Fulminant hepatic failure (FHF) is the clinical syndrome associated with acute massive necrosis of the liver that has been functioning normally before the onset of illness. It is characterized by increasing jaundice, decrease in the size of the liver, fetor hepaticus, coagulopathy, renal failure, hepatic encephalopathy, and eventual death. The mortality rate for FHF when associated with grade IV coma is 80 to 95%.1,2 The symptoms of hepatic failure (especially encephalopathy) develop within eight weeks from the onset of illness.
Various modalities of medical treatment have been applied for FHF, but none of these has had a significant impact on the lethal prognosis.3–5 With the significant improvement in the results of liver transplantation over the past several years,6,7 liver replacement has become a practical therapeutic option for advanced FHF.8,9 We report here our experience with liver transplantation for FHF in 42 patients under the cyclosporine (CsA)/steroid immunosuppressive therapy.
CASE MATERIAL AND METHODS
Between January 1980 and December 1987, 1061 patients underwent orthotopic liver transplantation under CsA/steroid therapy. Of these, 42 patients (4%) had FHF of various causes. There were 21 males and 21 females. The ages of the patients ranged from eight months to 62 years, with a mean of 22.9 years (SD 15.9 years). FHF was suspected from the rapidly progressive clinical course (maximum of eight weeks from the onset of the illness to obvious liver failure) and was confirmed by histologic examination of the excised liver. The patients with histologic evidence of chronic liver disease (significant fibrosis and early cirrhosis) and/or a clinical course of longer than eight weeks were excluded from this study. Also excluded were the patients with the fulminant form of Wilson’s disease (subacute Wilson’s disease), acute exacerbation of previously unrecognized chronic liver disease, and retransplants.
The patients with FHF were closely observed and managed preoperatively in the intensive-care unit. The clinical course, signs and symptoms of hepatic failure, blood chemistry, coagulation parameters, the size of the liver, neurologic tests (CT, EEG, intracranial pressure), and liver biopsy findings (where feasible) were followed closely. The decision to proceed with OLTx was made by a group of experienced physicians and surgeons, all of whom agreed upon the inevitable death of the patient without liver replacement.
At the time of transplantation 15 patients (36%) were in grade I or II coma, 13 (31%) in grade III coma, and 14 (33%) in grade IV coma. All patients had uncorrectable profound coagulopathy, even after replacement therapy (prothrombin time > 20 seconds). Ten patients had clinical bleeding (from the GI tract and/or from needle puncture sites), 14 had renal failure, 18 required respiratory support, seven had hypoglycemia, four had sepsis (positive blood culture), three had hemodynamic instability requiring large amounts of vasopressor support, and five had uncorrectable metabolic acidosis.
The survival rates as of August 1, 1988, were calculated by the life table method of Kaplan-Meyer. The statistical comparisons were made by the chi-square test and the Cox-Mantel method. The p value of <0.05 was considered statistically significant.
RESULTS
Causes of FHF and Histopathology
The causes of FHF were hepatitis B in eight patients, toxic hepatitis in nine patients, so-called non-A, non-B viral hepatitis (HNANB), or hepatitis of undetermined etiology in 24 patients, and hepatic artery thrombosis after excision of a pheochromocytoma in one patient. Of the nine patients with toxic hepatitis, anesthetic agents such as halothane were suspected in three, valproic acid (Depakene) in one, gold sodium thiomalate in one, phenytoin sodium (Dilantin) in one, methyl-ethyl-ketone (paint solvent) in one, and probably acetaminophen in two.
The weight of the excised liver in the adult-size patients (>45 kg) ranged from 420 g to 3200 g, with a mean of 1002 g (SD 586 g). Twenty-seven patients had massive necrosis, and 15 had submassive necrosis of the hepatocytes. None of these patients had either significant fibrosis or cirrhosis.
