Abstract
Although adrenergic receptors (AR) and hyperhomocysteinemia (HHcy) are implicated in heart failure, their role in diabetic cardiomyopathy is not completely understood. We tested the hypothesis that glucose mediated depletion of beta2-AR and HHcy impair contractile function of cardiomyocytes leading to diabetic cardiomyopathy. To prove the hypothesis, cardiac function was assessed in twelve week male diabetic Ins2+/− Akita and C57BL/6J mice by echocardiography, pressure-volume loop and contractile function of cardiomyocytes. The results revealed cardiac dysfunction in Akita. To investigate the mechanism, the levels of beta2-AR, GLUT4, sercoplasmic reticulum calcium ATP-ase –isoform 2 (SERCA-2) and homocysteine (Hcy) metabolic enzymes -cystathionine beta synthase (CBS), cystathionine gamma lyase (CTH) and methyl tetrahydrofolate reductase (MTHFR) were determined in the heart. It revealed down regulation of beta2-AR, GLUT4, SERCA-2, CBS, CTH and MTHFR in Akita. Attenuation of beta2-AR in hyperglycemic condition was also confirmed in cardiomyocytes at in vitro level. Interestingly, the ex vivo treatment of cardiomyocytes with beta2-AR antagonist deteriorated whereas beta-AR agonist ameliorated contractile function. It points to the involvement of beta2-AR in diabetic cardiomyopathy. We conclude that degradation of beta2-AR and impairment of Hcy metabolism is implicated in diabetic cardiomyopathy.
Keywords: Diabetes, Akita, cardiac dysfunction, contractile function
1. Introduction
Adrenergic receptors (AR) are important components of sympathetic system that regulate the contractile function of the heart and thereby implicated in pathological cardiac remodeling. AR is categorized into alpha (excitatory) and beta (inhibitory) by Ahlquist [1]. The beta- adrenergic receptors are further classified into beta1-, beta2- and beta3- subtypes. All the three receptor subtypes have highly conserved sequences across the species and are affected by diabetes [13]. Although both beta1- and beta2-AR co-exist in cardiomyocytes, their mechanisms of action differ in several conditions such as beta1-AR stimulation induces apoptosis [10] whereas beta2-AR stimulation inhibits apoptosis [7]. They also differ in G -protein coupling and cAMP handling [28]. It might be due to higher affinity of beta1-AR to noradrenaline and less affinity to G-protein coupled proteins receptor (GPCR). On contrary, beta2-AR has higher affinity to GPCR but less affinity to noradrenaline. In failing heart, due to release of noradrenaline from sympathetic nerve, beta1-AR is first to be stimulated. However, it is exhausted or depressed due to excess noradrenaline and the stimulated beta2-AR maintains the function of heart. The diabetic heart is comparable to the failing heart where beta2-AR has important role to maintain the cardiac function.
Heart failure is also associated with elevated level of homocysteine (Hcy - a non–protein coding amino acid), a condition called hyperhomocysteinemia (HHcy) [2]. In diabetes, plasma Hcy level is elevated. HHcy is caused mainly due to impairment of metabolic enzymes involved in either remethylation (methyl tetrahydrofolate reductase –MTHFR) or transsulfuration (cystathionine beta synthase -CBS and cystathionine gamma lyase -CTH) pathways [21]. The Hcy level can be partially normalized by supplying exogenous folic acid, which acts as a cofactor in the remethylation pathway [6]. Folic acid also has independent effect and has been tested as therapeutic drug either alone or in combinations in several diseases including heart failure [5;24;26].
It is often found that in diabetes glucose transportation is perturbed. One of the important glucose transporter molecules that is attenuated during diabetic heart failure is GLUT4 [12]. The cardiac remodeling either due to down –regulation of GLUT4 or other factors ultimately lead to contractile dysfunction of myocytes. Calcium plays important role in contractile function of cardiomyocytes and intracellular calcium flux is maintained by sercoplasmic reticulum calcium ATP-ase –isoform 2 (SERCA-2). Therefore, deficiency of SERCA-2 is implicated in contractile dysfunction leading to heart failure. Heart failure is determined by decrease in percentage fractional shortening (assessed by echocardiography), ejection fraction (measured by pressure- volume (P-V) loop) and contractile function (±dL/dt) of myocytes. To investigate the mechanism of heart failure at in vitro level, HL1 cell line having phenotypic characteristics of adult cardiomyocytes has been preferred [9;19]. In diabetes, hyperglycemia ultimately leads to cardiac dysfunction. To induce hyperglycemia, alloxan and streptozotocin chemical treatment has been used. However, Akita having genetic defect in insulin 2 (Ins2+/−) serve as a better model system because it induces hyperglycemia spontaneously. In Akita, proinsulin2 cannot be exocytose due to mutation in insulin 2 gene. The accumulation of proinsulin triggers apoptosis causing death of beta cells that ultimately leads to hypoinsulinemia and diabetes.
