Abstract
Many cardiac diseases coincide with changes in cell size and shape. One example of such a disease is cardiac hypertrophy. It is established that cardiac impulse propagation depends on the cell size, as well as other factors, but interrelations between conduction velocity (CV), cell size, and gap junction (GJ) conductance (gGJ) are complex. Furthermore, cardiac diseases are often accompanied by connexin (Cx) lateralization. To analyze the effects of cell size and Cx lateralization in cardiac disease, a two-dimensional computer simulation of ventricular myocytes based on the Luo-Rudy model was used. Control cells (80 μm/20 μm (length/diameter)), long cells (160 μm/20 μm), and wide cells (80 μm/40 μm) were simulated as was a redistribution of lateral GJs (constant lateral gGJ and increased lateral gGJ). CV in long cells showed high stability, i.e., it declined very slowly when gGJ was gradually reduced. Wide cells, however, were more affected by reduced gGJ, resulting in early transition to discontinuous propagation and low CV. Conduction block occurred earlier in enlarged cells than in control cells due to increased cell capacitance. Increased lateral gGJ stabilized longitudinal CV, which was a result of two-dimensional effects during planar wave propagation. Therefore, Cx lateralization may compensate for cardiac inhomogeneities. High lateral gGJ and enhanced cell diameter increased the susceptibility to conduction block at tissue expansion, providing a substrate for arrhythmia.
Introduction
Hypertrophic heart diseases coincide with ventricular arrhythmia and increased mortality due to sudden cardiac death (1–6). Arrhythmogenesis depends on multiple factors, because the electrophysiological behavior of cardiac tissue is a result of active and passive properties. Active properties include transmembrane ionic currents and channel kinetics, and passive properties include intercellular and intracellular resistances, gap junction (GJ) distribution, degree of fibrosis, and cell geometry. Electrical remodeling, of K+ channels, e.g., in hypertrophied and failing hearts can result in prolonged duration of action potentials (7–9), widening the vulnerable window for unidirectional conduction block and increasing the risk of arrhythmia. Hypertrophied hearts tend to prolonged QT intervals (10), which are known to be associated with sudden cardiac death (11). However, effects of increased cell size and GJ distribution in hypertrophied hearts are difficult to study experimentally. It is known that cell size can have a strong effect on impulse propagation (12–15). In a study by Spach et al. (13), an increase in cell size resulted in higher conduction velocity (θ),in accordance with findings of Ghali et al. (15), who showed that decreased cell size can negatively affect θ. In contrast, McIntyre et al. (16) found a negative correlation between cell diameter and θ in human ventricular tissue, which seems paradoxical, because an increase in cell diameter reduces the intracellular resistance. This indicates that the effects of cell size may be complex and are not completely understood. This study shall therefore provide a systematic analysis of effects of cell size on cardiac impulse propagation. Since impulse propagation strongly depends on GJ conductance (gGJ), it was appropriate to take into account the finding that hypertrophic, ischemic, and inflammatory cardiac diseases are often accompanied by altered cellular GJ distribution, with an increased number of connexin43 GJs (Cx43s) at cell sides (17–24), leading to an increased ratio of lateral to polar Cx43s (lateralization). Atrial fibrillation can also be accompanied by Cx lateralization (25). However, it is difficult to assess whether lateralized Cx builds a functional GJ. There are few studies of this issue. Polontchouk et al. (26) have shown that after stimulating rat atria for 24 h at a stimulation rate of 10 Hz, Cx43 was lateralized together with an increase in transverse θ (θT), giving evidence of functionality, whereas longitudinal θ (θL) was unchanged. In this study, we provide experimental evidence that this may also occur in human atria, although there are studies showing that Cx lateralization can be accompanied by decreased θ (19,27). Besides quantitative alterations, a more uniform spatial distribution of lateral Cx is observed (28). It is hardly possible to investigate separately the effects of altered cell size and GJ distribution in experiment, because usually they are accompanied by additional alterations, e.g., altered transmembrane currents (7–9) or fibrosis (29). Computer simulations make it possible to vary isolated parameters. Therefore, a two-dimensional (2D) computer model based on the Priebe-Beuckelmann (9) and Luo-Rudy (30) models was used. Different types of hypertrophy and different degrees of GJ lateralization were simulated and analyzed under normal and pathological conditions.
Methods
Mapping and immunostaining of atrial human tissue
A detailed description of Cx43 immunostaining and mapping experiments can be retrieved from the Supporting Material.
Computer model
For details, see the Supporting Material. A 2D computer simulation model with each cell composed of several cell segments 10 μm × 10 μm in size was used (Fig. 1). Different degrees of overlapping are discussed in Fig. S2 in the Supporting Material. Control cells were assigned a length and diameter of 80 μm and 20 μm, respectively (23,24,31), polar and lateral gGJ of 3.7 μS and 2.6 μS, respectively, and cytoplasmatic resistivity of 1.5 Ωm (32,33). Throughout the simulations, transmembrane current densities and kinetics were assumed to be unaltered. It is known that θ in elliptic waves can be smaller than in planar waves (34). Both were simulated, revealing a difference of 3–7%. If not otherwise specified, all results obtained were for planar waves.
Figure 1.
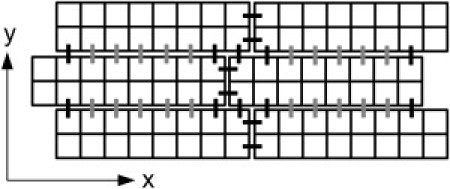
Schematic illustration of the bricklike cell arrangement and GJ distribution used in the simulations. Squares represent cell segments of 10 μm × 10 μm. Horizontal and vertical bars between blocks of cell segments indicate longitudinal and transverse GJs, respectively. Control cells had a nonuniform lateral GJ distribution, i.e., 90% of the lateral conductance was restricted to GJs near the cell poles (black vertical bars). Under simulation conditions of uniform connexin distribution, gray and black GJs had the same conductance.
