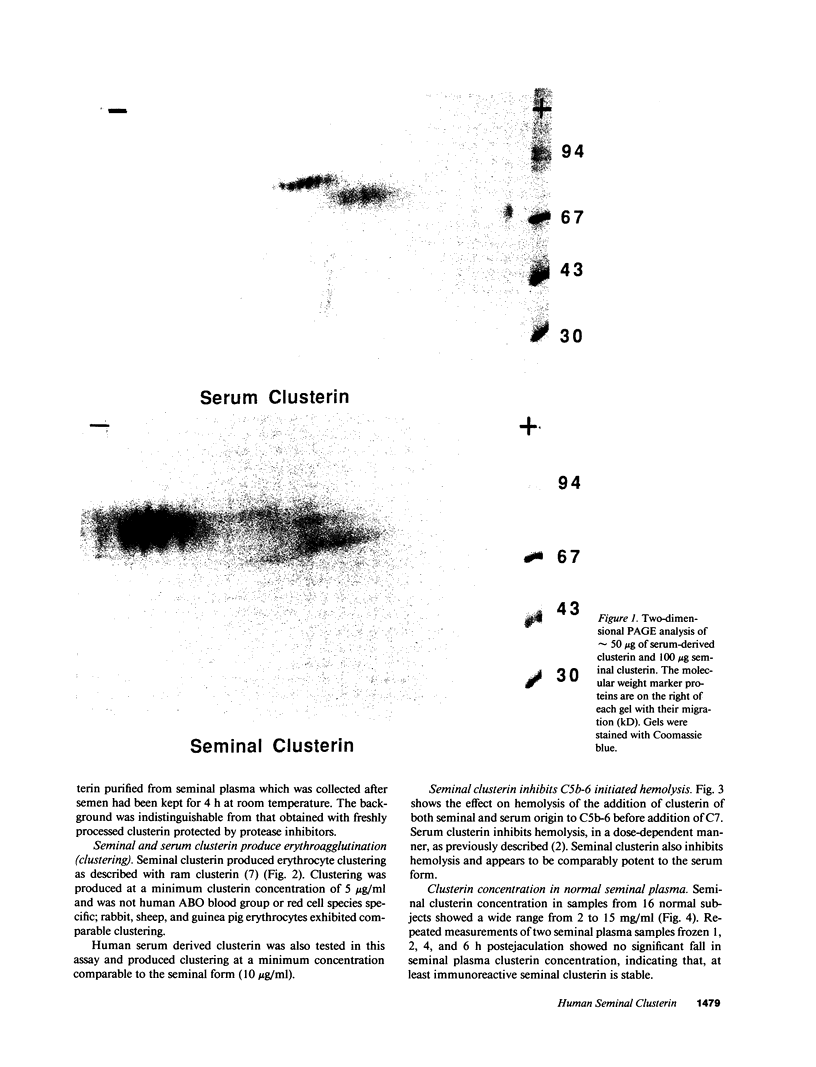
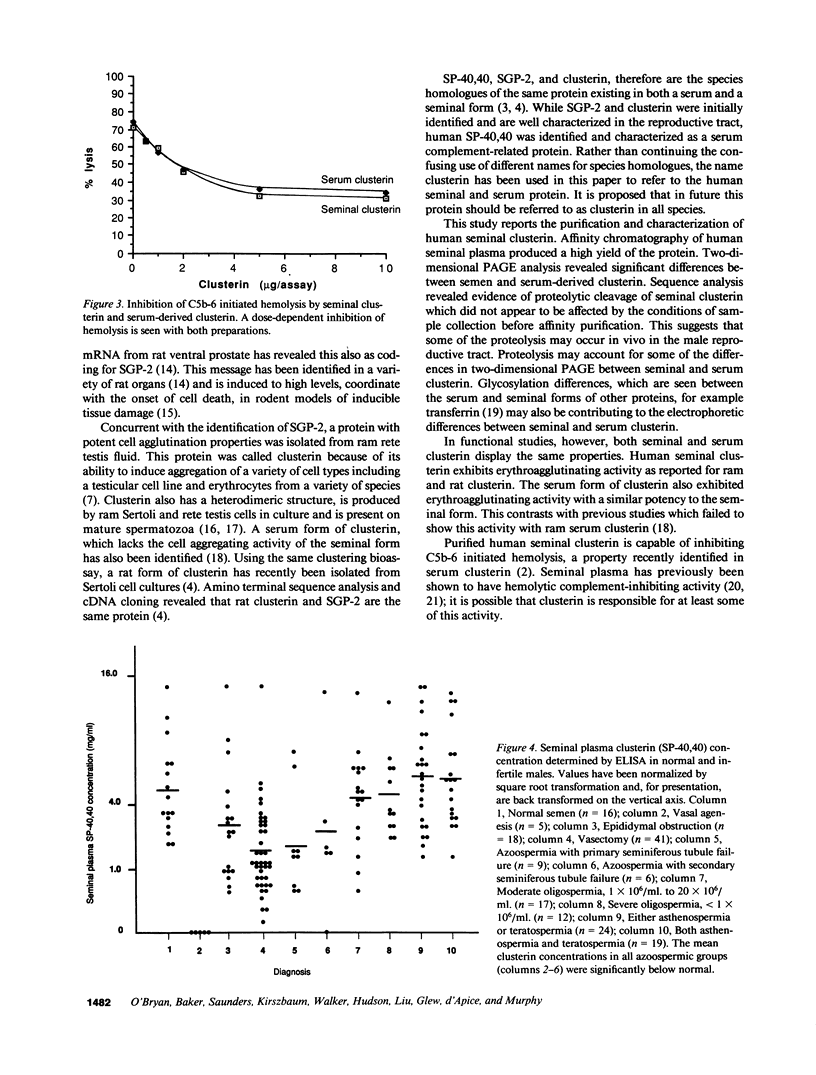
Molecular cloning of the human complement inhibitor SP-40,40, has revealed strong homology to a major rat and ram Sertoli cell product, sulfated glycoprotein-2, known also as clusterin. This study reports the purification and characterization of human seminal clusterin. Two-dimensional gel electrophoresis revealed charge differences between clusterin purified from semen and the serum-derived material. Both preparations demonstrate comparable hemagglutination (clustering) activity and inhibition of C5b-6 initiated hemolysis. The average clusterin concentration in normal seminal plasma is considerably higher than that found in serum. Mean seminal plasma clusterin concentrations were significantly lower in azoospermia caused by obstruction or seminiferous tubule failure than with oligospermia or normospermia. Only men with vasal agenesis had undetectable seminal clusterin, suggesting that some of the seminal clusterin is produced by the seminal vesicles. Immunofluorescence of human spermatozoa revealed that clusterin was detected on 10% of spermatozoa, predominantly those that were immature or had abnormal morphology. A pilot study of 25 patients suggests that seminal clusterin concentration, together with sperm motility and morphology, is correlated with the fertilization rate in vitro. The function of seminal clusterin is unknown. Its extensive distribution in the male genital tract and its high concentration in seminal plasma suggests an important role in male fertility.