2. Methods
2. 1. Animal models
The C57 BL/6J (WT) and diabetic (Ins2+/− Akita) mice were procured from Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facility of the University of Louisville. The animal care and use programs were carried out according to standard protocol and guidelines of National Institute of Health (NIH) and Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 86-23, revised 1985) and the regulation of the Animal Welfare Act. Twelve week male mice were used. Folic acid (0.03 mg/L) in drinking water was used for treatment for the duration of four weeks. The drinking water with folic acid was changed every alternate day. All mice were sacrificed after deep anesthesia and hearts were snap frozen immediately after extraction and stored at -80°C.
2. 2. Glucose level test
Blood glucose was tested randomly in twelve week male mice by taking 1–2 drops of blood collected from tail vein and using OneTouch Ultra Glucometer (LifeScan, Inc. Milpitas, CA).
2. 3. Echocardiography
A transthoracic M-mode echocardiography was performed with a 12 –MHz ultrasound system (Philips SONO-5500). Mice were anaesthetized with tribromoethanol (TBE- 2mg/ kg body weight) for duration of recording [20]. The percentage fractional shortening was determined from that echocardiogram where systolic and diastolic curves were consistent.
2. 4. Hemodynamic Measurements
The P-V loop was determined by1.4 French Millar’s catheter (Millar Inc., Houston, Texas). Mice were anaesthetized by pentobarbital (5 mg/ml) using the dose of 70 mg/kg body weight, i. p. The protocol from the company was followed.
2. 5. The isolation of ventricular cardiomyocytes
The cardiomyocytes were isolated from the left ventricle following the exact protocol as described elsewhere [18]. They were treated with different doses of β-AR agonist (isoproterenol) to obtain dose dependent curve. The dose at highest response was used for the experiments. Similarly, dose dependent curve for β2-AR antagonist (ICI 118,551 hydrochloride) was also obtained for use of specific amount of ICI 118,551 hydrochloride.
2. 6. Determination of rate of systolic contraction and diastolic relaxation
The video-edged Ion –Optics device was used for measurement of rate of contraction and relaxation of cardiomyocytes as described elsewhere [18].
2. 7. RNA extraction and quality assessment
The mirVanaTM miRNA isolation kit (Ambion, Part number #AM1560) was used to isolate the total RNA heart following the protocol of kit. The quality of total RNA was assessed by NanoDrop ND-1000 and only highly pure quality RNA (260/280-2.00 and 260/230 -2.0) was used for RT-PCR.
2. 8. RT-PCR
The RT-PCR was performed for mRNA expression of beta-2 AR, CBS, CTH and MTHFR in WT and Akita using ImProm-II TM Reverse Transcription system kit (Promega Corporation, Madison, WI, USA, Cat # A3800) as described elsewhere [19]. For gene amplification the RT-PCR program was 95°C- 7.00 min, [95°C-0.50min, 55°C-1.00 min, 72°C-1.00 min] × 34, 72°C-5.00 min, 4°C-∞. The primers for RT-PCR are described in the Table 1. The RT –PCR product was electrophoresed on 1% agarose gel in TAE with 0.008% Ethidium bromide.
Table 1.