Survival
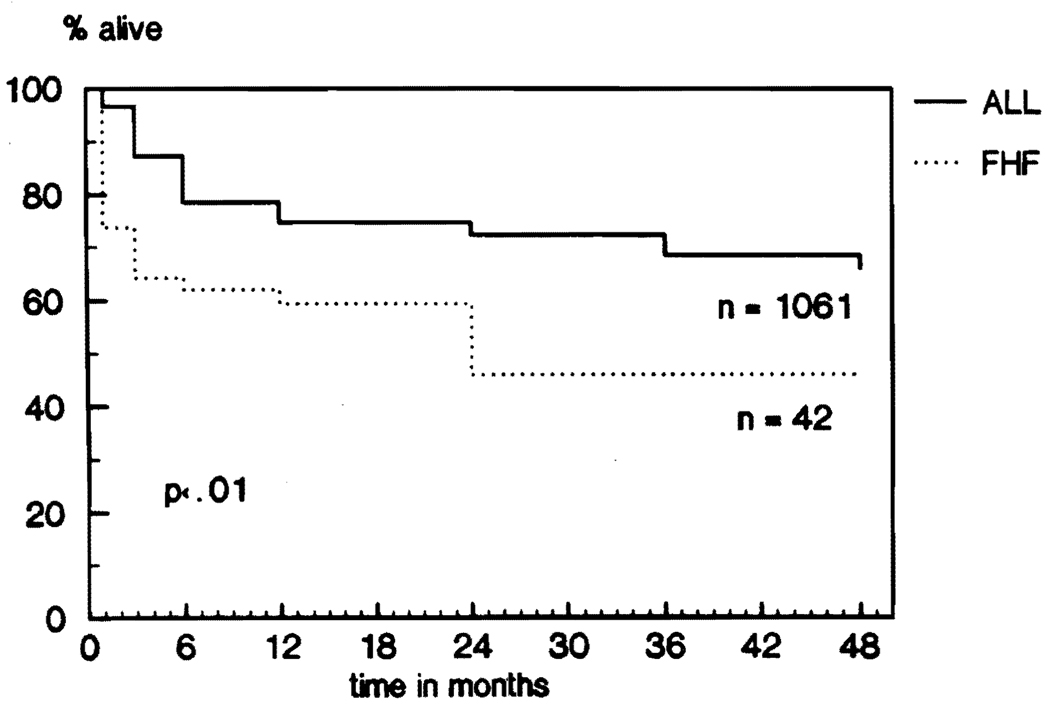
The overall actuarial survival rates as of August 1, 1988, are shown in Figure 1: 73.8% at one month, 64.3% at three months, 61.9% at six months, 59.4% at one year, and 45.8% at two, three, and four years.
Fig 1.
FHF versus general OLTx population: survival.
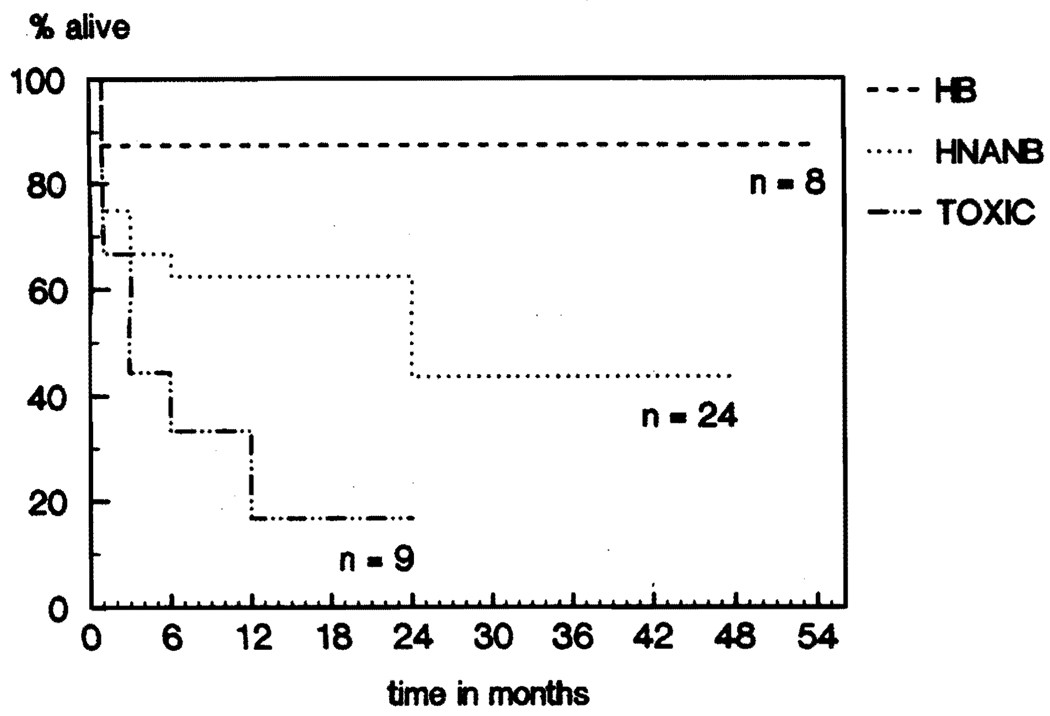
Seven of the eight (87.5%) patients with hepatitis Bare alive (Fig 2). The only death in this group occurred intraoperatively, as a result of pulmonary emboli during the use of veno-venous bypass. The survival rates of the nine patients with toxic hepatitis were 66.7% at one month, 44.5% at three and six months, 33.3% at one year, and 16.7% at two years; only two of the nine patients (22%) are still alive (Fig 2). The survival rates for the 24 patients with so called non-A, non-B hepatitis were 75% at one month, 66.7% at three months, 62.5% at six months and one year, and 43.8% at two, three, and four years (Fig 2). Finally, one patient with hepatic artery thrombosis after resection of an extensive retroperitoneal pheochromocytoma died soon after transplantation.
Fig 2.
FHF: Survival by diagnosis.
Risk Factors
Several risk factors were identified; their influence upon one-and three-month survival is analyzed and summarized in Table 1.
Table 1.
Number of Deaths at One and Three Months Without or With the Risk Factor