Full text
PDF



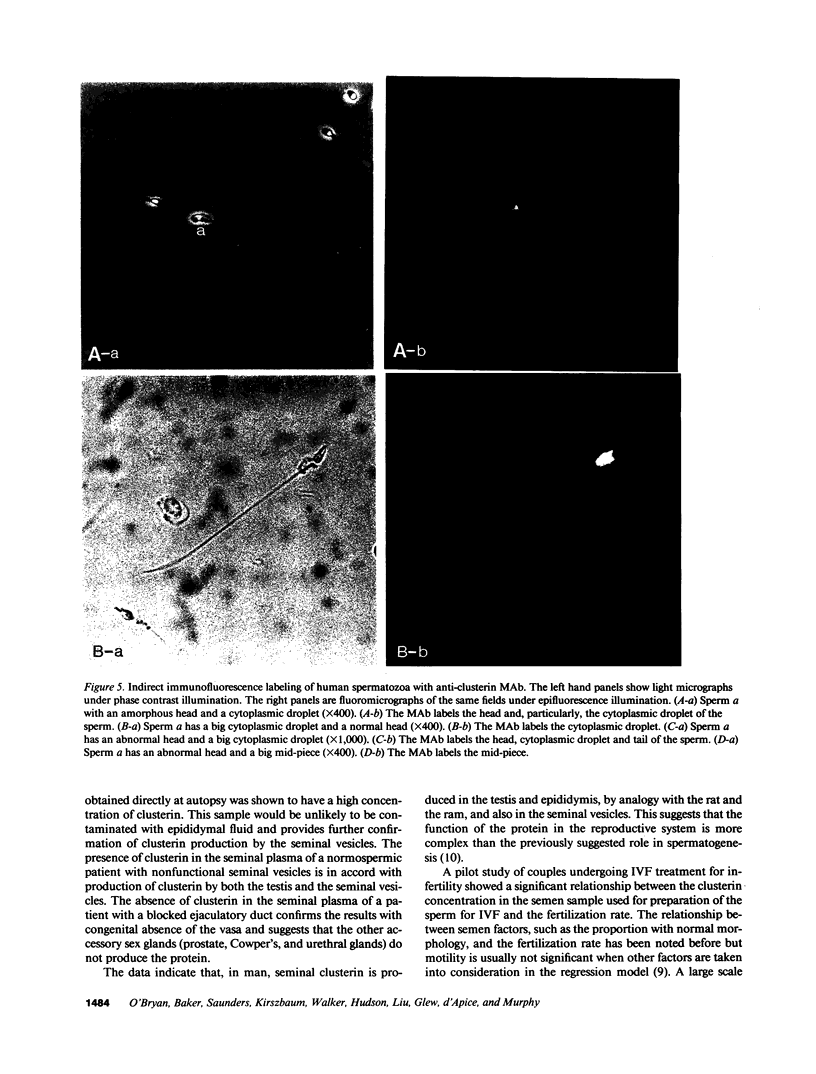
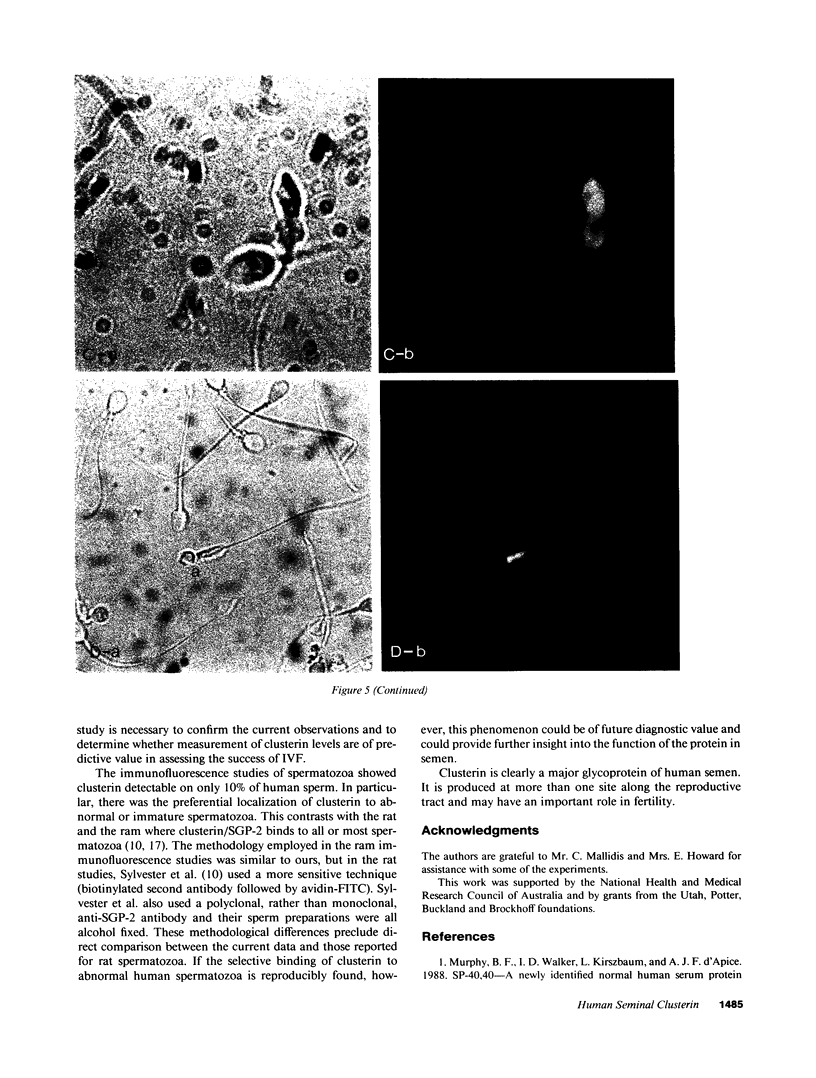

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettuzzi S., Hiipakka R. A., Gilna P., Liao S. T. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989 Jan 1;257(1):293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Chen C. L., Feng Z. M., Marshall A., Bardin C. W. Rat clusterin isolated from primary Sertoli cell-enriched culture medium is sulfated glycoprotein-2 (SGP-2). Biochem Biophys Res Commun. 1988 Aug 30;155(1):398–404. [PubMed] [Google Scholar]
- Cheng C. Y., Mathur P. P., Grima J. Structural analysis of clusterin and its subunits in ram rete testis fluid. Biochemistry. 1988 May 31;27(11):4079–4088. doi: 10.1021/bi00411a026. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Fritz I. B., Burdzy K., Sétchell B., Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983 Jun;28(5):1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- Griswold M. D., Morales C., Sylvester S. R. Molecular biology of the Sertoli cell. Oxf Rev Reprod Biol. 1988;10:124–161. [PubMed] [Google Scholar]
- Griswold M. D., Roberts K., Bishop P. Purification and characterization of a sulfated glycoprotein secreted by Sertoli cells. Biochemistry. 1986 Nov 18;25(23):7265–7270. doi: 10.1021/bi00371a003. [DOI] [PubMed] [Google Scholar]
- Husted S., Hjort T. Microtechnique for simultaneous determination of immobilizing and cytotoxic sperm antibodies. Methodological and clinical studies. Clin Exp Immunol. 1975 Nov;22(2):256–264. [PMC free article] [PubMed] [Google Scholar]