Simulating hypertrophy and Cx lateralization
Junctional conductance
Hypertrophied cells have a greater membrane area (Am) and, thus, a higher cell capacitance. If GJ density (number of GJs/Am) remains constant, gGJ will increase proportionally to Am. If the number of GJs remains constant, gGJ will remain constant. Because experimental data indicate that both situations may occur (35,17–19,23,24,36), both were simulated. Increased gGJ is simulated under assumption I, and constant gGJ under assumption II. Regarding Cx lateralization, it was necessary to decide whether a lateralization of Cx involves creation of new GJs or only redistribution of the existing GJs. Experimental studies suggest that both occur (19,27,24,26,37), so both were simulated. GJs were simulated as passive ohmic resistors.
Cellular parameters
Hypertrophy is often accompanied by increased cell diameter (31,38). To examine this effect, cell diameter was increased from 20 μm to 40 μm. Because cell length may also be increased (31) or decreased (27) in hypertrophy, it was increased from 80 μm to 160 μm (see Table 1). Cell capacitance increased from 113 pF in control cells to 214 pF in long and 251 pF in wide cells, corresponding well with experimental data (29,38). Table 2 shows the electrophysiological parameters.
Table 1.
Geometrical parameters of control cells and enlarged cells
| l | d | Am | V | C | Am/V | |
|---|---|---|---|---|---|---|
| Control | 80 μm | 20 μm | 5655 μm2 | 25133 μm3 | 113pF | 0.225 μm−1 |
| Increased width | 80 μm | 40 μm | 12566 μm2 | 100531 μm3 | 251pF | 0.125 μm−1 |
| Increased length | 160 μm | 20 μm | 10681 μm2 | 50265 μm3 | 214pF | 0.213 μm−1 |
Parameters represented are as follows: l, length; d, diameter; Am, geometrical membrane area; V, volume, C, capacitance; Am/V, area/volume ratio.
Table 2.
Electrophysiological parameters of simulated conditions
|
gIC |
gGJ |
gGJ/CnS/pF |
RGJ/RIC |
ρ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| L (μS) | T (μS) | L (μS) | T (μS) | L | T | L (Ω cm) | T (Ω cm) | ||
| Control | 2.6 | 42 | 3.7 | 2.6 | 111 | 0.7 | 16 | 256 | 2575 |
| Increased width I | 10.5 | 42 | 8.2 | 5.8 | 111 | 1.3 | 7 | 341 | 1242 |
| Increased width II | 10.5 | 42 | 3.7 | 2.6 | 50 | 2.8 | 16 | 575 | 2575 |
| Increased length I | 1.3 | 84 | 7.0 | 4.9 | 111 | 0.2 | 17 | 178 | 2716 |
| Increased length II | 1.3 | 84 | 3.7 | 2.6 | 59 | 0.4 | 32 | 203 | 5000 |
| GJ lateralization | 2.6 | 42 | 3.7 | 13.0 | 295 | 0.7 | 3 | 256 | 635 |
Parameters are for 100% of coupling. L, longitudinal; T, transverse; gIC, intracellular conductance; gGJ, gap junction conductance; gGJ/C, ratio of total cellular gGJ in two dimensions to cell capacitance; RGJ/RIC, ratio of junctional to intracellular resistance; ρ, tissue resistivity.
Cx lateralization
Some studies have shown that Cx lateralization is accompanied by increased θT in ventricular and atrial tissue from rats (26,37), indicating functional Cx and increased lateral gGJ. In this study we provide evidence that this is also possible in human cardiac tissue (Fig. 2). Recent experimental findings show that in diseased hearts, lateral Cx can be increased up to fivefold (24,27). Human tissue samples indicated an even more pronounced increase in atrial fibrillation (Fig. 2). Therefore, Cx lateralization was simulated by a fivefold increase in lateral gGJ. However, since some studies show that Cx lateralization can coincide with unchanged or diminished total Cx (18,19,23,27), we also simulated a redistribution with constant lateral gGJ (Fig. 1).
Figure 2.
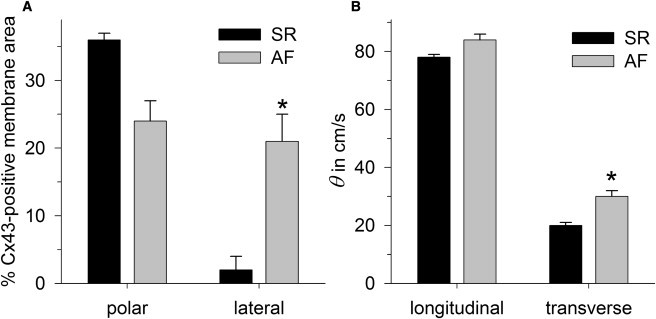
Experimental data obtained from atrial tissue samples from patients with sinus rhythm (SR) and atrial fibrillation (AF). (A) Percentage of the Cx43-positive membrane area, detected by immunostaining at cell poles (polar) and cell sides (lateral). (B) Conduction velocities (θ) obtained from mapping in longitudinal and transverse directions. An asterisk indicates a significant difference (p < 0.05) between SR and AF.
Conduction block
Polar and lateral gGJ were gradually reduced from 100% until the conduction block. Conductance was defined as 100% of coupling (Table 2). Maximum sodium channel conductivity (gNa) was gradually reduced from 100% (16 mS/μF) to 10% (1.6 mS/μF). A common method for assessing susceptibility to conduction block is the tissue expansion model (39,40). The critical width (hc) of a 4-mm-long cell strand (current source) sufficient to maintain propagation in the bulk area (4 mm × 4 mm; current sink) was determined.
Limitations
See the Supporting Material.
Results
Mapping and immunostaining of atrial human tissue
Atrial tissue samples (10 mm × 10 mm) of patients with sinus rhythm (SR) and atrial fibrillation (AF) were paced at a central electrode (1 Hz, 0.6 mA, 1-ms pulses) and simultaneously mapped using 64 electrodes. Immunohistological and morphometric analysis revealed that in SR, 36 ± 1% of the polar membrane length was positive for Cx43, whereas only 2 ± 2% of the lateral membrane was Cx43-positive. In AF, lateral Cx43 expression was strongly increased to 21 ± 4% (p < 0.05), whereas polar expression was slightly diminished to 24 ± 3% (Fig. 2 A). Mapping data revealed that θL was 78 ± 1 cm/s in SR and 84 ± 2 cm/s in AF (not significant). θT, which was 20 ± 1 cm/s in SR, significantly increased to 30 ± 2 cm/s (p < 0.05) in AF (Fig. 2 B). Micrographs of immunostaining are given in Fig. S1.