Forward and reverse primer sequences for beta2-Adrenergic receptors (Beta2-AR), cystathionine gamma lyase (CTH), cystathionine beta synthase (CBS) and methyl tetrahydrofolate reductase (MTHFR).
| mRNA | orientation | primer |
|---|---|---|
| Beta2-AR | forward | 5′GAGCACAAAGCCCTCAAGAC3′ |
| reverse | 5′GTTGACGTAGCCCAACCAGT3′ | |
| CTH | forward | GACCTCAATAGTCGGCTTCGTT |
| reverse | CAGTTCTGCGTATGCTCCGTAA | |
| CBS | forward | TGCGGAACTACATGTCCAAG |
| reverse | TTGCAGACTTCGTCTGATGG | |
| MTHFR | forward | CTACCAGAGCCCAAGACAGC |
| reverse | ATTGCAGTTGCTCCTTGTCC |
2. 9. Western Blotting
It was followed as from Mishra et al [19]. The primary antibodies were: beta2 –AR (Santa Cruz Biotechnology, USA, cat # sc-569), GLUT-4 (Abcam, MA, USA, cat # ab654) and beta actin (Sigma, Missouri, USA, cat # A-2228). The secondary antibodies used were rabbit (Santa Cruz Biotechnology, USA, cat # sc-2054) and mouse (Santa Cruz Biotechnology, USA, cat # sc 2005).
2. 10. Confocal microscopy
The 5 μM heart sections of Akita and WT were fixed with 4% paraformaldehyde, permeabilized with 0.02 % Triton × 100 in PBS and blocked with 1% BSA in PBS. The sections were incubated with SERCA-2 (same primary antibody as Western blotting) and FITC tagged secondary antibody. Dapi was used for staining the nucleus. The sections were observed under Olympus confocal microscope.
2. 11. Cell culture
HL1 cardiomyocytes were cultured following the same method as described elsewhere [19]. These cells were treated with 5 and 25 mM of glucose (Sigma cat# G-8270) for 24 hours. After treatment, protein was extracted using the same protocol as described elsewhere [19].
2. 12. Statistics
The results were represented as average ± standard deviation. Two tailed t-test with unequal variance was used for comparing two groups and significance level was denoted with “*” symbol.
3. Results
3.1. Hyperglycemia and cardiac dysfunction in diabetic Akita
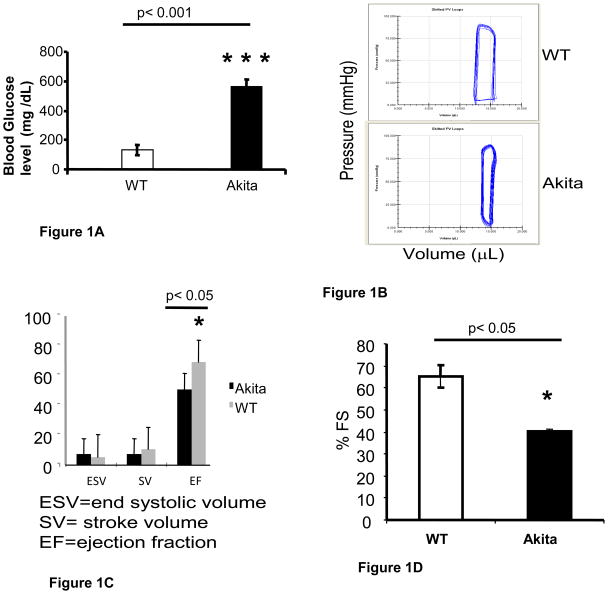
There was robust hyperglycemia in Akita as compared to the WT (Figure 1A). To investigate the effect of hyperglycemia on cardiac function, we performed P-V loop and echocardiography. The hemodynamic measurement by P-V loop showed increase in end systolic volume while decrease in stroke volume and ejection fraction in Akita (Figure 1B, C, D). M-mode echocardiogram showed significant decrease in percentage fractional shortening in Akita (Figure 1D).
Figure 1.
A comparison of plasma glucose level and cardiac function of 12 week male Akita with C57BL/6J (WT): A. Akita showed robust hyperglycemia. The graph represents mean and standard deviation. n=10. B. A representative Pressure - Volume loop with Millar catheter showing reduction in stroke volume in Akita. C. Bar graph representing average and standard deviation of the end systolic volume, stroke volume and percentage ejection fraction. n= 5. D. Transthoracic M-mode echocardiography revealed significant decrease in percentage fractional shortening (% FS) in Akita. n= 5.