- Kirszbaum L., Sharpe J. A., Murphy B., d'Apice A. J., Classon B., Hudson P., Walker I. D. Molecular cloning and characterization of the novel, human complement-associated protein, SP-40,40: a link between the complement and reproductive systems. EMBO J. 1989 Mar;8(3):711–718. doi: 10.1002/j.1460-2075.1989.tb03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. Y., Du Plessis Y. P., Nayudu P. L., Johnston W. I., Baker H. W. The use of in vitro fertilization to evaluate putative tests of human sperm function. Fertil Steril. 1988 Feb;49(2):272–277. doi: 10.1016/s0015-0282(16)59715-5. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. F., Saunders J. R., O'Bryan M. K., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40 is an inhibitor of C5b-6-initiated haemolysis. Int Immunol. 1989;1(5):551–554. doi: 10.1093/intimm/1.5.551. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rosenior J., Tung P. S., Fritz I. B. Biosynthesis and secretion of clusterin by ram rete testis cell-enriched preparations in culture. Biol Reprod. 1987 Jun;36(5):1313–1320. doi: 10.1095/biolreprod36.5.1313. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Cosand W. L., Griswold M. D. Purification and characterization of testicular transferrin secreted by rat Sertoli cells. Biochem J. 1984 Mar 1;218(2):313–320. doi: 10.1042/bj2180313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester S. R., Skinner M. K., Griswold M. D. A sulfated glycoprotein synthesized by Sertoli cells and by epididymal cells is a component of the sperm membrane. Biol Reprod. 1984 Dec;31(5):1087–1101. doi: 10.1095/biolreprod31.5.1087. [DOI] [PubMed] [Google Scholar]
- Tarter T. H., Alexander N. J. Complement-inhibiting activity of seminal plasma. Am J Reprod Immunol. 1984 Jul-Aug;6(1):28–32. doi: 10.1111/j.1600-0897.1984.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. Immunolocalization of clusterin in the ram testis, rete testis, and excurrent ducts. Biol Reprod. 1985 Aug;33(1):177–186. doi: 10.1095/biolreprod33.1.177. [DOI] [PubMed] [Google Scholar]