Effects of increased cell size
Assumption I: increased gGJ
In control cells (length/diameter of 80 μm/20 μm), θL was 59 cm/s and θT was 16 cm/s. When we assumed that gGJ increases proportionally to Am (Fig. 3 A), both enhanced length (160 μm/20 μm) and enhanced diameter (80 μm/40 μm) led to a comparable increase in θL: +22% in long cells (72 cm/s) and +15% in wide cells (68 cm/s). However, wide cells showed a much faster reduction of θL during gradual uncoupling. As a result, θL in wide cells dropped below θL in control cells when coupling was ≤15% and θL ≤30 cm/s. In contrast, θL in long cells declined very slowly. At 12.5% of coupling, θL was reduced to 51% (30 cm/s) in control cells, only to 68% (49 cm/s) in long cells, and to 44% (30 cm/s) in wide cells (Fig. 3 C). One explanation for this is that the increase in θL in wide and in long cells is based on different mechanisms. Increased cell length prolongs the distance between GJs, i.e., the proportion of intracellular resistance (RIC) to total resistance becomes greater. Accordingly, longitudinal tissue resistivity (ρ) and the ratio of GJ resistance (RGJ) to RIC are lower than in the control, i.e., the effect of gGJ on ρ is smaller. An increase in diameter, however, increases longitudinal ρ. Higher θL is the result of an approximately twofold lower ratio of Am to cell volume (Am/V). See Tables 1 and 2 for details. Since a twofold increase in diameter results in a fourfold smaller RIC, the RGJ/RIC ratio is greater in wide cells. As a result, θL decreases faster in wide cells than in control cells. θT was considerably higher in wide cells (31 cm/s) than in control cells (16 cm/s), because apart from lowering the Am/V, the longer distance between GJs decreases transverse ρ. Long cells revealed a θT similar to that of control cells (15 cm/s vs. 16 cm/s), because transverse ρ and Am/V were almost unchanged. In contrast to θL, the relative decrease of θT during gradual uncoupling was comparable in all groups (Fig. 3 C), a result of the higher RGJ/RIC ratios in the transverse direction: RGJ represents almost 100% of the transverse ρ, i.e., an increase in RGJ by a given factor increases transverse ρ by the same factor in all groups. Note that this is a reason why anisotropy (θL/θT) becomes greater at lower gGJ (see Fig. S3).
Figure 3.
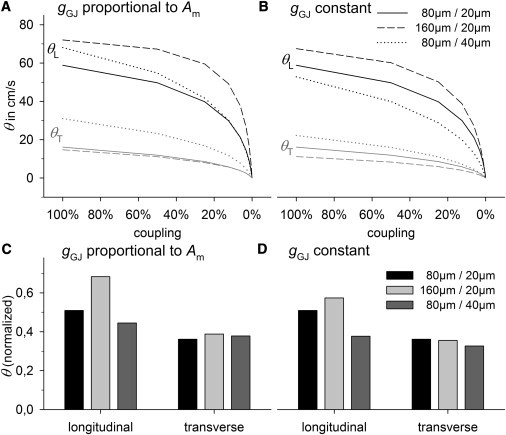
θL and θT in control cells (80 μm/20 μm (length/width)), long cells (160 μm/20 μm), and wide cells (80 μm/40 μm) with gradual reduction in coupling. Cell size was increased, assuming that gGJ is proportional to Am (left) and that gGJ remains constant (right). (A and B) Plots of absolute values of θL and θT versus coupling. (C and D) θL and θT at 12.5% of coupling, normalized to their values at 100% of coupling.
Assumption II: constant gGJ
When we assumed constant gGJ, the ratio of gGJ to cell capacitance was lower in enlarged cells (Table 2). Accordingly, θ was lower than under assumption I (Fig. 3 B). However, θL in long cells was higher (68 cm/s) than in control cells (59 cm/s), which was a result of a still lower longitudinal ρ (Table 2). The RGJ/RIC ratio was also still lower than in control cells, i.e., θL decreased more slowly during gradual uncoupling, but less significantly compared to assumption I (Figs. 3 C and D). Regarding θT, there was a considerable reduction to 11 cm/s (−30%). Wide cells at constant gGJ revealed decreased θL (52 cm/s) and higher θT (22 cm/s). θL declined even faster than under assumption I (Fig. 3 D). It is interesting to note that long cells under assumption II had the same θL (68 cm/s) as did wide cells under assumption I (compare Fig. 3, A and B), although gGJ was more than twofold higher in wide cells and cell capacitance was similar. At lower gGJ (25% of coupling), θL was even higher in long cells compared to wide cells (50 cm/s vs. 41 cm/s).
Gradual uncoupling
As indicated by Fig. 3, cell size affected the sensitivity to uncoupling: θL declined faster in wide cells with both increased and constant gGJ, suggesting a mechanism independent of gGJ. To analyze cell-size effects independent of θ, in the following simulations, gGJ was adapted in enlarged cells to obtain the same θ (±5%) as in control cells. Fig. 4 gives insight into the basic effects of uncoupling. At high coupling (Fig. 4 A), the membrane potential (ϕ) spreads almost continuously, because RIC and RGJ are in the same order of magnitude. As a result, there is a steady spatial curve at the wave front, leading to continuous impulse propagation, indicated by continuous activation time curves (Fig. 4 A, inset). There were no differences among the different cell sizes, and the curve of discrete cells corresponded well to the ideal curve of a continuous model with the same average ρ. The distance over which ϕ spreads is characterized by the space (also the length) constant (λ), which is the distance from the point of maximum ϕ (ϕmax) to the point where ϕ = ϕmax/e, i.e., where ϕ is ∼37% of ϕmax. At 100% of coupling and θL ∼ 60 cm/s, λ was ∼300 μm, which was almost fourfold longer than the length of control cells. Thus, the discrete tissue character did not interfere with the impulse propagation. However, at low coupling (Fig. 4 B), λ becomes smaller because of increased ρ. Cable theory predicts that λ is proportional to 1/√ρ. An eightfold increase of ρ would lead to an approximately threefold decrease in λ. Accordingly, at 12.5% of coupling and a velocity of ∼30 cm/s, λ was ∼100 μm in the continuous model. This was in the range of the cell length. Fig. 4 B shows that when λ was equal to or smaller than the cell length, impulse propagation became discontinuous. Low coupling, with RGJ >> RIC, does not allow a steady spatial spread of ϕ. In contrast, cells become equipotential with high ϕ gradients along GJs. Each cell acts as a minimum discrete capacitance, being depolarized and activating at once. As a result, λ cannot drop below the cell length and θ becomes lower than predicted by the cable theory (where θ is proportional to 1/√ρ). As an indicator for discontinuous propagation we calculated junctional and intracellular activation time delays (JD and ID). A high JD/ID ratio indicates discontinuity. During longitudinal propagation, control cells and cells of increased length revealed similar values: their respective JD/ID ratios were 0.7 and 0.6 at 100% of coupling and 4.6 and 3.9 at 12.5% of coupling. In contrast, wide cells showed significantly higher values: 1.4 at 100% of coupling and 13.5 at 12.5% of coupling, i.e., propagation in wide cells became discontinuous earlier. This is an additional explanation of why θL decreased most rapidly in wide cells (Fig. 3). During transverse propagation, ID was already negligible at 100% of coupling, with very high JD/ID ratios (between 27 and 37), indicating that in the transverse direction there is always discontinuous propagation. This fits with the result that θT showed a similar decay during gradual uncoupling in all cell size groups and that θT was more affected than θL by uncoupling (Fig. 3) (for details, see Fig. S4).
