Abstract
Background
The current staging system of hepatocellular carcinoma established by the International Union Against Cancer and the American Joint Committee on Cancer does not necessarily predict the outcomes after hepatic resection or transplantation.
Study Design
Various clinical and pathologic risk factors for tumor recurrence were examined on 344 consecutive patients who received hepatic transplantation in the presence of nonfibrolamellar hepatocellular carcinoma to establish a reliable risk scoring system.
Results
Multivariate analysis identified three factors as independently significant poor prognosticators: 1) bilobarly distributed tumors, 2) size of the greatest tumor (2 to 5 cm and > 5 cm), and 3) vascular invasion (microscopic and macroscopic). Prognostic risk score (PRS) of each patient was calculated from the relative risks of multivariate analysis. The patients were grouped into five grades of tumor recurrence risk: grade 1: PRS = 0 to < 7.5; grade 2: PRS = 7.5 to ≤ 11.0; grade 3: PRS > 11.0 to 15.0; grade 4: PRS ≥ 15.0; and grade 5: positive node, metastasis, or margin. The proposed PRS system correlated extremely well with tumor-free survival after liver transplantation (100%, 61%, 40%, 5%, and 0%, from grades 1 to 5, respectively, at 5 years), but current pTNM staging did not.
Conclusions
1) Patients with grades 1 and 2 are effectively treated with liver transplantation, 2) patients with grades 4 and 5 are poor candidates for liver transplantation, and 3) patients with grade 1 do not benefit from adjuvant chemotherapy.
The current staging system of hepatocellular carcinoma (HCC) established by the International Union Against Cancer1 and the American Joint Committee on Cancer2 does not necessarily predict the outcomes after hepatic resection or transplantation.3,4 Although modifications of the current system have been suggested,3,5,6 they are not widely accepted because of their complexity.
Various clinical and pathologic risk factors for tumor recurrence and mortality were examined on 344 consecutive patients who underwent hepatic transplantation in the presence of HCC to establish a prognostic scoring system of HCC that can predict the prognosis after liver transplantation better than the current staging system.1,2
METHODS
During the 18-year period between 1981 and 1998, 344 consecutive patients underwent orthotopic liver transplantation in the presence of HCC at the University of Pittsburgh Medical Center. Fibrolamellar variant of HCC was excluded from this study because this variant carried a better prognosis than ordinary HCC.7 Basic immunosuppressive therapies were cyclosporine and steroid before 1989, and tacrolimus replaced cyclosporine after 1989.
All surviving patients were followed closely at the outpatient clinic and the tumor recurrence was monitored by α-fetoprotein and CT every 3 months for the first 3 years and semiannually thereafter. Suspicious lesions were biopsied for confirmation of recurrence.
There were 257 men and 87 women. Their ages ranged from 2.8 to 76.8 years (mean ± SD: 52.9 ± 13.3 years). In 317 of the 344 patients HCC developed in the cirrhotic liver, and in the remaining 27 patients it developed in the noncirrhotic liver. Hepatitis B surface antigen (HBsAg) was positive in 75 of the 344 patients and antihepatitis C virus antibody (HCV-Ab) was positive in 105 of the 221 patients tested.
The most common cause of the associated liver disease was HCV-Ab–positive cirrhosis (94 patients), followed by HBsAg-positive cirrhosis (58 patients) and alcoholic cirrhosis (49 patients). In addition there were 11 cirrhotic patients who tested positive for both HBsAg and HCV antibodies. There were 59 patients of so-called non-A, non-B cirrhosis, which was diagnosed before testing of HCV-Ab became available. Other liver diseases included inborn errors of metabolism (hemochromatosis, tyrosinemia, α-1 antitrypsin deficiency, and others; 23 patients), primary biliary cirrhosis (9 patients), autoimmune hepatitis (9 patients), biliary atresia (3 patients), and other various diseases (11 patients). The remaining 18 patients with HCC did not have any associated liver disease.
The size of HCC ranged from 0.3 to 25.0 cm (mean ± SD: 4.1 ± 4.1 cm). The number of gross tumors ranged from one to five (mean ± SD: 2.22 ± 1.47). The tumors were distributed bilobarly in 108 patients and unilobarly in 236 patients. Microscopic examination of HCC revealed well-differentiated tumor in 94 patients, moderately differentiated in 224 patients, and poorly differentiated in 26 patients. The HCC invaded vessels macroscopically in 62 patients and microscopically in 87 patients. The tumor did not show any vascular invasion in 195 patients. Regional lymph node was involved by tumor in 9 patients and was not involved in 335 patients. Metastatic lesions were present in 18 patients (diaphragm, omentum, extrahilar node) and were absent in 326 patients. Although all gross tumors were removed, postoperative pathologic examination revealed that surgical margins were microscopically involved by tumor in 18 patients and were not involved in 326 patients.
