Abstract
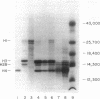
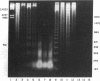
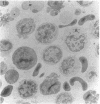
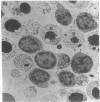
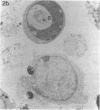
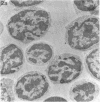
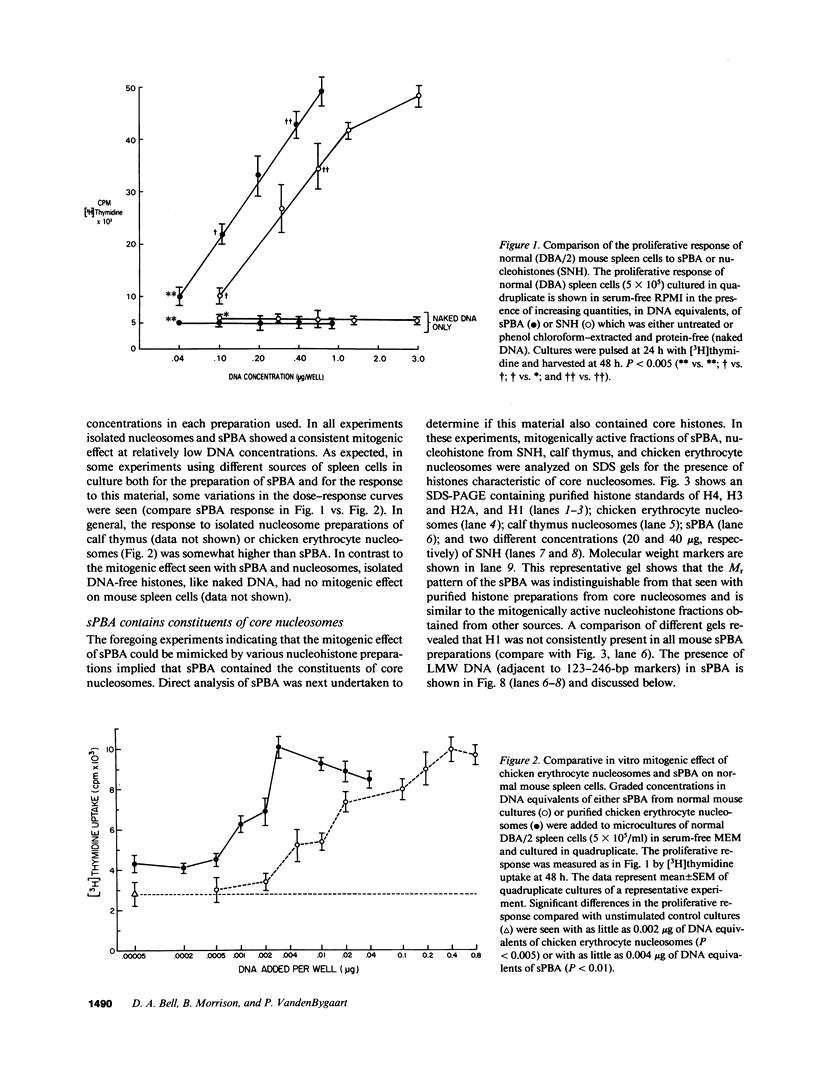
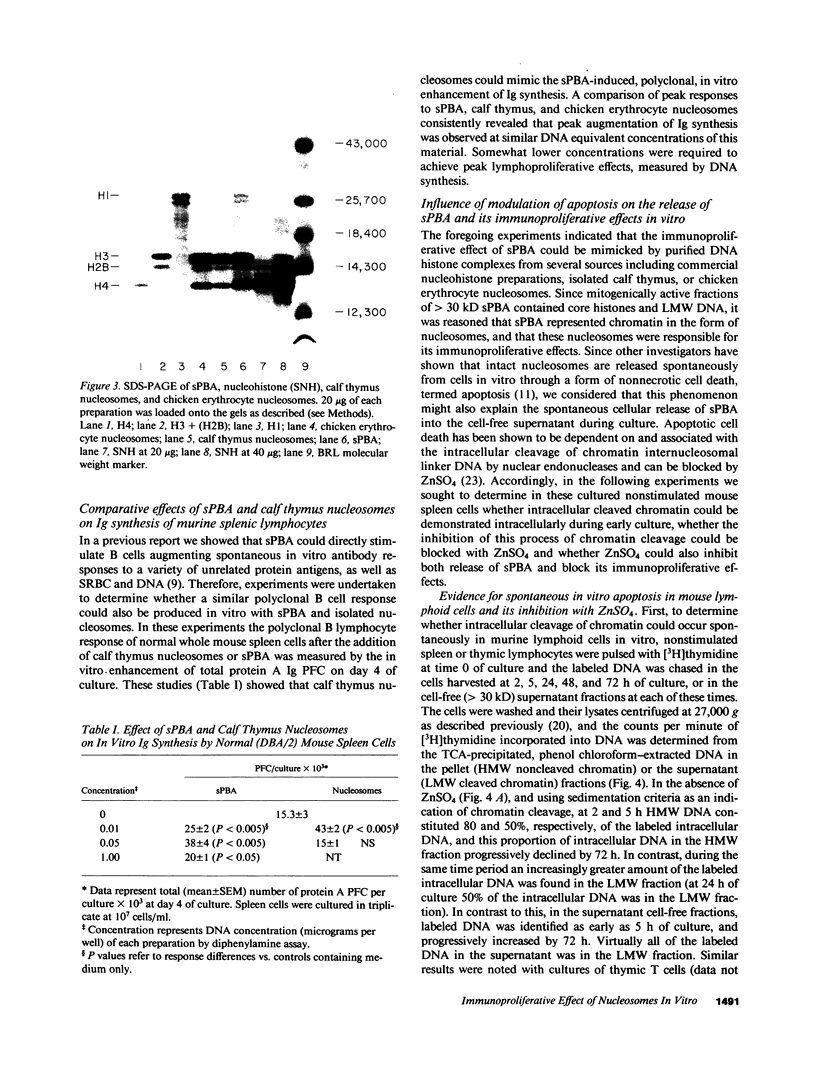
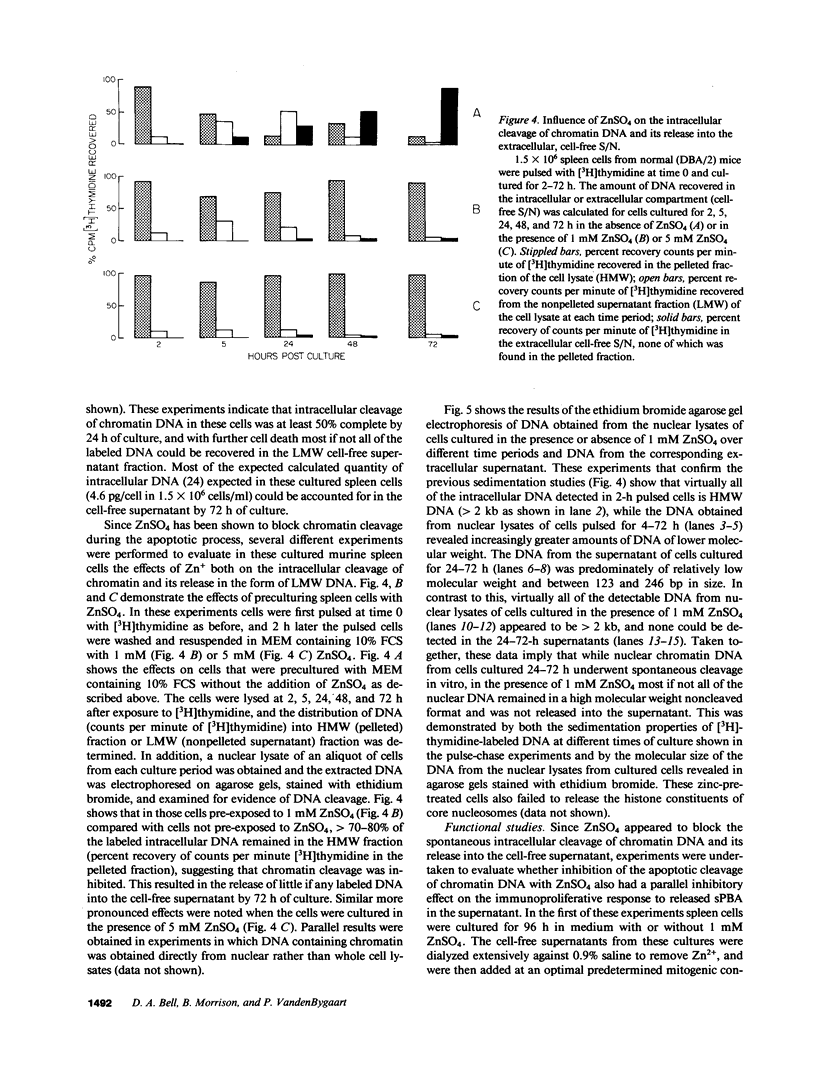
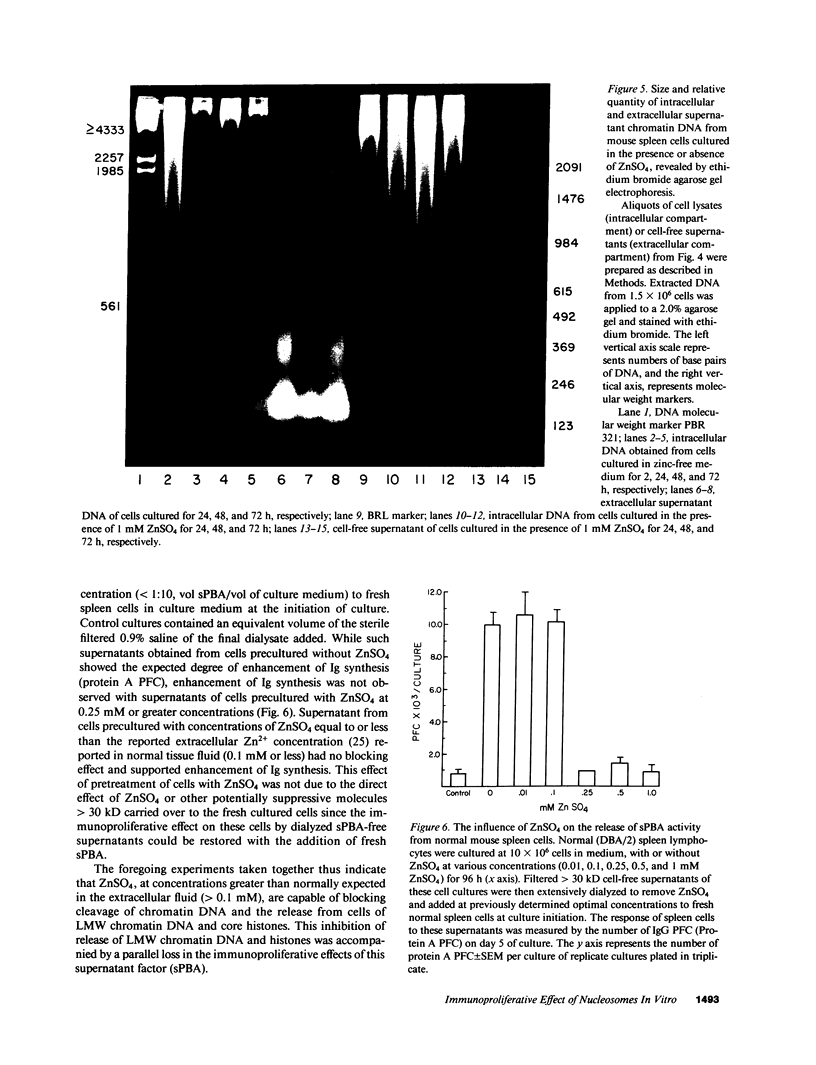
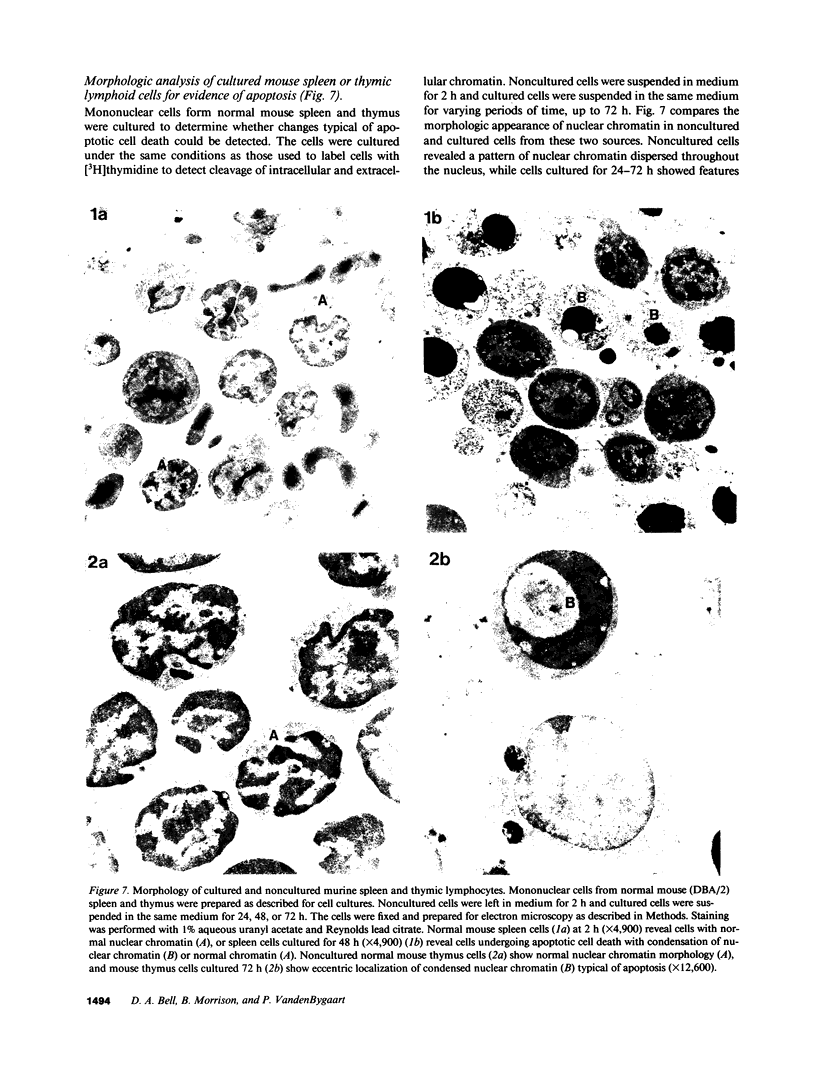
The cell-free supernatants of normal spleen and thymus lymphocytes in short-term culture release low molecular weight (LMW) DNA protein molecules that have an immunoproliferative effect (polyclonal B cell activation) in vitro. We have determined that the protein-LMW DNA complexes responsible for these effects are nucleosomal constituents of chromatin, since the mitogenically active fractions of these cell-free supernatants contain the constituents of core histones (H3, H2A, H2B, H4) together with LMW DNA (140-180 bp), and since the immunoproliferative effects of these cell-free supernatants could be mimicked by various other nucleoprotein preparations (including calf thymus and chicken erythrocyte nucleosomes). The spontaneous cellular release of cleaved chromatin constituents in vitro can be attributed to a form of programmed cell death termed apoptosis, since the cultured spleen cells exhibited (a) morphologic evidence consistent with this process