In doing liver transplantation, it is important to know the effect of immunosuppressive agents on hepatic regeneration. In many such patients, livers are transplanted that are not the ideal size for a particular recipient. Accommodation of these organs to recipient size depends on poorly understood growth homeostasis which conceivably could be altered by the immunosuppressive agents. In fact, it has been reported that the regeneration response is inhibited by azathioprine and adrenal cortical steroids. 1,2
CyA AND REGENERATION
Largely because of such concerns, Makowka et al3 tested hepatic generation after partial hepatectomy in rats treated with CyA and reported that the regeneration response was actually greater than normal. This finding has been confirmed by several investigators.4–6
FK 506 AND REGENERATION
More recently, we have demonstrated an even more dramatic augmentation of hepatic regeneration by FK 506. CyA and FK 506 have completely different molecular structures (Fig 1), but they have in common the relatively specific inhibition of the T cell component of the immune system.7,8 Because of this similar mechanism. we have suggested that hepatic regeneration may be subject to immune modulation.6
Fig 1.
Structural formulas of FK 506 and CyA.
The Immune modulation hypothesis was supported by the data summarized in Fig 2. In essence, the increase in DNA and mitotic activity which is characteristic of a 70% hepatic resection in rats was increased significantly with CyA and nearly doubled when FK 506 was administered for 3 days preoperatively and for a final time just after completing the hepatic resection.
Fig 2.
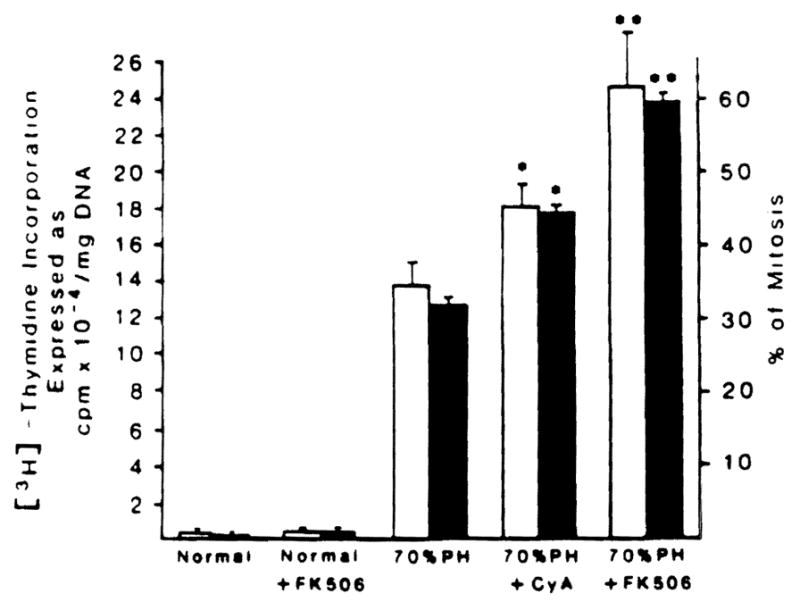
[3H]-thymidine incorporation and percentage of mitosis in normal and 70% hepatectomized rats treated or not treated with FK 506 and CyA. Control and immunosuppressive drug-treated animals were given intramuscular injections of vehicle and CyA and FK 506, 1 mg/kg body weight, respectively, for 3 days before surgery and again just after completing hepatic resection. The values represented by the bars are the means from at least 15 rats ± SD. *Significantly different from 70% hepatectomized rats (P< 0.05). **Significantly different from 70% hepatectomized rats (P < 0.01) and CyA-treated 70% hepatectomized rats (P < 0.05).
IN VITRO MECHANISMS
What was the explanation for these curious results? The first step was to establish pure hepatocyte cultures9 and to determine if the resting rate of mitoses was altered by the direct addition of CyA or FK 506 to the medium. A wide range of doses of both drugs was completely without effect (Fig 3).
Fig 3.