| Risk Factor | No of Deaths | No | Yes | p |
|---|---|---|---|---|
| Coma stage | 1 mo | 4/28 (14.3%)* | 7/14 (50%)† | <0.05 |
| 3mo | 8/28 (28.5%)* | 10/14 (71.4%)† | <0.05 | |
| Clinical bleeding | 1 mo | 2/32 (6.3%) | 6/10 (60%) | <0.001 |
| 3mo | 4/32 (13%) | 7/10 (70%) | <0.01 | |
| Renal failure | 1 mo | 2/32 (9.4%) | 5/10 (50%) | <0.05 |
| 3mo | 6/32 (18.8%) | 61 10 (60%) | <0.05 | |
| Respiratory support | 1 mo | 1/24 (4.2%) | 7/18 (38.9%) | <0.05 |
| 3mo | 4/24 (16.7%) | 8/18 (44.4%) | <0.1 | |
| Metabolic acidosis | 1 mo | 6/37 (16.2%) | 5/5(100%) | <0.001 |
| Hypoglycemia | 1 mo | 7/35 (20%) | 4/7 (57.1%) | <0.1 |
| Hemodynamic instability | 1 mo | 3/3 | ‡ | |
| Sepsis | 1 mo | 2/4 | ‡ | |
| 3mo | 3/4 | ‡ |
Grades I to III coma.
Grade IV coma.
Too few cases for analysis.
Stage IV coma, clinical bleeding, and renal failure were identified as significant risk factors (p < 0.05). The need for pretransplant respiratory support and hypoglycemia were probable risk factors (0.05 < P < 0.1). Sepsis, uncorrectable metabolic acidosis, and hemodynamic instability requiring high doses of vasopressors appeared to be significant risk factors, but the numbers were too small for meaningful analysis.
Neurologic Recovery
The neurologic recovery was studied in 36 patients who lived for more than one week with satisfactory graft function.
