Abstract
The disparity between the number of patients waiting for kidney transplantation and the limited supply of kidney allografts has renewed interest in the benefit from kidney transplantation experienced by different groups. This study evaluated kidney transplant survival benefit in prior non-renal transplant recipients (kidney after liver, KALi; lung, KALu; heart, KAH) compared to primary isolated (KA1) or repeat isolated kidney (KA2) transplant. Multivariable Cox regression models were fit using UNOS data for patients wait listed and transplanted from 1995–2008. Compared to KA1, the risk of death on the wait list was lower for KA2 (p<0.001;HR=0.84;CI=0.81–0.88), but substantially higher for KALu (p<0.001;HR=3.80;CI=3.08–4.69), KAH (p<0.001;HR=1.92;CI=1.66–2.22), and KALi (p<0.001;HR=2.69;CI=2.46–2.95). Following kidney transplant, patient survival was greatest for KA1, similar among KA2, KALi, KAH, and inferior for KALu. Compared to the entire wait list, renal transplantation was associated with a survival benefit among all groups except KALu (p=0.017;HR=1.61;CI=1.09–2.38), where post-transplant survival was inferior to the wait list population. Recipients of KA1 kidney transplantation have the greatest post-transplant survival and compared to the overall kidney wait list, the greatest survival benefit.
Introduction
Prior kidney transplant recipients and recipients of a non-renal solid organ transplant who subsequently develop advanced chronic kidney disease are commonly evaluated and wait listed for a kidney transplant.1–2 In 2004, 19% of the kidney waiting list in the United States was composed of recipients who had a prior transplant of any kind.3 Among prior transplant recipients, the etiology of renal failure may be multifactorial; calcineurin inhibitor nephrotoxicity is often suspected to be one of the most important contributors.4–7 The number of these patients will probably grow further given an expanding population of solid organ transplant recipients, increasing experience with kidney transplantation in this group, and widespread use of calcineurin therapy.8–13
Patients with a prior organ transplant pose a particular challenge to the guiding principles of kidney allocation: equity for individual candidates and efficient use of organs. In 1998, the Department of Health and Human Services set forth the “Final Rule”, which stipulated specifically that allocation policy should “achieve equitable allocation” while also aiming to achieve “best use” of donated organs and “to avoid wasting organs.”14 The current system for kidney allocation emphasizes equity by placing a high priority on individual waiting time. Since 1998, however, several issues have driven interest in changing allocation to place more emphasis on maximizing survival benefit from each kidney: 1) waiting time for a kidney transplant has risen, 2) mortality on the waiting list remains very high, and 3) there is recognition that many kidney recipients die with a functioning allograft, suggesting that potential transplant benefit from these organs is lost.15–17 Kidney transplantation after prior organ transplantation may conflict with equity in that a single individual receives more than one organ while others wait for their first, and may not maximize the efficient use of the kidney allograft if the survival of the non-renal transplant recipient is less than it would have been for other individuals on the waiting list.18
These two ethical problems suggest that kidney transplantation among prior solid organ transplant recipients should be carefully examined as a part of ongoing efforts to revise kidney allocation in a way that respects the Final Rule.19–21 However, limited data are available about the survival benefit from kidney transplantation among prior organ recipients compared to primary isolated kidney transplant recipients. The aims of this study were to assess the risk of death on the kidney wait list, the estimated survival benefit of kidney transplantation, and post-transplant survival among patients with a previous non-renal transplant compared to recipients of a primary or repeat isolated kidney transplant.
Methods
Study Design
This is a retrospective cohort study of kidney transplant candidates and transplant recipients between January of 1995 and August of 2008 using registry data from the United Network for Organ Sharing (UNOS). The Hospital of the University of Pennsylvania Institutional Review Board approved the study.