Figure 4.
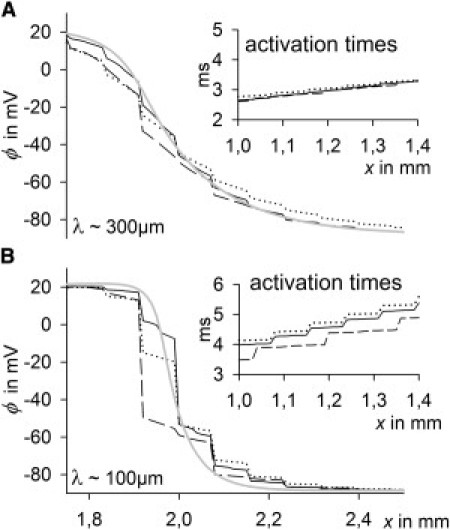
Membrane potential (ϕ) curves in the x-direction during longitudinal impulse propagation of control cells (black solid line), long cells (dashed line), and wide cells (dotted line), and a continuous model (gray solid line) in which resistivity is that same as in the control cell. gGJ was adapted in enlarged cells to obtain the same θL and θT at 100% of coupling as in the control (±5%). (Insets) Activation times in the x-direction (right), and λ (left), the space constant. (A) 100% of coupling. (B) 12.5% of coupling.
Conduction block
As described above, λ cannot drop below a certain value in a medium with discrete capacitive units, i.e., below cell length in longitudinal and below cell width in transverse propagation. A consequence would be that the whole-cell capacitance must be charged until its activation threshold, i.e., the lowest charge (Q) that must be provided by already-activated cells is proportional to the cell size. To verify this prediction, we calculated the Q accumulated by nonactivated tissue in front of the activation wave (Fig. 5 A). At 100% of coupling, equal values were obtained from different cell sizes and a continuous model with the same ρ. At 12.5% of coupling, tissue with discrete elements (cells) accumulated more Q than did the continuous model and enlarged cells accumulated more than did control cells. This was more pronounced at 1.56% of coupling. As expected, long cells, having the highest discrete capacitance/tissue width in the longitudinal direction, revealed the highest Q accumulation. Accordingly, in the transverse direction, wide cells showed the highest Q values (not shown). Coupling was further reduced until conduction block occurred (Fig. 5 B). Minimum θL and θT correlated well with the cell length and width, respectively. Block occurred at ∼5 nS in control cells, ∼10 nS in long cells, and ∼12 nS in wide cells, corresponding well to the minimum discrete capacitance (C), i.e., Ccell (113 pF, 214 pF, and 251 pF, respectively). Thus, gGJ/Ccell was constant (∼45 pS/pF). Note that in a continuous model there is no conduction block. If block occurs, a smaller value of Δx or Δy, reducing the minimum capacitive unit, always allows conduction to be maintained.
Figure 5.
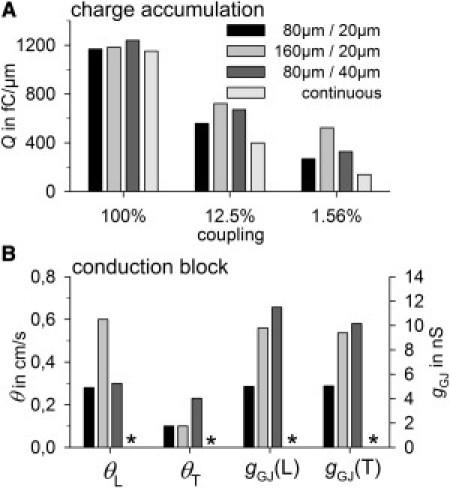
(A) Charge accumulated by nonactivated cells during longitudinal impulse propagation at 100%, 12.5%, and 1.56% of coupling in control cells (80 μm/20 μm), long cells (160 μm/20 μm), and wide cells (80 μm/40 μm), and in a continuous model with the same resistivity as the control. Note that this charge corresponds to the area under the curve (ϕ) in Fig. 4 within the x-interval from the point where ϕ ∼ –40 mV to xmax, multiplied by the specific membrane capacity (in pF/μm2). For details, see Fig. S5, Fig. S6, and Fig. S7. (B) Minimum longitudinal (L) and transverse (T) θ and minimum gGJ directly before conduction block due to uncoupling. There is no block in a continuous model (asterisks).
Reduced excitability
To analyze the influence of reduced membrane excitability on different cell sizes, gNa was reduced gradually from 100% (16 mS/μF) to 10% (1.6 mS/μF). The effect of reduced gNa on the spatial curve of ϕ is contrary to the effect of reduced gGJ. Whereas a reduction of gGJ decreased the space constant (λ), a reduction of gNa led to an increase in λ. As a consequence, the effects of low gNa did not differ from those in a continuous model and did not reveal differences in enlarged cells. Conduction block occurred at 13% of gNa in all groups (Fig. S8).