3.2. Degradation of beta2-AR and down regulation of GLUT4 and SERCA-2
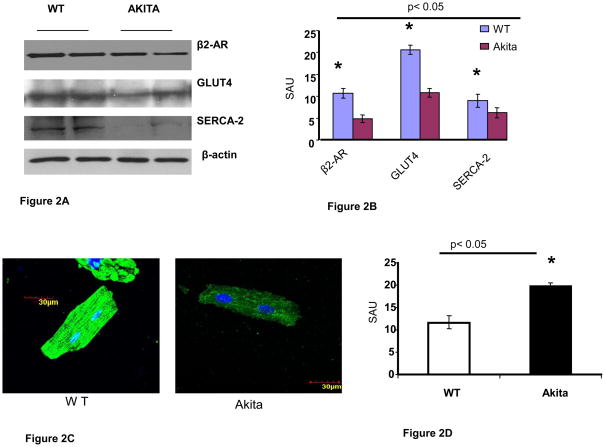
The Western blotting of total proteins from heart of Akita and WT revealed attenuation of beta2-AR, GLUT4 and SERCA-2 in Akita (Figure 2A, B). The confocal microscopy of cardiomyocytes revealed that SERCA-2 was significantly decreased in Akita (Figure 2C, D).
Figure 2.
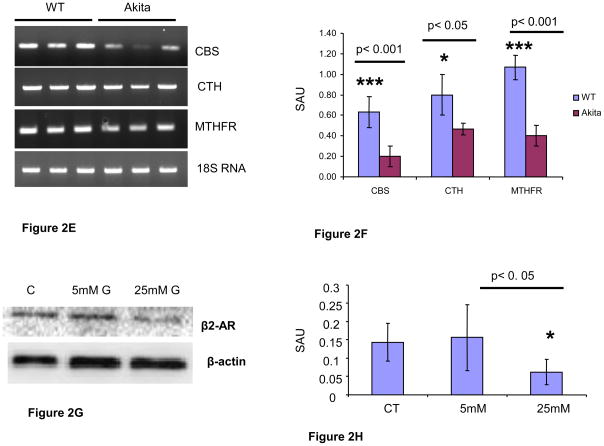
Analyses of cardiac mRNA and protein in 12 week male WT and Akita: A. Western blotting of heart tissue of Akita showed down regulation of beta 2-AR, GLUT4 and SERCA-2 in Akita. Beta-actin was used as a loading control. B. Bar graph represents the scanned arbitrary unit (SAU) of the bands for expression of beta2-AR, GLUT4 and SERCA-2. n=6. C. Confocal microscopy of cardiomyocytes: SERCA-2 stained with green FITC was attenuated in Akita. The nucleus is stained with dapi. D. Bar graph from the intensity of FITC shows significant decrease in SERCA-2 in Akita. n=6. E. Reverse Transcription Polymerase Chain Reaction (RT-PCR) showing attenuation of homocysteine metabolic enzymes-cystathionine beta synthase (CBS), cystathionine gamma lyase (CTH) and methyl tetrahydrofolate reductase (MTHFR). 18S RNA was used as a loading control. F. Bar graph from scanned arbitrary unit of the bands shows significant decrease in CBS, CTH and MTHFR in Akita. n=6. G. In vitro study on HL1 cardiomyocytes showing reduction of beta 2-AR after treatment with 25mM of glucose for 24 hours. Beta-actin was used as a loading control. n=6. H. Bar graph from scanned arbitrary unit of bands shows attenuation of beta 2-AR in HL1 cardiomyocytes after treatment with 25 mM of glucose for 24 hours.
3.3. Homocysteine metabolism in Akita
The mRNA expression of Hcy metabolic enzymes CBS, CTH and MTHFR was determined in the heart tissue of Akita and WT. All the three enzymes were attenuated in Akita (Figure 2E, F).
3.4. Glucose treatment on HL1 cardiomyocytes
To simulate the diabetic condition, HL1 cardiomyocytes were treated with glucose (5 mM- normal dose and 25 mM –high dose). The protein analyses showed significant down regulation of beta2-AR in hyperglycemic HL1 (Figure 2G, H).