The UNOS kidney, thoracic, and liver data sets were used to identify patients who were listed for or who received a kidney transplant in the following clinical scenarios: primary isolated kidney transplant (KA1), second isolated kidney transplant (KA2), kidney after lung transplant (KALu), kidney after heart transplant (KAH), and kidney after liver transplant (KALi).
Patients less than 17 years of age at the time of listing, patients listed for multiple organs, simultaneous transplants, and patients with prior or subsequent transplants other than the initial kidney, lung, heart, or liver, were excluded. Those patients missing patient age at the time of listing, date of listing, date of transplant, donor age, and waitlist and pre-transplant dialysis status were excluded from the analysis. A total of 264,558 KA1, 47,072 KA2, 362 KALu, 1,029 KAH, and 2,237 KALi wait list patients were identified. Among the wait list patients, 132,856 KA1, 14,242 KA2, 170 KALu, 512 KAH, and 689 KALi underwent living or deceased donor kidney transplantation.
Survival Analyses
The primary endpoints of the study were 1) wait list mortality defined as death on the wait list or delisting due to severe illness 2) death after a kidney transplant and 3) death-censored graft survival. Multivariable Cox hazard models were constructed to compare KA1, KA2, KALu, KALi, and KAH. Independent variables nominally associated with the outcome (p<0.10) in univariate analysis were entered into the multivariable models. Covariates not meeting entry threshold were also included if considered clinically significant. The wait list model included the following covariates: candidate age, race, gender, diabetes, dialysis at time of listing, and panel reactive antibody (PRA). Post transplant survival models included Recipient characteristics of age, race, gender, diabetes, dialysis status at time of transplant, hepatitis C seropositivity, and peak PRA, as well as Donor characteristics of age, race, gender, cold ischemic time, and hepatitis C.
Transplant Survival Benefit
To quantify the potential benefit that a kidney, lung, heart, or liver transplant recipient obtained from a deceased donor kidney transplant, the risk of death following transplantation was compared to the risk of death on the waiting list. A time dependent model was constructed in which waiting list survival was compared to transplant survival.9,22 Survival time for wait list candidates was measured from the day of listing, with events occurring at the time of wait list death or delisting due to severe illness. Wait list patients were censored at the time of entry to the transplant group. All transplant recipients contributed survival time to the waiting list population, and also to the transplant population after transplantation. Comparing the kidney recipients to patients on the kidney wait list allowed a more realistic assessment of survival benefit versus a comparison to all patients on dialysis, since patients listed for a kidney transplant are implicitly recognized as achieving medical suitability for kidney transplantation.
Survival of the transplant population in a specific clinical setting (e.g. KA2) was compared to that group’s wait list survival using multivariable Cox hazard regression analysis. Independent variables nominally associated with the outcome (p<0.10) in univariate analysis, and variables considered clinically significant, were entered into the multivariable models. The models were restricted to wait list covariates which included candidate age, race, gender, diabetes, dialysis status, and etiology of renal failure.
Assessment of Relative Benefit from Kidney Transplant
In order to understand the relative survival benefit of a deceased donor kidney allograft in the different clinical settings, transplant survival hazards for each clinical setting were compared to the risk of wait list removal due to death or illness of the wait list population as a whole. This analysis was limited to deceased donor kidneys in order to provide insight into the effective use of deceased donor kidney allografts in different clinical situations.
Missing Data
Among the wait list cohort, data were missing for PRA (8.7%) and dialysis at the time of wait listing (1.9%). Among the recipient cohort, data were missing for hepatitis C seropositivity (11.9%), peak PRA (7.1%), and dialysis at the time of transplant (0.1%). Within the donor cohort, data were missing for cold ischemic time (21.8%), diabetes (16.2%), and hepatitis C seropositivity (4.3%). In our primary analyses, multivariate models were constructed using patients with complete data. In order to demonstrate that eliminating patients with incomplete data did not significantly affect the primary relationships of interest, we conducted a sensitivity analysis with imputation of missing covariates for variables missing greater than 5 percent of data. In addition, multiple sensitivity analyses were performed in which missing values for PRA and cold ischemic time were imputed using values corresponding to the 90%, 10%, and mean among patients who did have data for these variables. Similarly, sensitivity analyses were performed in which patients with missing data for binary covariates were imputed as having or not having hepatitis C, pre-transplant dialysis, and diabetes. These secondary analyses demonstrated similar results as the primary analyses and are not shown.