GJ lateralization
Fig. 6 shows the effects of fivefold-enhanced lateral gGJ (from 2.6 to 13 μS) under conditions of normal lateral GJ distribution (Fig. 6 A) and uniform lateral GJ distribution (Fig. 6 B). It is interesting to note that under normal distribution conditions, enhanced lateral gGJ increased not only θT, from 16 cm/s to 30 cm/s, but also θL, from 59cm/s to 63cm/s. Furthermore, it stabilized both θL and θT against uncoupling (Fig. 6 C). Under conditions of uniform GJ distribution, enhanced lateral gGJ had a negligible effect on θL but increased θT to 40 cm/s, 33% higher than with normal GJ distribution, but lower lateral gGJ (2.6 μS) effected an increase in θT of only 12% (from 16 to 18 cm/s) under uniform compared to normal distribution. Hence, at a given θT, uniform distribution is more strongly affected by gGJ. The reason is that if lateral GJs are concentrated near the cell poles (normal distribution), currents must flow in the longitudinal cell direction also to activate the downstream cell (zig-zag-like), whereas this is not necessary if GJs are uniformly distributed (for better understanding, see Fig. 6, A and B, insets, or Fig. 1). As a result, longitudinal intracellular resistance (R) contributes to total R during transverse impulse propagation. Thus, if lateral gGJ is changed, the resulting change in total R is higher in cells with uniform lateral GJ distribution.
Figure 6.
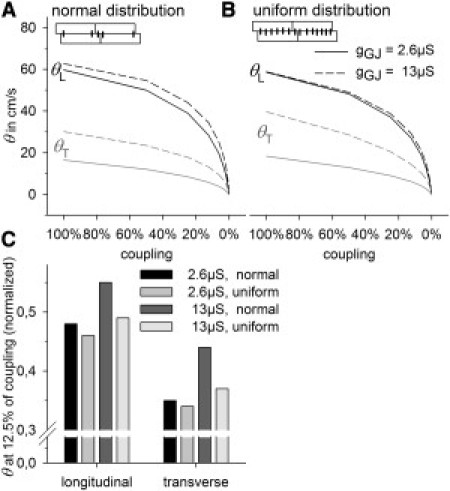
Effects of increased lateral gGJ on θL and θT. Lateral gGJ was increased from 2.6 μS to 13 μS. (A) Normal lateral GJ distribution with 90% of lateral gGJ near the poles (see inset). (B) Uniform lateral GJ distribution. (C) θL and θT at 12.5% of coupling, normalized to their values at 100% of coupling.
Microscopic effects
Increased lateral gGJ was able to stabilize θL against uncoupling (Fig. 6 C), i.e., it enhanced θL more when coupling and θL were low. This effect is a result of overlapping cells and the decrease of the space constant λ at low gGJ (Fig. 7). As a consequence of cell arrangement, the activation wave front is not completely planar (Fig. 7 A, inset). Thus, there is a ϕ gradient in the transverse direction, which is the driving force for lateral currents. A smaller λ in the longitudinal direction (low coupling) causes higher transverse ϕ gradients, i.e., the driving force for lateral currents increases as coupling decreases. Accordingly, high lateral gGJ had more of an effect at low polar gGJ (Fig. 7 B). Note that high lateral gGJ was able to partially counteract cell-size effects at low polar gGJ. At polar gGJ 0.06 μS and lateral gGJ 0 μS, control cells, long cells, and wide cells had θL values of 8 cm/s, 23 cm/s, and 4 cm/s, respectively. That is, θL was around sixfold higher in long than in wide cells. If lateral gGJ was 3.7 μS, θL values were 32 cm/s, 41 cm/s, and 23 cm/s, respectively, i.e., θL was less than twofold higher in long than in wide cells.
Figure 7.
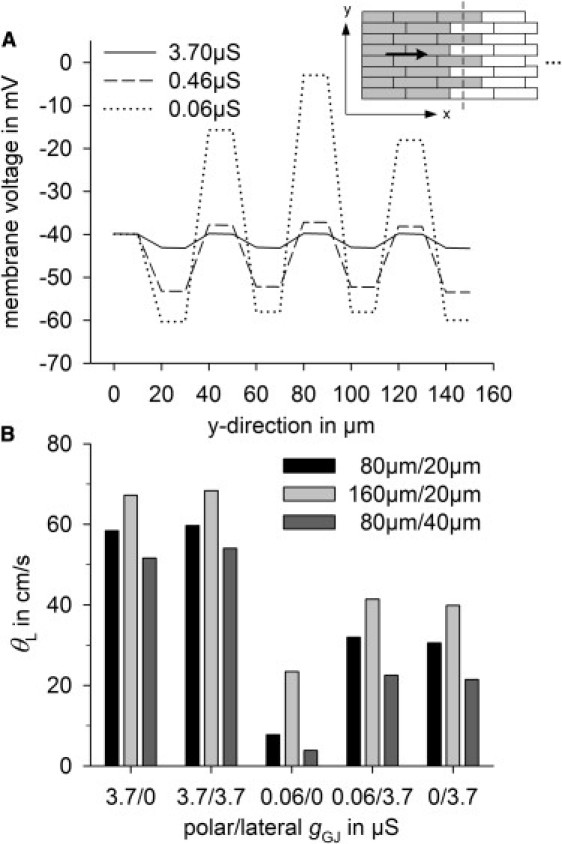
(A) Membrane potential gradients in the transverse direction (y) during longitudinal impulse propagation (see inset, where activated cells are gray) at 100%, 12.5%, and 1.56% of coupling in control cells, with polar and lateral gGJs of 3.7 μS, 0.46 μS, and 0.06 μS, respectively. (B) Effects of polar and lateral gGJ on θL in control cells (80 μm/20 μm), long cells (160 μm/20 μm), and wide cells (80 μm/40 μm).
Macroscopic effects
Similar effects of increased lateral gGJ were observed on a more macroscopic scale (Fig. 8). To simulate heterogeneity, a tissue stripe of 4 mm × 1 mm was initialized with differing polar gGJ and stimulated to obtain longitudinal propagation. In the upper half of the stripe, polar gGJ was decreased to 12.5% (0.5 μS), whereas it was normal in the lower half (3.7 μS) (Fig. 8 A). A fivefold increase in lateral gGJ from 2.6 μS to 13 μS was able to smooth the activation wavefront (Fig. 8 B) and to enhance θL in the partially uncoupled area from 33 cm/s to 42 cm/s, whereas θL was unchanged in the well-coupled area (58 cm/s). Furthermore, the tissue activation time was reduced from 10.2 ms to 8.8 ms (Fig. 8 C).