Thirteen of the 14 (92.9%) patients with grades I or II coma had complete neurologic recovery. One had partial recovery, but died from an intracranial hemorrhage 20 days after the first and 12 days after the second transplant. All of the 12 (100%) patients with grade III coma had a complete neurologic recovery. However, the neurologic recovery was complete in only five of the 10 (50%) patients with grade IV coma. Severe cerebral dysfunction persisted in two (20%) patients, and the coma progressed to brain death in three (30%) of the 10 patients (p < 0.05).
Recurrence of the Primary Disease
Of the eight patients with hepatitis B, all were positive for HBsAg, four were positive for HBeAg, and two had the antidelta antibody at the time of transplantation. All of the seven patients who survived the operation received a large dose (50 to 100 ml) of anti–hepatitis B immunoglobulin during the anhepatic phase of OLTx, then one day, one month, and two months after transplantation. All seven patients became HBsAg negative and HBsAb positive for one to three months. Later, however, five of the seven patients became HBsAg positive again. Two of the seven remain HBsAg negative 18 months and 4.5 years after transplantation, respectively, and both have developed HBcAb. Two of the four patients who were HBeAg positive became negative after transplantation; however, two other patients who were negative for HBeAg preoperatively acquired the antigen after transplantation.
Two patients who lost the HBsAg after transplantation have not developed any clinical or histologic evidence of recurrent hepatitis B. Three of the five patients who are still positive for HBsAg after transplantation developed clinical and histologic recurrence of the disease. One of these required retransplantation after 21 months for hepatitis B, and two are waiting for retransplantation because of chronic rejection and hepatitis B.
Of the 15 patients with so-called non-A, non-B viral hepatitis who survived more than six months, two became positive for HBsAg after transplantation. One patient required retransplantation for chronic active hepatitis B at 18 months, and the other still had normal liver function four years later. Although difficult to document, three other patients have developed recurrence of their non-A, non-B hepatitis by histologic examination of the liver. Two of the three patients have good to fair liver function after 2.5 and four years. The other patient is deeply jaundiced and waiting for retransplantation 27 months after his first liver transplantation.
Causes of Death
A total of 21 patients died during the observation period. Six patients died primarily from infectious complications. Five deaths were primarily neurologic, including three from brain death. Despite the high incidence of primary non function of the initial grafts (9 of 42, or 21.4%), only three patients died as a result, and the remaining six patients were rescued by timely retransplantation. Three patients died of hepatic failure due to acute and chronic rejection with or without regrafting. Three patients died of technical failure, and one patient died of a posttransplant lymphoproliferative disorder.
Current Status of Surviving Patients
Fourteen of the 21 survivors (66.7%) have good liver function (SGOT < 100 U and/or total bilirubin < 2 mg%) between seven months and four-plus years after transplantation. Four (19%) have fair liver function (SGOT 100 to 200 U and/or total bilirubin 2 to 4 mg%) between one and four years, and three (14.3%) have poor liver function between one and two years after transplantation. These three patients are waiting for retransplantation.
Aplastic Anemia
Four of the 24 (16%) patients with non-A, non-B viral hepatitis developed aplastic anemia, a known serious complication of non-A, non-B viral hepatitis. 10,11 Three of the four patients recovered from aplastic anemia, but one still remains severely pancytopenic ten months after transplantation.
DISCUSSION
Survival rates after liver transplantation for fulminant hepatic failure were significantly worse than for the 1061 patients who received liver grafts during the same period (Fig 1). The difference was most significant during the first three months. The lower survival rates of patients with FHF were related to the grave condition of most of the recipients prior to liver replacement. Grade IV encephalopathy, renal failure, obvious bleeding diathesis, metabolic acidosis, hypoglycemia, hemodynamic instability, sepsis and respiratory failure are, singularly or in combination, ominous prognostic signs of FHF (Table 1). The survival rates of those who did not have these risk factors were as good as the overall survival rates of the other 1061 transplant recipients.