3.5. Effect of beta-AR agonist on contractility of cardiomyocytes
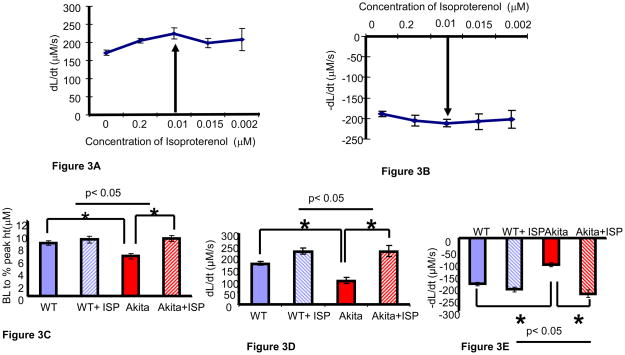
The cardiomyocytes from WT were treated with different concentrations of isoproterenol (beta-AR agonist) and changes in rate of contraction (dL/dt) and relaxation (−dL/dt) was measured. The dose dependent curve showed 0.01 μM of isoproterenol as the most effective dose for increasing the contractile function of cardiomyocytes (Figure 3A, B). Treatment of cardiomyocytes from Akita with 0.01 μM of isoproterenol significantly increased the baseline to percentage peak height (Figure 3C), rate of contraction (Figure 3D) and rate of relaxation (Figure 3E), which is comparable to WT treated mice (Figure 3C, D, E). There was no significant difference in the baseline to percentage peak height, rate of contraction and relaxation after treatment with 0.01 μM of isoproterenol on WT cardiomyocytes (Figure 3C, D, E).
Figure 3.
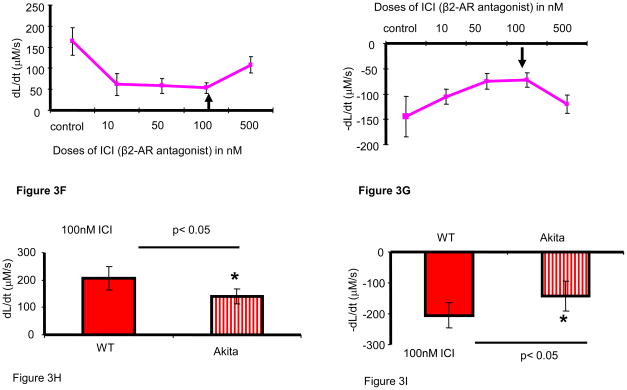
Ex vivo studies showing effect of beta-AR agonist Isoproterenol and beta 2-AR antagonist ICI on cardiomyocyte contractile function. A and B. Standardization of doses of Isoproterenol (ISP) suggests 0.01μM is the optimum dose for improving the rate of contraction (A) and relaxation (B) of cardiomyocytes. The treated myocytes were stimulated at 1Hz and at least 20 myocytes from each set of experiment was examined. n=5. The arrow shows the highest effective dose of ISP for myocytes. C. A comparison of baseline (BL) to percentage peak height of cardiomyocytes during contraction and relaxation among WT, WT treated with ISP, Akita and Akita treated with ISP. n=6. D. The rate of contraction (dL/dt) was compared among the above four groups. n=6. E. The rate of relaxation (−dL/dt) was compared among the above four groups. n=6, *, p< 0.05. F and G. Standardization of dose of ICI - a beta2-AR antagonist following the same protocol as performed for ISP showed 100 nM or 0.01μM as the highest effective dose for maximum attenuation of contraction (F) and relaxation (G) of cardiomyocytes. n=5.
3.6. Effect of beta2-AR antagonist on contractility of cardiomyocytes
To determine the role of beta2-AR on contractile function of cardiomyocytes, WT cardiomyocytes were treated with different doses of ICI118, 551 hydrochloride (β2-AR antagonist). The 100nM dose of ICI118, 551 hydrochloride had the maximum effect on rate of contraction and relaxation (Figure 3F, G). Therefore, cardiomyocytes from WT and Akita were treated with 100 nM of ICI118, 551 hydrochloride. There was significant decrease in the rate of contraction (Figure 3H) and relaxation (Figure 3I) in Akita as compared to WT after treatment with 100nM dose of ICI118, 551 hydrochloride.
3.7. Effect of folic acid on contractility of cardiomyocytes
The remethylation pathway of Hcy metabolism requires folic acid as co-factor. Therefore, Akita was treated with folic acid and rate of contraction and relaxation of cardiomyocytes were compared between treated and untreated mice. As a control, WT was also treated with same dose of folic acid for the same period of time. There was no significant change found in the rate of contraction and relaxation of Akita cardiomyocytes after treatment with folic acid.