Figure 8.
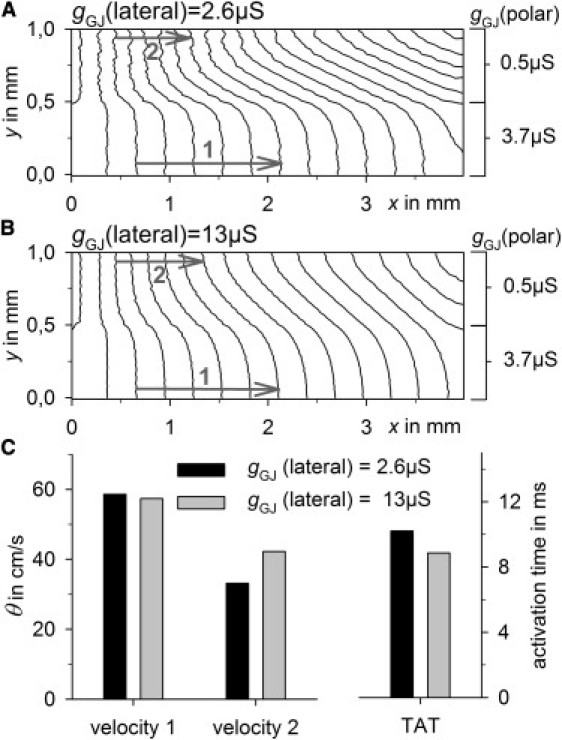
A tissue stripe of 4 mm × 1 mm was simulated with different polar gGJs (3.7 μS and 0.5 μS). θ was measured in the areas of high polar coupling (velocity 1) and low polar coupling (velocity 2) (gray arrows). (A) Isochrones of tissue activation for a lateral gGJ of 2.6 μS. (B) Isochrones of tissue activation for a lateral gGJ of 13 μS. Distance between isochrones is 0.5 ms. (C) Velocity 1, velocity 2, and tissue activation time (TAT) at normal (black) and increased (gray) lateral gGJ.
Conduction block at tissue expansion
We concluded by investigating the susceptibility of different cell sizes and degrees of GJ lateralization to conduction block at elliptic wave propagation, using a tissue expansion model (see Fig. 9 A, inset). The critical width of a cell strand at which propagation could be maintained in the expanded area, hc,was determined. Fig. 9 A plots hc at different cell sizes against gNa, showing that with increased cell diameter, hc increases from 50 μm (control) to 80 μm (+60%) if gGJ is proportional to Am (assumption I). A reduction of gNa to 40% potentiated this, leading to an hc of 120 μm in control and 220 μm (+83%) in wide cells. If gGJ was constant (assumption II), the effects were similar but less significant. In long cells, however, hc was equal to or smaller than the control cell value at both increased and constant gGJ. GJ lateralization (Fig. 9 B) was able to increase hc strikingly. Whereas at normal lateral gGJ (2.6 μS), a uniform lateral GJ distribution had no effect, at fivefold-higher gGJ (13 μS), hc increased from 50 μm to 80 μm (+60%) with normal GJ distribution and 110 μm (+120%) with uniform GJ distribution. When gNa was reduced to 40%, this effect became more pronounced: hc increased from 120 μm in control to 220 μm (+83%) with normal and 280 μm (+133%) with uniform GJ distribution.
Figure 9.
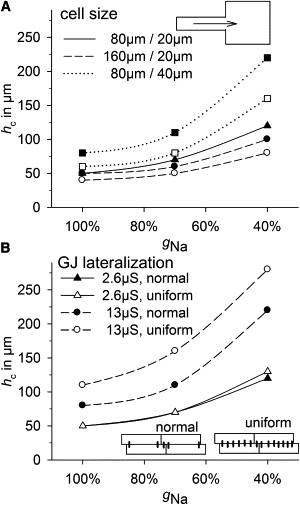
Susceptibility to conduction block determined by a tissue expansion model. The critical width (hc) of a 4-mm-long source strand at which propagation can be maintained in the 4 mm × 4 mm bulk area (see inset in A) was determined at 100%, 70%, and 40% of the maximum sodium channel conductance (gNa). (A) hc versus gNa in control cells (80 μm/20 μm; solid line), long cells (160 μm/20 μm; dashed lines), and wide cells (80 μm/40 μm; dotted lines). Assumption I (gGJ proportional to Am) is represented by solid symbols, and assumption II (constant gGJ) by open symbols. (B) hc versus gNa in control cells with normal (solid symbols) or uniform (open symbols) GJ distribution (insets) while lateral gGJ was 2.6 μS (solid lines) or 13 μS (dashed lines).
Discussion
This study demonstrates that there is a complex interplay between cell size, cell arrangement, and gap junction (GJ) distribution, showing that cell size, shape, and lateral GJ conductance (gGJ) are important factors in modulating the influence of uncoupling on conduction velocity (θ).
Cell size not only affected θ at normal conditions, but also modulated the effects of partial uncoupling on θ. Increasing the cell diameter or the cell length led to an approximately twofold-higher cell capacitance (Ccell) but resulted in significant differences in θL and θT. θL in long cells was high and showed a very slow decline during uncoupling, whereas θL in wide cells decreased much more quickly. We were able to show that there are two underlying mechanisms. On the one hand, long cells have a lower RGJ/RIC ratio, i.e., total R is less affected by increased RGJ, contrary to the situation in wide cells. On the other hand, impulse propagation in wide cells becomes discontinuous more rapidly, also reducing θL. Spach et al. (13) supposed that increasing cell size at a given gGJ increases discontinuity. The results of this study confirm that supposition for increased diameter but not for increased length. However, θT was significantly greater in wide cells, resulting in lower anisotropy and higher risk of conduction block at tissue expansion (Fig. 9), which is a result of mismatches between current sources and sinks (34,39,40). It was also shown that Ccell is directly related to the occurrence of conduction block at very low gGJ, since Ccell/gGJ was constant. The discontinuous nature of propagation does not allow capacitive units smaller than the cell size, leading to earlier block (at higher θ and at higher gGJ) in enlarged cells. In summary, small cells and cells with a high length/width ratio seem to have advantageous properties regarding θL, anisotropy (θL/θT), effects of uncoupling, and conduction blocks. This fits well with the results of Ghaly et al. (15) and Nygren et al. (27), who found a “reduced conduction reserve”, together with Cx lateralization and shorter cells, in diabetic hearts. Unfortunately, they did not discriminate between θL and θT and did not measure gGJ. This study explains that reduced conduction reserve (Fig. 3 and Table 2), since shorter cells are more affected by uncoupling due to an increased RGJ/RIC ratio. The aforementioned studies (15,27) also found higher sensitivity to reduced gNa, which cannot be explained by altered cell size (Fig. S8). Spach et al. (29) demonstrated a positive correlation between cell size and θ, whereas McIntyre et al. (16) found a negative correlation. Both can be explained by the results of this study. Increased diameter reduces θL if gGJ remains constant, whereas it increases θL if gGJ is proportional to Am, suggesting that gGJ/Ccell and Am/V (Tables 1 and 2) are more important in analyzing cardiac disease than is gGJ.