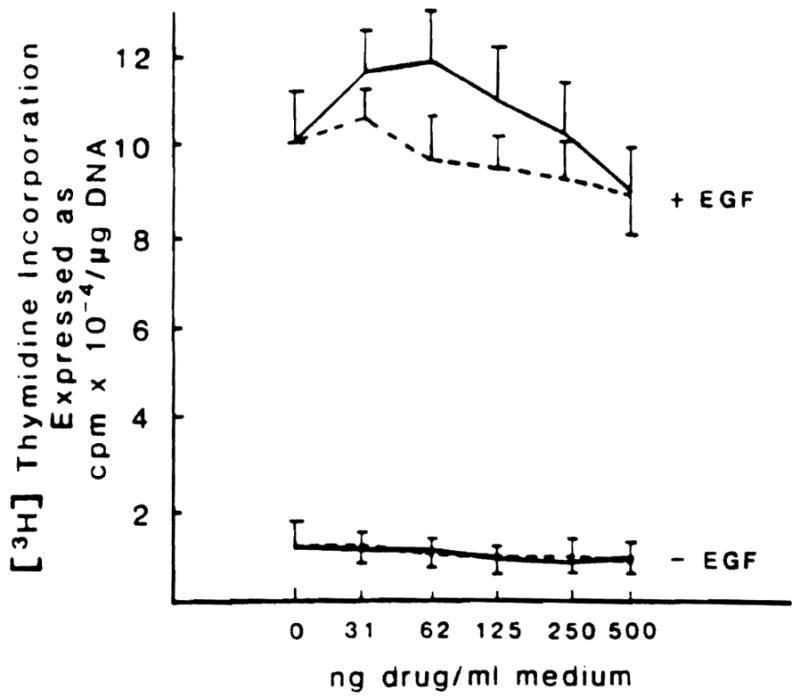
Effect of different doses of FK 506 and CyA on DNA synthesis in hepatocytes cultured in presence or not of epidermal growth factor (EGF). (Solid line, FK 506; broken line, CyA.) Hepatocytes were isolated as previously described.9 They were plated at a density of 1.5 × 105 cell/dish in 1.5 ml of minimum essential medium (MEM) + 5% fetal calf serum + insulin (10−7 mol/L) and maintained at 37°C in a 5% CO2 atmosphere. Following a 3-hour attachment period, the medium was substituted with 1.5 ml of fresh MEM and insulin with or without EGF (10 mg/ml). DNA content and [3H]-thymidine incorporation were determined as previously described.6 When added to the incubation medium, FK 506 and CyA vehicles were used at concentrations <0.2 μl/ml, which did not affect hepatocyte proliferation (data not shown). The values reported represent the means of five experiments ± SD.
Hepatocyte cultures were now used for additional experiments under two different conditions that are known to increase the basal rate of mitoses. In the first experiment, epidermal growth factor was added to the hepatocyte culture which, as expected.10 increased the basal mitotic rate approximately 10-fold. The addition of CyA and FK 506 had no further effect (Fig 3).
In the second experiment, normal rat serum, which in tissue culture systems is mitogenic in low doses.9 was added to the hepatocyte culture in graded quantities (Table 1). The expected increase in mitoses was seen at first with an inhibition of this effect as larger volumes of normal rat serum were added (Table 1). As in the first experiment, there was no augmentation effect with CyA on FK 506.
Table 1.
Effect of CyA and FK 506* on Hepatocyte DNA Synthesis.
| Group No. | Addition to the Incubation Medium (MEM + Insulin) | [3H]-Thymidine incorporation (cpm × 10−3/mg DNA) |
|||||
|---|---|---|---|---|---|---|---|
| % Serum | 5 | 10 | 20 | 30 | 40 | ||
| 1 | NRS | 110 ± 15 | 88 ± 9 | 71 ± 14 | 53 ± 5 | 63 ± 7 | |
| 2 | NRS + CyA | 92 ± 10 | 82 ± 10 | 56 ± 8 | 57 ± 7 | 38 ± 7 | |
| 3 | NRS + FK 506 | 86 ± 11 | 83 ± 4 | 59 ± 9 | 52 ± 6 | 36 ± 7 | |
| 4 | Vehicle-treated rat serum | 130 ± 18 | 124 ± 10 | 106 ± 6 | 82 ± 10 | 68 ± 8 | |
| 5 | CyA-treated rat serum | 140 ± 7 | 167 ± 11* | 196 ± 12* | 170 ± 11* | 114 ± 10* | |
| 6 | FK 506.treated rat serum | 154 ± 16 | 306 ± 28† | 286 ± 26† | 244 ± 17† | 225 ± 15† | |
Note: Culture conditions are the same as described in Fig 3, the only difference being that rat serum was used as mitogen instead of epidermal growth factor. In groups 2 and 3, the concentration in the incubation medium of FK 506 and CyA vehicles was <0.2 mg/ml; this concentration did not affect hepatocyte proliferation (data not Shown). The animals used in groups 4–6 to provide serum were treated as described in Fig 2, the difference being that on the fourth day, they only received the last injection and the blood was drawn 2 hours later. The values reported are the means of five experiments ± SD.
Abbreviations: MEM, minimum essential medium: NRS, normal rat serum.
Significantly different from group no. 4 (P < 0.05).
Significantly different from groups no. 4 (P < 0.02) and 5 (P < 0.01).