To improve the survival rate after liver transplantation for FHF, the potential recipients should be transferred to liver transplant centers where the candidates can be closely monitored and aggressively treated by experienced staff. Should a liver replacement be deemed unavoidable, it must be carried out immediately before ominous complications develop. The potentially dangerous procedure of liver biopsy will be important in the assessment of some candidates. With accumulation of experience in the treatment of FHF, more accurate and earlier prognostic indicators of acute massive hepatic necrosis can hopefully be established.
It is encouraging to note that none of the viral hepatitis recurred in a fulminant form after successful liver transplantation, including hepatitis B. Although the number of cases was small, the survival rates of patients with fulminant hepatitis B after transplantation were better than the overall survival rates (Figs 1 and 2). Two of the seven one-year survivors have lost the HBsAg and acquired HBcAb and have remained antigen negative for 18 months and nearly five years. The liver function tests of these two patients are completely normal. The remaining five patients became positive for HBsAg again a few months after liver replacement, and all five have developed clinical and histologic recurrence of hepatitis B. Two of the five will require retransplantation because of chronic rejection and recurrent hepatitis B. One patient was retransplanted for recurrent hepatitis B, and although the HBsAg remains positive, the patient has normal liver function five months after retransplantation. The remaining two patients have elevated levels of transaminases with normal serum bilirubin.
The recurrence of non-A, non-B viral hepatitis was observed in only three of the 15 six-month survivors, although its documentation is quite difficult. Nevertheless, 11 of the 12 surviving patients have normal liver function tests eight to 54 months after transplantation.
These data indicate that viral hepatitis, including hepatitis B, does not seem to be a contraindication for liver transplantation.
SUMMARY
The actuarial survival rates of patients with fulminant hepatic failure were 73.8% at one month, 64.3% at three months, 61.9% at six months, 59.4% at one year, and 45.8% at two, three, and four years after liver transplantation. These survival rates were significantly lower than the overall survival rates of 1061 patients transplanted during the same period, mainly because of the grave condition of these patients. Fulminant viral hepatitis, including hepatitis B, is not a contraindication for liver transplantation. Liver transplantation has established its significant role in the treatment of fulminant hepatic failure of various causes.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant no. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Trey C, Davidson CS. In: Progress in Liver Diseases. Popper H, Schaffner F, editors. vol 3. New York: Grune and Stratton; 1970. pp. 282–298. [Google Scholar]
- 2.Acute Hepatic Failure Study Group. Rakela J. Gastroenterology. 1979;77:A33. [Google Scholar]
- 3.EASL. Gut. 1979;20:620. doi: 10.1136/gut.20.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimson AES, Braude S, Mellon PJ, et al. Lancet. 1982;1:681. doi: 10.1016/s0140-6736(82)90711-5. [DOI] [PubMed] [Google Scholar]
- 5.Redeker AG, Yamahiro HS. Lancet. 1973;1:3. doi: 10.1016/s0140-6736(73)91220-8. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwatsuki S, Starzl TE, Todo S, et al. Transplantant Proc. 1988;20:498. [Google Scholar]
- 8.Iwatsuki S, Esquivel CO, Gordon RD, et al. Semin Liver Dis. 1985;5:325. doi: 10.1055/s-2008-1040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieber AC, Ambrosino G, Van Thiel DH, et al. In: Clinics in Gastroenterology. Van Thiel DH, Makowka L, editors. Philadelphia: Saunders; 1988. p. 157. [PMC free article] [PubMed] [Google Scholar]
- 10.Zeldis JB, Dienstag JL, Gale RP. Am J Med. 1983;74:64. doi: 10.1016/0002-9343(83)91119-1. [DOI] [PubMed] [Google Scholar]
- 11.Tzakis AG, Arditi M, Whitington PF, et al. N Engl J Med. 1988;319:393–396. doi: 10.1056/NEJM198808183190702. [DOI] [PMC free article] [PubMed] [Google Scholar]