Chemotherapy was administered before transplantation in 46 patients (including the 34 patients with intraarterial chemotherapy) and it was given after transplantation but before tumor recurrence in 29 patients. The chemotherapeutic regimens were highly variable because of the prolonged study period.
The results were summarized as of April 1, 1999 with a median followup period of 91.0 ± 44.9 (SD) months. Survival curves were generated by the method of Kaplan-Meier and were compared using the log-rank test. A multivariate stepwise Cox’s regression analysis (backward elimination method) was performed to identify the factors that were independently associated with mortality and tumor recurrence. A two-sided p value of < 0.05 was considered statistically significant.
RESULTS
Overall patient and tumor-free survivals
As of April 1, 1999, 145 patients were alive, free of HCC; 5 patients were alive with recurrent HCC; 104 patients were dead without HCC; 78 patients were dead with recurrent HCC; and 12 patients were lost to followup (they were free of recurrence at the last followup). One-, 3-, 5-, and 10-year overall patient survivals were 73.0 ± 2.4% (SE), 58.8 ± 2.8%, 49.4 ± 3.0%, and 32.7 ± 3.9%, respectively, and those of tumor-free survivals were 81.9 ± 2.3%, 73.0 ± 2.8%, 68.7 ± 3.0%, and 64.4 ± 4.0%, respectively.
Clinical and pathologic risk factors of mortality and tumor recurrence
Tumor recurrence developed within 2 years after transplantation in all of the 26 patients who had lymph node involvement, metastasis, or positive microscopic surgical margins. These 26 patients with lethal risk factors were excluded from additional analysis but they were considered as a lethal risk group, or noncandidates for transplantation.
The results of univariate analysis on the 11 risk factors in the remaining 318 patients (excluding 26 patients with lethal factors) are shown in Table 1. Tumor number, size, lobar distribution, vascular invasion, and differentiation were significant risk factors of both patient survival and tumor-free survival. Positive HBsAg was a significant risk factor of tumor-free survival, but it was not a significant risk factor of patient survival. Anecdotally, positive HCV-Ab was a significant good prognostic factor both in patient and tumor-free survival. Additional examination revealed that significantly more patients with positive HBsAg were included in the HCV-Ab–negative patients than in the HCV-Ab–positive patients (p < 0.03). When the patients with positive HBsAg were excluded, both patient and tumor-free survivals were similar between HCV-Ab–positive and –negative patients. The absence of cirrhosis and the use of neoadjuvant/adjuvant chemotherapy were significant poor prognostic factors. The HCCs in the noncirrhotic liver and the HCCs treated with chemotherapy were disproportionately more advanced than those in the cirrhotic liver or those without chemotherapy, respectively.
Table 1.
Influences of Various Clinicopathologic Risk Factors on Overall Patient and Tumor-Free Survival (Univariate Analysis)
| Risk factor | n | Patient survival (y) Mean ± SE |
p Value | Tumor-free survival (y) Mean ± SE |
p Value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 235 | 6.9 ± 0.5 | NS | 10.3 ± 0.5 | NS |
| Female | 83 | 8.6 ± 0.9 | 12.5 ± 0.8 | ||
| Age (y) | |||||
| ≤65 | 277 | 7.8 ± 0.5 | NS | 11.7 ± 0.5 | NS |
| > 65 | 41 | 5.4 ± 0.7 | 8.2 ± 0.8 | ||
| HBsAg | |||||
| Positive | 66 | 5.4 ± 0.6 | NS | 6.8 ± 0.7 | < 0.002 |
| Negative | 252 | 7.5 ± 0.5 | 12.6 ± 0.5 | ||
| HCV antibody | |||||
| Positive | 101 | 7.0 ± 0.4 | < 0.001 | 9.0 ± 0.3 | < 0.0001 |
| Negative | 111 | 5.2 ± 0.4 | 6.8 ± 0.3 | ||
| No. of tumors | |||||
| 1–2 | 221 | 9.4 ± 0.9 | 13.9 ± 0.5 | ||