by electron microscopy, and (b) evidence of intracellular cleavage of chromatin which, like apoptosis, could be blocked with ZnSO4. This resulted in inhibition of the extracellular release of nucleosomal constituents as well as the immunoproliferative effects of the cell-free supernatants. The immunoproliferative effect of nucleosomes released from cells during apoptosis could be responsible for previously observed spontaneous in vitro anti-DNA and anti-histone antibody responses of murine spleen cells, and in vivo in normal lymphoid tissues, resulting in renewed cellular proliferation after cell death. In pathological states, this could result in abnormal polyclonal B cell proliferation and autoantibody formation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. J., Bell D. A., Singhal S. K. A naturally occurring polyclonal B cell activator of normal and autoantibody responses. J Immunol. 1985 Oct;135(4):2524–2533. [PubMed] [Google Scholar]
- Bell D. A., Cairns E., Cikalo K., Ly V., Block J., Pruzanski W. Antinucleic acid autoantibody responses of normal human origin: antigen specificity and idiotypic characteristics compared to patients with systemic lupus erythematosus and patients with monoclonal IgM. J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):127–131. [PubMed] [Google Scholar]
- Bennett R. M., Gabor G. T., Merritt M. M. DNA binding to human leukocytes. Evidence for a receptor-mediated association, internalization, and degradation of DNA. J Clin Invest. 1985 Dec;76(6):2182–2190. doi: 10.1172/JCI112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. M., Kotzin B. L., Merritt M. J. DNA receptor dysfunction in systemic lupus erythematosus and kindred disorders. Induction by anti-DNA antibodies, antihistone antibodies, and antireceptor antibodies. J Exp Med. 1987 Oct 1;166(4):850–863. doi: 10.1084/jem.166.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns E., Block J., Bell D. A. Anti-DNA autoantibody-producing hybridomas of normal human lymphoid cell origin. J Clin Invest. 1984 Sep;74(3):880–887. doi: 10.1172/JCI111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns E., Kwong P. C., Misener V., Ip P., Bell D. A., Siminovitch K. A. Analysis of variable region genes encoding a human anti-DNA antibody of normal origin. Implications for the molecular basis of human autoimmune responses. J Immunol. 1989 Jul 15;143(2):685–691. [PubMed] [Google Scholar]
- Chung D. G., Lewis P. N. Intermolecular histone H4 interactions in core nucleosomes. Biochemistry. 1986 Apr 22;25(8):2048–2054. doi: 10.1021/bi00356a032. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Datta S. K., Stollar B. D., Schwartz R. S. Normal mice express idiotypes related to autoantibody idiotypes of lupus mice. Proc Natl Acad Sci U S A. 1983 May;80(9):2723–2727. doi: 10.1073/pnas.80.