4. Discussion
The propensity of diabetes due to genetic predisposition is unclear. It causes a large human population to succumb to this disease in an unnoticed manner. The heterozygous mutation in “Insulin 2” gene in Akita serves as a unique model for type1 diabetes (T1D) that is more relevant to human in the sense that diabetic patient may be “Insulin 2” mutant [14]. The mutation causes impaired trafficking of Insulin 2 polypeptide resulting in accumulation and subsequent death of pancreatic beta-cells leading to hypoinsulinemia and hyperglycemia. It is a better model than the others such as NOD-the autoimmune depletion of beta-cells, OVE 26- the non-specific gene expression in beta-cells and Streptozotocin or Alloxan chemical mediated destruction of beta-cells [15]. The genetic screening for “Insulin 2” mutation can be a plausible method to warn people against their vulnerability to diabetes and to take proper precautionary measures to escape diabetic complications. It is supported by the recent finding that Ins2+/− mutation causes neonatal diabetes in human [14].
Cardiac dysfunction is one of the earliest manifestations of diabetes. The impairment of pumping capacity of heart can be assessed by increase in end systolic volume and decrease in ejection fraction and percentage fractional shortening. In twelve week Akita, there was robust hyperglycemia (Figure 1A), which is accompanied by decrease in end systolic volume, stroke volume and ejection fraction (Figure 1B, C), and percentage fractional shortening (Figure 1D). It suggests that diabetic Akita has impaired cardiac function due to defective left ventricular function.
Beta-AR plays a pivotal role in maintaining the sympathetic tone. The pharmacological beta-AR agonist and blockers are in clinical practice for maintaining the sympathetic drive in cardiovascular diseases [4;29]. The empirical evidences suggest their role in diabetic cardiomyopathy [3]. During heart failure there is remarkable down regulation of beta-AR [4;29]. To investigate the impact of sympathetic tone on diabetic cardiomyopathy, in vivo (Akita Vs WT), ex vivo (cardiomyocytes from Akita and WT treated with beta- AR agonist and beta2-AR antagonist) and in vitro (HL1 cells were treated with high dose of glucose) approaches were used. Interestingly, the attenuation of beta2-AR protein in Akita (Figure 2A, B) and glucose treated HL1 (Figure 2G, H) provides concrete evidence that there is depletion of beta2-AR in diabetic condition. It is supported by the previous finding that beta2-AR density decreases in diabetes [13]. However, there is possibility that beta2-AR are non –responsive / insensitive in diabetes. Nevertheless, the improvement in contractile function of Akita cardiomyocytes after treatment with beta-AR agonist (Figure 3C, D, E) and deterioration of contractility after treatment with beta2-antagonist (Figure 3H, I) points to the responsive nature of beta2-AR in diabetic cardiomyocytes. Therefore, it is concluded that AR and especially beta2-AR is one of the important molecules that mediate diabetic cardiomyopathy and can be used as a therapeutic target for ameliorating cardiac dysfunction.
Beta2-AR also influence GLUT4 trafficking independent of insulin stimulation [23]. The impaired glucose transport due to defective GLUT 4 is one of the major causes of cardiomyopathy in diabetes [11;17]. The decrease in the expression of GLUT4 suggests impairment of glucose transport in Akita (Figure 2A, B) that ultimately leads to diabetic cardiomyopathy. The decrease in cardiac function is mainly caused by contractile dysfunction of cardiomyocytes where intracellular calcium uptake and release play a crucial role. The calcium handling during contraction and relaxation of cardiomyocytes is maintained by SERCA-2 [27]. Therefore, SERCA-2 was measured both at cellular level in cardiomyocytes and protein level in the heart tissue. The mitigation of SERCA-2 (Figure 2A, B, C, D) explains the contractile dysfunction in Akita.