| 3–4 | 68 | 7.1 ± 0.6 | < 0.00001 | 7.8 ± 1.1 | < 00001 |
| ≥ 5 | 29 | 4.2 ± 1.3 | 3.9 ± 1.0 | ||
| Distribution | |||||
| Unilobar | 232 | 8.8 ± 0.6 | < 0.0001 | 14.0 ± 0.5 | < 0.00001 |
| Bilobar | 86 | 4.3 ± 0.5 | 5.0 ± 0.6 | ||
| Tumor size | |||||
| ≥ 2 cm | 149 | 7.2 ± 0.6 | 11.6 ± 0.5 | ||
| 2–5 cm | 108 | 8.0 ± 0.8 | < 0.007 | 10.9 ± 0.8 | < 0.00001 |
| > 5 | 61 | 4.9 ± 0.8 | 6.4 ± 1.1 | ||
| Vascular invasion | |||||
| V0 (none) | 195 | 9.1 ± 0.6 | 14.8 ± 0.5 | ||
| V1 (micro) | 80 | 7.8 ± 0.9 | < 0.00001 | 10.0 ± 0.9 | < 0.00001 |
| V2 (macro) | 43 | 1.7 ± 0.3 | 1.5 ± 0.4 | ||
| Differentiation | |||||
| Good | 94 | 9.4 ± 0.9 | 14.0 ± 0.7 | ||
| Moderate | 205 | 7.1 ± 0.6 | < 0.002 | 10.9 ± 0.6 | < 0.002 |
| Poor | 19 | 4.2 ± 1.3 | 7.0 ± 2.1 | ||
| Cirrhosis | |||||
| Yes | 300 | 8.0 ± 0.5 | < 0.02 | 12.1 ± 0.5 | < 0.0001 |
| No | 18 | 3.8 ± 0.5 | 4.2 ± 1.3 | ||
| Chemotherapy | |||||
| Yes | 64 | 5.9 ± 0.6 | NS | 6.6 ± 0.7 | < 0.002 |
| No | 254 | 7.8 ± 0.5 | 12.8 ± 0.5 |
HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
The results of multivariate analysis of the factors that were significant by univariate analysis for tumor-free survival and those of patient survival are shown in Tables 2 and 3, respectively, with relative risk and 95% confidence interval. For tumor-free survival, bilobar tumors, large tumor size (> 2 cm, > 5 cm), and the presence of vascular invasion (micro- and macroscopic) were independently significant poor prognostic factors. For patient survival the number of tumors and vascular invasion were independently significant prognostic factors.
Table 2.
Risk Factors of Tumor-Free Survival Found to Be Significant (p < 0.05) by Backward Cox Proportional Hazard Regression (Multivariate Analysis)
| Variable | RR | 95% CI |
|---|---|---|
| Bilobar tumor (p < 0.0001) | 3.1 | [1.7,5.4] |
| Compared with unilobar | ||
| Tumor size (p < 0.0003) | ||
| Compared with size ≤ 2 cm | ||
| 2–5 cm | 4.5 | [1.5, 13.0] |
| > 5 cm | 6.7 | [2.2, 19.9] |
| Vascular invasion (p < 0001) | ||
| Compared with none | ||
| Micro | 4.4 | [2.1, 9.5] |
| Macro | 15.0 | [6.7, 33.8] |
CI, confidence interval; RR relative risk.
Table 3.
Risk Factors of Overall Survival Found to Be Significant (p < 0.05) by Backward Cox Proportional Hazard Regression (Multivariate Analysis)
| Variable | RR | 95% CI |
|---|---|---|
| No. of tumors (p < 0.02) | ||
| Compared with 1–2 | ||
| 3–4 | 1.4 | [0.9, 2.1] |
| > 4 | 2.1 | [1.3, 3.5] |
| Vascular invasion (p < 0.0001) | ||
| Compared with none | ||
| Micro | 1.1 | [0.7, 1.7] |
| Macro | 3.5 | [2.2, 5.4] |
CI, confidence interval; RR relative risk.
Risk scores for tumor recurrence
Using the relative risks obtained by the Cox regression analysis (Table 2), the risk score of tumor recurrence was calculated additively on each patient who had negative surgical margins, no lymph node invasion, or no metastasis. The risk scores ranged from 0 (none of the three risk factors present) to 24.8 (all of the three risk factors present at the highest level) and was a monotonous function of the likelihood of tumor recurrence. The mean risk score was 6.8 ± 7.4 (SD). The risk scores were computed and the various groups of risk scores were searched to fulfill that the number of groups with distinctly different outcomes was maximized while the variance of outcomes inside each group was minimized. The following five groups of risk scores satisfied the criteria mentioned earlier: grade 1: risk score 0 to < 7.5; grade 2: risk score 7.5 to ≤ 11.0; grade 3: risk score > 11.0 to 15.0; and grade 4: risk score ≥ 15.0. The final group (grade 5) consisted of the patients who had positive surgical margins, lymph node, or distant metastasis (Table 4).
Table 4.