Statistical analysis
Means of continuous variables were compared between groups using ANOVA. Categorical variables were compared between groups using the chi-square test. All analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, Il). Microsoft Excel 2003 was utilized for construction of some figures.
Results
Wait List Demographics
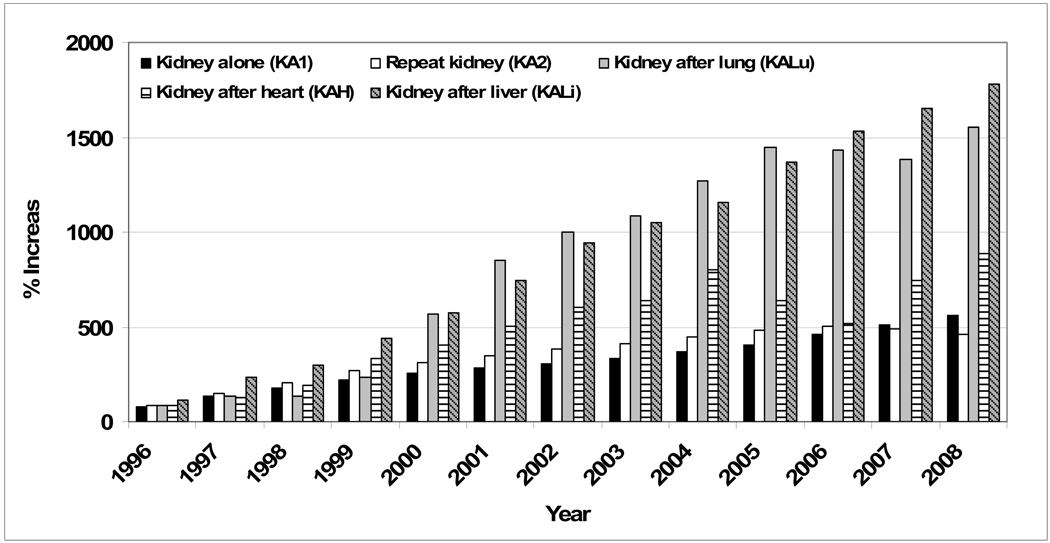
From 1995 to 2007, the number of KA1 and KA2 patients on the kidney wait list at the end of each year increased from 13,011 to 79,405 and 2,402 to 14,200 respectively. Although fewer in number, the rate of increase of wait listed non-renal transplant candidates increased more rapidly; KALu from 6 to 89, KAH from 30 to 254, and KALi, 36 to 632 (Figure 1). Over 70 percent of KA1 and KA2 patients were receiving renal replacement therapy at the time of listing as compared to 56.4 percent for KALu, 62.1 percent for KAH, and 63.4 percent for KALi (Table 1), suggesting earlier referral for addition to the waiting list. The number of years from the time of the first transplant to listing for a kidney transplant varied among the groups : KA2 6.2 (4.4), KALu 6.5 (3.1), KAH 7.1 (4.3), KALi 6.1 (4.0), p < 0.001. The most common indication for transplant at the time of listing was diabetes for KA1, retransplantation/graft failure for KA2, and calcineurin inhibitor nephrotoxicity for KALu, KAH, and KALi. During the study period, KALu, KAH, and KALi had the greatest percentage of patients dying or requiring delisting due to illness while awaiting a transplant; 32.0, 28.1, and 32.6 percent respectively, compared to 16.6 and 12.5 percent for KA1 and KA2.
Figure 1.