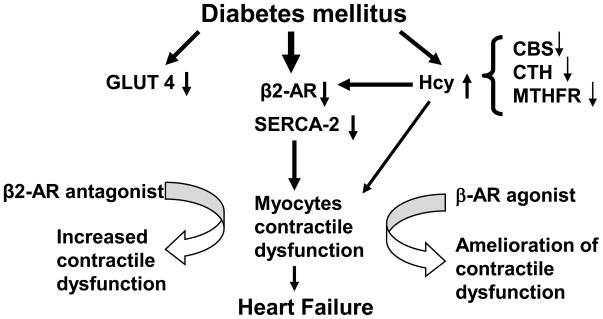
It is found that plasma Hcy is elevated in Akita. The effect of Hcy is independent of insulin in diabetes [22]. The competitive binding of s-adenosyl –homocysteine (SAH) with beta-AR provides the evidence of direct interaction of Hcy with beta-AR [16]. Additional evidence is the fact that beta2-AR response is maintained by stimulatory (Gs) and inhibitory (Gs) signals from adenylyl cyclase dependant protein kinase (a G-coupled proteins located on transmembrane domain), and HHcy inhibits Gs signal [25]. In Akita, HHcy is due, in part to the impairment of its metabolic enzymes CBS, CTH and MTHFR (Figure 2E, F), which supports the findings of Chiang et al [8]. At one hand beta2-AR is degraded due to hyperglycemia while on the other hand it is inhibited by HHcy that ultimately results into contractile dysfunction in Akita. To reduce the effect of HHcy, Akita was treated with folic acid, which acts as a co-factor in the remethylation pathway of Hcy. However, we did not find any significant improvement in the contractile function of Akita cardiomyocytes after treatment with folic acid. This can be explained by the fact that folic acid can ameliorate the remethylation of Hcy but it does not have effect on transsulfuration pathway. As there was down regulation of CBS and CTH enzymes in Akita, folic acid could not ameliorate HHcy and Hcy mediated degradation of β2-AR. Based on our findings, we propose that glucose mediated metabolic derangement in diabetes leads to depletion of beta-2 AR, attenuation of GLUT4 and SERCA-2 and induction of HHcy by inhibiting CBS, CTH and MTHFR enzymes in the myocardium leading to cardiomyopathy. The beta2-AR antagonist treatment exacerbates deterioration whereas beta-AR agonist ameliorates cardiac dysfunction suggesting that beta2-AR and HHcy have crucial role in diabetic cardiomyopathy. We propose that in diabetes, attenuation of beta 2-AR, SERCA-2, GLUT4 and Hcy metabolic enzymes results into myocyte contractile dysfunction leading to cardiomyopathy and beta-AR agonist have therapeutic implications (Figure 4).
Figure 4.
Diabetic cardiomyopathy involves impairment of homocysteine (Hcy) metabolism, attenuation of beta2-AR, SERCA-2 and GLUT4 that leads to cardiomyocytes contractile dysfunction. Treatment with beta2-AR antagonist exacerbates while beta-AR agonist ameliorates the contractile dysfunction of Akita. It shows the association of HHcy with degradation of beta-AR during diabetic cardiomyopathy. In addition, it supports the role of beta-AR as a therapeutic target for diabetic heart failure.
Acknowledgments
A part of the study was supported by National Institute of Health grants HL-71010, HL-74185 and HL-88012.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 2.Alter P, Rupp H, Rominger MB, Figiel JH, Renz H, Klose KJ, Maisch B. Association of hyperhomocysteinemia with left ventricular dilatation and mass in human heart. Clin Chem Lab Med. 2010;48:555–560. doi: 10.1515/CCLM.2010.102. [DOI] [PubMed] [Google Scholar]
- 3.Bhuiyan MS, Fukunaga K. Cardioprotection by vanadium compounds targeting Akt-mediated signaling. J Pharmacol Sci. 2009;110:1–13. doi: 10.1254/jphs.09r01cr. [DOI] [PubMed] [Google Scholar]
- 4.Bilginoglu A, Seymen A, Tuncay E, Zeydanli E, Aydemir-Koksoy A, Turan B. Antioxidants but not doxycycline treatments restore depressed beta-adrenergic responses of the heart in diabetic rats. Cardiovasc Toxicol. 2009;9:21–29. doi: 10.1007/s12012-009-9032-8. [DOI] [PubMed] [Google Scholar]
- 5.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, Jacques PF, McKenney J. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152:448–7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Brustolin S, Giugliani R, Felix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43:1–7. doi: 10.1590/s0100-879x2009007500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 8.Chiang EP, Wang YC, Chen WW, Tang FY. Effects of insulin and glucose on cellular metabolic fluxes in homocysteine transsulfuration, remethylation, S-adenosylmethionine synthesis, and global deoxyribonucleic acid methylation. J Clin Endocrinol Metab. 2009;94:1017–1025. doi: 10.1210/jc.2008-2038. [DOI] [PubMed] [Google Scholar]