Prognostic Risk Score Grading for Tumor Recurrence
| Grade 1 | 0 ≤ Risk score < 7.5 |
| Grade 2 | 7.5 ≤ Risk score ≤11.0 |
| Grade 3 | 11.0 < Risk score <15.0 |
| Grade 4 | Risk score ≥ 15.0 |
| Grade 5 | Positive margin, lymph node, or metastasis |
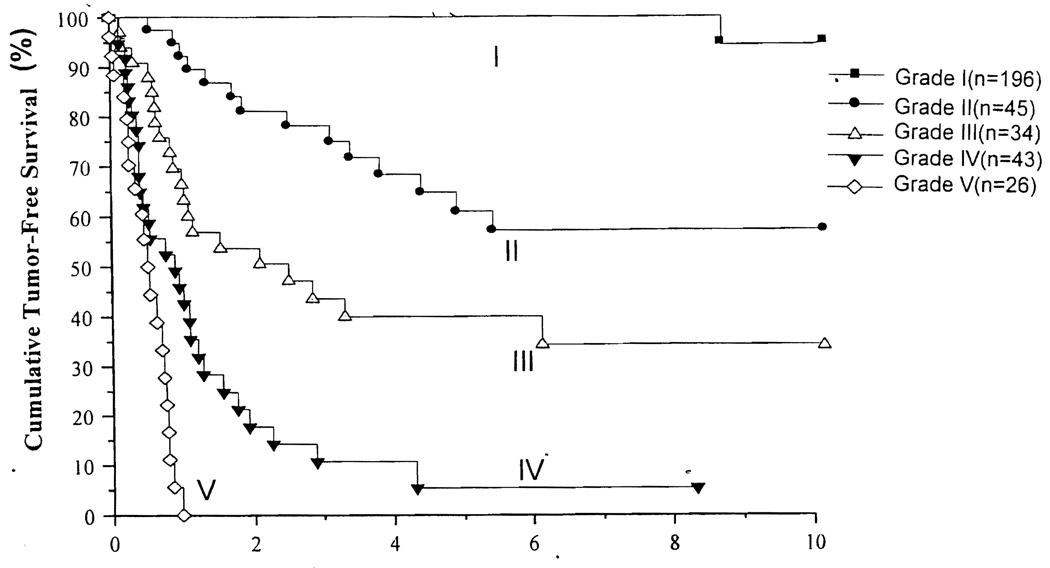
The actuarial tumor-free survivals of the five grades of risk scores are shown in Figure 1. Five-year tumor-free survivals of grades 1 to 5 were 100%, 61%, 40%, 5%, and 0%, respectively. It should be noted that the differences in survivals are statistically significant in any comparison.
Figure 1.
Actuarial tumor-free survival stratified by proposed risk score grading.
DISCUSSION
Various risk factors for tumor recurrence and mortality after hepatic resection and transplantation for HCC have been identified in numerous reports,8–19 but there has been no prognostic scoring system that can reliably predict HCC recurrence after surgical treatment. The development of such a system for subtotal hepatectomy is more complex than that for total hepatectomy with replacement (orthotopic liver transplantation), because in the former de novo HCCs and metastatic HCCs constitute tumor recurrence. After liver replacement de novo HCCs are extremely rare and the prediction of tumor recurrence should be possible only from the metastic potential of the treated HCCs.