Lateral GJ distribution proved to be crucial not only for θT but also for θL. It has been shown by other authors (41,42) that a bricklike cell arrangement can increase θL, but those studies lacked a detailed explanation of why this would be the case. This study provides a systematic insight and explains this mechanism, showing that the 2D effects present in planar wave propagation cannot be taken into account by one-dimensional models (Fig. 7). High lateral gGJ can stabilize θL against uncoupling (Figs. 6 and 7) and can balance out inhomogeneities on a microscopic and a macroscopic scale, decreasing activation time (Fig. 8). Therefore, GJ lateralization may be a compensating mechanism in cardiac disease, which is often accompanied by increased inhomogeneity, e.g., due to fibrosis (29). However, there may also be negative effects of increased lateral gGJ. As indicated by Fig. 9, the susceptibility to conduction block at tissue expansion becomes significantly higher, as do the effects of reduced gNa. Cx lateralization with constant gGJ decreased θL slightly and increased θT by up to 30%, in accordance with other studies (28), but also allowed much higher θT (Fig. 6). The question remains whether lateralized Cx increases lateral gGJ. Experimental data show that Cx43 lateralization coincides with enhanced θT in human atria (Fig. 2), giving a rationale for the simulations. However, the findings discussed here refer to ventricular hypertrophy. In vivo, hypertrophy is often accompanied by fibrosis, with collagen separating lateral cell strands, reducing θT (29), i.e., θT can hardly be used as an indicator of lateral gGJ in hypertrophied ventricles. However, fibrotic strands are not evenly distributed, i.e., areas showing the changes obtained from simulations of high lateral gGJ may be adjacent to areas with transverse uncoupling.
Conclusions
The gGJ/Ccell ratio and the cell shape can be more important than absolute values, indicating a lack of experimental data regarding the combination of geometrical and functional measurements, because there are very few data on the functionality of lateralized Cx and on specific alterations of cell size, shape, and gGJ in cardiac diseases. This study required several assumptions. Some effects of increased cell size and GJ lateralization found in this study, e.g., the higher risk of conduction block in wide cells and cells with GJ lateralization, as well as the faster decline of θL in wide cells, are proarrhythmic. It is important to note that cardiac diseases often coincide with altered transmembrane currents (7–9,43), higher degrees of inhomogeneity, and fibrosis (29,44–46), which may further increase the risk of arrhythmia. Taking this into account together with cell-size effects could be the subject of further studies.
Acknowledgments
This research was supported by the Faculty of Medicine of the University of Leipzig (T.S.).
Supporting Material
References
- 1.Kozhevnikov D.O., Yamamoto K., El-Sherif N. Electrophysiological mechanism of enhanced susceptibility of hypertrophied heart to acquired torsade de pointes arrhythmias: tridimensional mapping of activation and recovery patterns. Circulation. 2002;105:1128–1134. doi: 10.1161/hc0902.104711. [DOI] [PubMed] [Google Scholar]
- 2.Tomaselli G.F., Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc. Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 3.Winterton S.J., Turner M.A., Sheridan D.J. Hypertrophy causes delayed conduction in human and guinea pig myocardium: accentuation during ischaemic perfusion. Cardiovasc. Res. 1994;28:47–54. doi: 10.1093/cvr/28.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Qin D., Zhang Z.H., el-Sherif N. Cellular and ionic basis of arrhythmias in postinfarction remodeled ventricular myocardium. Circ. Res. 1996;79:461–473. doi: 10.1161/01.res.79.3.461. [DOI] [PubMed] [Google Scholar]
- 5.Kohya T., Yokoshiki H., Kitabatake A. Regression of left ventricular hypertrophy prevents ischemia-induced lethal arrhythmias. Beneficial effect of angiotensin II blockade. Circ. Res. 1995;76:892–899. doi: 10.1161/01.res.76.5.892. [DOI] [PubMed] [Google Scholar]
- 6.Virmani R., Burke A.P., Farb A. Sudden cardiac death. Cardiovasc. Pathol. 2001;10:211–218. doi: 10.1016/s1054-8807(01)00091-6. [DOI] [PubMed] [Google Scholar]
- 7.Beuckelmann D.J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 8.Kääb S., Dixon J., Tomaselli G.F. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 9.Priebe L., Beuckelmann D.J. Simulation study of cellular electric properties in heart failure. Circ. Res. 1998;82:1206–1223. doi: 10.1161/01.res.82.11.1206. [DOI] [PubMed] [Google Scholar]
- 10.Swynghedauw B., Baillard C., Milliez P. The long QT interval is not only inherited but is also linked to cardiac hypertrophy. J. Mol. Med. 2003;81:336–345. doi: 10.1007/s00109-003-0437-8. [DOI] [PubMed] [Google Scholar]
- 11.George A.L., Jr. Common genetic variants in sudden cardiac death. Heart Rhythm. 2009;6(11, Suppl):S3–S9. doi: 10.1016/j.hrthm.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyner R.W. Effects of the discrete pattern of electrical coupling on propagation through an electrical syncytium. Circ. Res. 1982;50:192–200. doi: 10.1161/01.res.50.2.192. [DOI] [PubMed] [Google Scholar]
- 13.Spach M.S., Heidlage J.F., Barr R.C. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ. Res. 2000;86:302–311. doi: 10.1161/01.res.86.3.302. [DOI] [PubMed] [Google Scholar]
- 14.Fast V.G., Kléber A.G. Anisotropic conduction in monolayers of neonatal rat heart cells cultured on collagen substrate. Circ. Res. 1994;75:591–595. doi: 10.1161/01.res.75.3.591. [DOI] [PubMed] [Google Scholar]
- 15.Ghaly H.A., Boyle P.M., Nygren A. Simulations of reduced conduction reserve in the diabetic rat heart: response to uncoupling and reduced excitability. Ann. Biomed. Eng. 2010;38:1415–1425. doi: 10.1007/s10439-009-9855-2. [DOI] [PubMed] [Google Scholar]