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978 Nov 14;17(23):4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Gohill J., Cary P. D., Couppez M., Fritzler M. J. Antibodies from patients with drug-induced and idiopathic lupus erythematosus react with epitopes restricted to the amino and carboxyl termini of histone. J Immunol. 1985 Nov;135(5):3116–3121. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Thomas J. O. Antibodies to histones in systemic lupus erythematosus: localization of prominent autoantigens on histones H1 and H2B. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7410–7414. doi: 10.1073/pnas.80.24.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Searle J. The digestion of cellular fragments within phagolysosomes in carcinoma cells. J Pathol. 1972 Sep;108(1):55–58. doi: 10.1002/path.1711080107. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayus J. L., Pisetsky D. S. Expression of a highly conserved anti-DNA idiotype in normal and autoimmune mice. Clin Immunol Immunopathol. 1985 Mar;34(3):366–378. doi: 10.1016/0090-1229(85)90185-0. [DOI] [PubMed] [Google Scholar]
- Miovic M. L., Pizer L. I. Characterization of RNA synthesized in isolated nuclei of herpes simplex virus type 1-infected KB cells. J Virol. 1980 Jan;33(1):567–571. doi: 10.1128/jvi.33.1.567-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancer L. B., Bell D. A., Singhal S. K. Induction of anti-ssDNA antibodies in normal and preautoimmune mice in vivo. J Immunol. 1980 Feb;124(2):939–946. [PubMed] [Google Scholar]