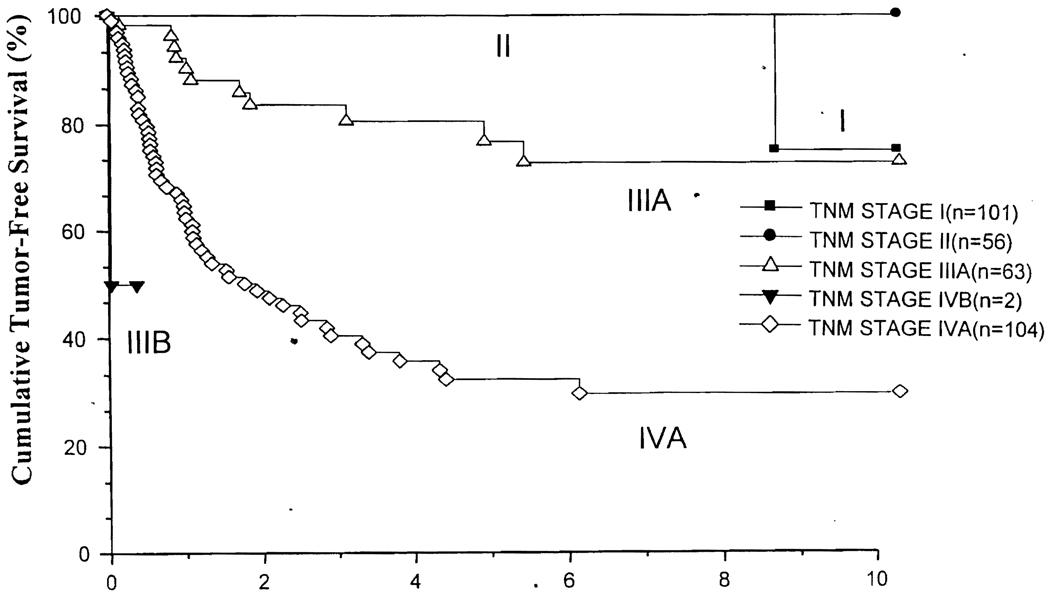
In this study various clinical and pathologic risk factors were examined in 344 patients and the three risk factors were found to be independently significant for tumor recurrence. The risk score was calculated in each patient by adding the relative risk of the Cox proportional model, and the scores were grouped into four grades. An additional group consisted of the patients who had positive surgical margin, lymph node, or metastasis. The tumor-free survivals of these five grades of risk scores are significantly different from each other (Fig. 1). Tumor-free survivals of our 326 patients with negative surgical margins are stratified by pTNM stages1, 2 and are shown in Figure 2. It is quite evident that pTNM staging does not correlate with tumor-free survival after liver transplantation: the tumor-free survivals of patients in stages I to III-A were similar.
Figure 2.
Actuarial tumor-free survival stratified by pTNM stages.
Our proposed scoring system must be verified by a large number of patients at other major centers. This type of clinical investigation is the essential next step after identifying independent risk factors to correctly select the patients for transplantation and also to determine the true effect of chemotherapy in the prevention of tumor recurrence. Our results indicate that 1) patients with grades 1 and 2 are effectively treated with liver transplantation, 2) patients with grades 4 and 5 are poor candidates for liver transplantation, 3) patients with grade 1 do not benefit from adjuvant chemotherapy, and 4) patients with grades 2 and 3 should be studied systematically to determine the effect of adjuvant chemotherapy. Our analysis of the 79 patients with grades 2 and 3 did not reveal any significant effect of neoadjuvant and adjuvant chemotherapy on tumor recurrence.
Footnotes
No competing interests declared.
References
- 1.TNM classification of malignant tumors. 5th ed. New York: Wiley-Liss; 1997. International Union Against Cancer; pp. 66–69. [Google Scholar]
- 2.AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven; 1997. American Joint Committee on Cancer; pp. 101–105. [Google Scholar]
- 3.Izumi R, Shimizu K, Tohru II, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the rumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–1577. doi: 10.1002/hep.510270616. [DOI] [PubMed] [Google Scholar]
- 5.Marsh JW, Dvorchik I, Subotin M, et al. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444–450. doi: 10.1002/hep.510260227. [DOI] [PubMed] [Google Scholar]
- 6.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pTNM classification system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. doi: 10.1002/(sici)1097-0142(20000201)88:3<538::aid-cncr7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Pinna A, Iwatsuki S, Randall L, et al. Treatment of fibrolamellar hepatoma with subtotal hepatectomy or transplantation. Hepatology. 1998;26:877–883. doi: 10.1002/hep.510260412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg. 1988;207:373–379. doi: 10.1097/00000658-198804000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagorney DM, van Heerden JA, Elstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740–749. [PubMed] [Google Scholar]
- 10.Ringe B, Wittelkind C, Bechstein WO, et al. The role of liver transplantation in hepatobiliary malignancy. Ann Surg. 1989;209:88–98. doi: 10.1097/00000658-198901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwatsuki S, Starzl TE, Sheaham DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–229. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–15l. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Liver Cancer Study Group of Japan. Predictive factors for long-term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer. 1994;74:2772–2780. doi: 10.1002/1097-0142(19941115)74:10<2772::aid-cncr2820741006>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Fuster J, Garcia-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996;223:297–302. doi: 10.1097/00000658-199603000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto J, Kosuge T, Takayama T, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219–1222. [PubMed] [Google Scholar]
- 16.Otto G, Heushen U, Hofman WJ, et al. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinomas: a retrospective study. Ann Surg. 1998;227:424–432. doi: 10.1097/00000658-199803000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau H, Fan ST, Ng IO, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer. 1998;83:2303–2311. [PubMed] [Google Scholar]
- 18.Poon RTP, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto J, Iwatsuki S, Kosuge T, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151–1158. doi: 10.1002/(sici)1097-0142(19991001)86:7<1151::aid-cncr8>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]