- 16.McIntyre H., Fry C.H. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J. Cardiovasc. Electrophysiol. 1997;8:887–894. doi: 10.1111/j.1540-8167.1997.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 17.Kostin S., Dammer S., Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc. Res. 2004;62:426–436. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Kostin S., Rieger M., Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol. Cell. Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 19.Akar F.G., Nass R.D., Tomaselli G.F. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 20.Sepp R., Severs N.J., Gourdie R.G. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart. 1996;76:412–417. doi: 10.1136/hrt.76.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J.H., Green C.R., Severs N.J. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am. J. Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 22.Luke R.A., Saffitz J.E. Remodeling of ventricular conduction pathways in healed canine infarct border zones. J. Clin. Invest. 1991;87:1594–1602. doi: 10.1172/JCI115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters N.S., Green C.R., Severs N.J. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 24.Salameh A., Krautblatter S., Janousek J. The signal transduction cascade regulating the expression of the gap junction protein connexin43 by β-adrenoceptors. Br. J. Pharmacol. 2009;158:198–208. doi: 10.1111/j.1476-5381.2009.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostin S., Klein G., Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc. Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 26.Polontchouk L., Haefliger J.A., Dhein S. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J. Am. Coll. Cardiol. 2001;38:883–891. doi: 10.1016/s0735-1097(01)01443-7. [DOI] [PubMed] [Google Scholar]
- 27.Nygren A., Olson M.L., Shimoni Y. Propagation of the cardiac impulse in the diabetic rat heart: reduced conduction reserve. J. Physiol. 2007;580:543–560. doi: 10.1113/jphysiol.2006.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabo C., Boyden P.A. Extracellular space attenuates the effect of gap junctional remodeling on wave propagation: a computational study. Biophys. J. 2009;96:3092–3101. doi: 10.1016/j.bpj.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhein S., Hammerath S.B. Aspects of the intercellular communication in aged hearts: effects of the gap junction uncoupler palmitoleic acid. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364:397–408. doi: 10.1007/s002100100462. [DOI] [PubMed] [Google Scholar]
- 30.Luo C.H., Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ. Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 31.Sawada K., Kawamura K. Architecture of myocardial cells in human cardiac ventricles with concentric and eccentric hypertrophy as demonstrated by quantitative scanning electron microscopy. Heart Vessels. 1991;6:129–142. doi: 10.1007/BF02058278. [DOI] [PubMed] [Google Scholar]
- 32.Botchway A.N., Turner M.A., Fry C.H. Electrophysiological effects accompanying regression of left ventricular hypertrophy. Cardiovasc. Res. 2003;60:510–517. doi: 10.1016/j.cardiores.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Cascio W.E., Yan G.X., Kléber A.G. Passive electrical properties, mechanical activity, and extracellular potassium in arterially perfused and ischemic rabbit ventricular muscle. Effects of calcium entry blockade or hypocalcemia. Circ. Res. 1990;66:1461–1473. doi: 10.1161/01.res.66.6.1461. [DOI] [PubMed] [Google Scholar]
- 34.Kléber A.G., Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 35.Peters N.S. New insights into myocardial arrhythmogenesis: distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts. Clin. Sci. 1996;90:447–452. doi: 10.1042/cs0900447. [DOI] [PubMed] [Google Scholar]
- 36.Dupont E., Matsushita T., Severs N.J. Altered connexin expression in human congestive heart failure. J. Mol. Cell. Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 37.Rojas Gomez D.M., Schulte J.S., Dhein S. α-1-adrenoceptor subtype selective regulation of connexin 43 expression in rat cardiomyocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:77–85. doi: 10.1007/s00210-007-0244-9. [DOI] [PubMed] [Google Scholar]
- 38.Aiello E.A., Villa-Abrille M.C., Cingolani H.E. Myocardial hypertrophy of normotensive Wistar-Kyoto rats. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1229–H1235. doi: 10.1152/ajpheart.00779.2003. [DOI] [PubMed] [Google Scholar]
- 39.Fast V.G., Kléber A.G. Block of impulse propagation at an abrupt tissue expansion: evaluation of the critical strand diameter in 2- and 3-dimensional computer models. Cardiovasc. Res. 1995;30:449–459. [PubMed] [Google Scholar]
- 40.Rohr S., Kucera J.P., Kléber A.G. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- 41.Hubbard M.L., Ying W., Henriquez C.S. Effect of gap junction distribution on impulse propagation in a monolayer of myocytes: a model study. Europace. 2007;9(Suppl 6):vi20–vi28. doi: 10.1093/europace/eum203. [DOI] [PubMed] [Google Scholar]
- 42.Hand P.E., Griffith B.E., Peskin C.S. Deriving macroscopic myocardial conductivities by homogenization of microscopic models. Bull. Math. Biol. 2009;71:1707–1726. doi: 10.1007/s11538-009-9421-y. [DOI] [PubMed] [Google Scholar]
- 43.Chorvatova A., Snowdon R., Hussain M. Effects of pressure overload-induced hypertrophy on TTX-sensitive inward currents in guinea pig left ventricle. Mol. Cell. Biochem. 2004;261:217–226. doi: 10.1023/b:mcbi.0000028759.22274.cf. [DOI] [PubMed] [Google Scholar]
- 44.Ehrlich J.R., Nattel S., Hohnloser S.H. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J. Cardiovasc. Electrophysiol. 2002;13:399–405. doi: 10.1046/j.1540-8167.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 45.Spach M.S., Boineau J.P. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin. Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 46.Boldt A., Wetzel U., Dhein S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