- Pancer L. B., Milazzo M. F., Morris V. L., Singhal S. K., Bell D. A. Immunogenicity and characterization of supernatant DNA released by murine spleen cells. J Immunol. 1981 Jul;127(1):98–104. [PubMed] [Google Scholar]
- Portanova J. P., Kotzin B. L., Coleman E. A., Claman H. N. Distribution of anti-histone-antibody-secreting cells in NZB/NZW mice. Cell Immunol. 1984 Sep;87(2):485–493. doi: 10.1016/0008-8749(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Purcell S. K., Hambidge K. M., Jacobs M. A. Zinc concentrations in mononuclear and polymorphonuclear leukocytes. Clin Chim Acta. 1986 Mar 16;155(2):179–183. doi: 10.1016/0009-8981(86)90281-0. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Bell D. A., Singhal S. K. Loss of self-tolerance to single-stranded deoxyribonucleic acid (sDNA) in vitro. J Immunol. 1978 Jul;121(1):29–37. [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Sperling R., Wachtel E. J. The histones. Adv Protein Chem. 1981;34:1–60. doi: 10.1016/s0065-3233(08)60517-3. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Wilson C. M., Hardin J. A. The major core histone antigenic determinants in systemic lupus erythematosus are in the trypsin-sensitive regions. FEBS Lett. 1984 Apr 9;169(1):90–96. doi: 10.1016/0014-5793(84)80295-1. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]