- 9.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 11.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P, Camici PG. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrois M, Sidell RJ, Gauguier D, King LM, Radda GK, Clarke K. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res. 2004;61:288–296. doi: 10.1016/j.cardiores.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Dincer UD, Bidasee KR, Guner S, Tay A, Ozcelikay AT, Altan VM. The effect of diabetes on expression of beta1-, beta2-, and beta3-adrenoreceptors in rat hearts. Diabetes. 2001;50:455–461. doi: 10.2337/diabetes.50.2.455. [DOI] [PubMed] [Google Scholar]
- 14.Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, Maestro MA, Alshaikh A, Bundak R, del CG, Deeb A, Deiss D, Fernandez JM, Godbole K, Hussain K, O’Connell M, Klupa T, Kolouskova S, Mohsin F, Perlman K, Sumnik Z, Rial JM, Ugarte E, Vasanthi T, Johnstone K, Flanagan SE, Martinez R, Castano C, Patch AM, Fernandez-Rebollo E, Raile K, Morgan N, Harries LW, Castano L, Ellard S, Ferrer J, Perez de NG, Hattersley AT. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci USA. 2010;107:3105–3110. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52:409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
- 16.Limas CJ. Effect of phospholipid methylation on beta-adrenergic receptors in the normal and hypertrophied rat myocardium. Circ Res. 1980;47:536–541. doi: 10.1161/01.res.47.4.536. [DOI] [PubMed] [Google Scholar]
- 17.Menard SL, Croteau E, Sarrhini O, Gelinas R, Brassard P, Ouellet R, Bentourkia M, van Lier JE, Des RC, Lecomte R, Carpentier AC. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. Am J Physiol Endocrinol Metab. 2010;298:E1049–E1057. doi: 10.1152/ajpendo.00560.2009. [DOI] [PubMed] [Google Scholar]
- 18.Mishra PK, Metreveli N, Tyagi SC. MMP-9 gene ablation and TIMP-4 mitigate PAR-1-mediated cardiomyocyte dysfunction: a plausible role of dicer and miRNA. Cell Biochem Biophys. 2010;57:67–76. doi: 10.1007/s12013-010-9084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra PK, Tyagi N, Kundu S, Tyagi SC. MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochem Biophys. 2009;55:153–162. doi: 10.1007/s12013-009-9063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993;43:189–192. [PubMed] [Google Scholar]
- 21.Sen U, Tyagi SC. Homocysteine and Hypertension in Diabetes: Does PPARgamma Have a Regulatory Role? PPAR Res. 2010;2010:806538. doi: 10.1155/2010/806538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla N, Angelini GD, Jeremy JY. The administration of folic acid reduces intravascular oxidative stress in diabetic rabbits. Metabolism. 2008;57:774–781. doi: 10.1016/j.metabol.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Shumay E, Song X, Wang HY, Malbon CC. pp60Src mediates insulin-stimulated sequestration of the beta(2)-adrenergic receptor: insulin stimulates pp60Src phosphorylation and activation. Mol Biol Cell. 2002;13:3943–3954. doi: 10.1091/mbc.E02-03-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirban A. Drugs for the treatment of diabetes complications. Zycose: a new player in the field? Drugs Today (Barc) 2008;44:783–796. doi: 10.1358/dot.2008.44.10.1183086. [DOI] [PubMed] [Google Scholar]
- 25.Vacek TP, Sen U, Tyagi N, Vacek JC, Kumar M, Hughes WM, Passmore JC, Tyagi SC. Differential expression of Gs in a murine model of homocysteinemic heart failure. Vasc Health Risk Manag. 2009;5:79–84. [PMC free article] [PubMed] [Google Scholar]
- 26.van Beynum IM, Kapusta L, Bakker MK, den HM, Blom HJ, de Walle HE. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp479. [DOI] [PubMed] [Google Scholar]
- 27.Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54:162–174. doi: 10.1016/s0008-6363(01)00565-x. [DOI] [PubMed] [Google Scholar]
- 28.Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac beta(2)-adrenergic signal transduction. Circ Res. 1999;85:1092–1100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- 29.Zeydanli EN, Bilginoglu A, Tanriverdi E, Gurdal H, Turan B. Selenium restores defective beta-adrenergic receptor response of thoracic aorta in diabetic rats. Mol Cell Biochem. 2010;338:191–201. doi: 10.1007/s11010-009-0353-5. [DOI] [PubMed] [Google Scholar]