From this we concluded that neither CyA nor FK 506 had a direct effect on hepatocyte mitoses, no matter whether the mitotic index was at a resting or heightened level at the time of addition of the drug to the medium.
In contrast, the serum from animals treated for 4 days with therapeutic doses of CyA and FK 506 had a pronounced effect on hepatocyte cultures (Table 1). These sera caused an increase in mitosis far exceeding that seen with comparable amounts of normal rat serum. The stimulatory potency of the rat serum was greater if the serum donors were treated with FK 506 than if therapy was with CyA.
ARE THESE GROWTH EFFECTS LIVER SPECIFIC?
We questioned whether CyA and FK 506 altered the regeneration of other organ systems. To test this possibility, DNA synthesis was measured in kidneys before and after right nephrectomy. The basal level of DNA synthesis was essentially doubled in the contralateral kidney after right nephrectomy, but this was not further increased by the treatment with FK 506 that enhanced liver regeneration (Table 2).
Table 2.
[3H]-Thymidine Incorporation In Kidney and Small intestine From Normal. Nephrectomized, or Small Intestine-Resected Rats Treated or Not With FK 506
| Group No. | Treatment (IM) | Surgery | [3H]-Thymidine Incorporation (cpm/mg DNA) |
|---|---|---|---|
| 1 | Vehicle | No | 2,930 ± 650 |
| 2 | Vehicle | Unilateral nephrectomy | 5,012 ± 559* |
| 3 | FK 506 | Unilateral nephrectomy | 4,971 ± 702* |
| 4 | Vehicle | No | 13,420 ± 16,216 |
| 5 | Vehicle | 40% small intestine resection | 73,711 ± 17,071† |
| 6 | FK 506 | 40% small intestine resection | 79,823 ± 30,973† |
Note: Surgical procedures have been described elsewhere.11 The treatment follows the same scheme used for the partial) hepatectomized animals described in Fig 2. The values reported are the means from five rats ± SD.
Significantly different from thymidine incorporation in kidneys from non-operated animals (P < 0.05).
Significantly different from thymidine incorporation in the small intestine from non-operated animals (P < 0.05).
With resection of 50% of the small bowel, DNA synthesis in the residual small bowel tripled, as has been described by others.11 This powerful regeneration response was neither increased or decreased by treatment of these animals with FK 506 (Table 2).
DISCUSSION AND CONCLUSIONS
These observations could have a deeper biologic significance than those practical questions which prompted us to begin these studies. It remains possible that the immune system has a “braking” effect on hepatocyte cell mitosis, and that this is unmasked by powerful T cell-specific immunosuppression. From the work presented here, it is probable that this control is at least relatively liver-specific: at least it could not be identified in regenerating kidney or intestine. The fact that CyA and FK 506 did not have a direct action on cultured hepatocytes. but that the serum of immunosuppressed animals was stimulatory to these same cell cultures, suggests that some circulating substance is induced by CyA and FK 506. CyA and FK 506 act by inhibiting IL2 production and binding. 7,8 It is conceivable that by inhibition of this second signal, both drugs could interrupt the secretion of liver regulatory factors, either by as yet unknown immune mechanisms or, possibly, by pathways that are not directly connected with the immune system.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD, and Grant 87/01291-44 from Consiglio Nazionale delle Ricerche, Italy.
References
- 1.Ganzalez EM, Krejczy K, Malt RA. Surgery. 1970;68:254. [PubMed] [Google Scholar]
- 2.Guzek JW. Nature. 1964;201:930. doi: 10.1038/201930a0. [DOI] [PubMed] [Google Scholar]
- 3.Makowka L, Svanas G, Esquivel C, et al. Surg Forum. 1986;37:352. [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn D, Lai HS, Romovacek H, et al. Transplant Proc. 1988;20(suppl 3):850. [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YI, Calne RY, Nagasue N. Surg Gynecol Obstet. 1988;166:317. [PubMed] [Google Scholar]
- 6.Francavilla A, Barone M, Todo S, et al. Lancet. 1989;2:1248. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto T, Kino T, Hatanaka H, et al. Transplant Proc. 1987;19:4. [PubMed] [Google Scholar]
- 8.Borel JF. In: Cyclosporine A: Proceedings of an International Conference on Cyclosporine A. White DJG, editor. Amsterdam: Elsevier; 1982. p. 5. [Google Scholar]
- 9.Michalopoulos G, Cianciulli HD, Novotny R, et al. Cancer Res. 1982;42:4673. [PubMed] [Google Scholar]
- 10.Richman RA, Cleus TH, Phiekis SJ, et al. Proc Natl Acad Sci USA. 1976;73:3589. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obertop H, Nundy S, Malamud D, et al. Gastroenterology. 1977;72:267. [PubMed] [Google Scholar]