Percent change in the size of the kidney waitlist compared to the 1995 kidney wait list population reference group, by year.
Table 1.
Subject characteristics of patients on the waiting list for a kidney transplant*.
| Kidney alone KA1 |
Repeat kidney KA2 |
Kidney after lung KALu |
Kidney after heart KAH |
Kidney after liver KALi |
|
|---|---|---|---|---|---|
| Number | 264,558 | 47,072 | 362 | 1,029 | 2,237 |
| Age at Listing - Years (SD) | 49 (13) | 45 (13) | 51 (13) | 58 (10) | 54 (10) |
| Gender | |||||
| Male | 59.6% | 59.3% | 47.0% | 81.4% | 65.7% |
| Female | 40.4% | 40.7% | 53.0% | 18.6% | 34.3% |
| Race | |||||
| White | 49.1% | 55.5% | 93.1% | 79.8% | 72.1% |
| Black | 28.8% | 27.0% | 3.9% | 14.0% | 10.4% |
| Other | 22.2% | 17.5% | 3.0% | 6.2% | 17.5% |
| Diabetes | 40.1% | 23.8% | 25.7% | 32.4% | 36.3% |
| Chronic Dialysis Treatment | 77.5% | 72.6% | 56.4% | 62.1% | 63.4% |
| Missing | 1.7% | 3.2% | 1.1% | 1.8% | 2.6% |
| Panel Reactive Antibody (SD) | 9.6 (23.4) | 28.2 (37.2) | 4.8 (14.2) | 6.9 (18.4) | 12.1 (24.9) |
| Missing | 8.1% | 7.3% | 10.2% | 11.3% | 16.7% |
| Cause of End Stage Renal Disease | |||||
| Diabetes | 32.7% | 11.3% | 3.3% | 7.6% | 11.0% |
| Hypertensive Nephrosclerosis | 18.5% | 11.0% | 3.6% | 5.3% | 4.0% |
| Glomerulonephritis | 5.7% | 5.6% | 1.4% | 1.0% | 3.4% |
| Polycystic Kidney | 7.3% | 5.9% | 0.6% | 1.0% | 1.7% |
| Reflux Nephropathy / Congenital Abnormality | 1.3% | 1.5% | 0.0% | 0.1% | 0.0% |
| Calcineurin Inhibitor Nephrotoxicity | 0.0% | 0.2% | 50.8% | 45.2% | 23.6% |
| Retransplantation / Graft Failure | 0.0% | 38.3% | 0.0% | 0.0% | 0.0% |
| Other | 22.3% | 17.4% | 12.7% | 9.5% | 16.7% |
| Other/Unspecified by UNOS | 12.2% | 8.8% | 27.6% | 30.3% | 39.6% |
| 1st Transplant to kidney wait list - Years (SD) | *** | 6.2 (4.4) | 6.5 (3.1) | 7.1 (4.3) | 6.1 (4.0) |
| Survival Status | |||||
| Waiting | 33.2% | 57.2% | 21.0% | 22.1% | 36.6% |
| Transplanted | 50.2% | 30.3% | 47.0% | 49.8% | 30.8% |
| Death or Too Sick for Transplant | 16.6% | 12.5% | 32.0% | 28.1% | 32.6% |
| Kidney listing to death or delisting - Years (SD) | 2.0 (2.0) | 2.1 (2.3) | 1.0 (1.1) | 1.4 (1.5) | 1.0 (1.4) |
| Dialysis patients | 2.0 (2.0) | 2.1 (2.2) | 0.9 (1.1) | 1.5 (1.5) | 1.0 (1.3) |
| Non-dialysis patients | 2.3 (2.2) | 2.2 (2.3) | 1.1 (1.3) | 1.2 (1.7) | 1.0 (1.6) |
| % death or delisting for those listed prior to needing dialysis | 12.5% | 11.4% | 22.1% | 20.8% | 29.2% |
| % death or delisting for those on dialysis prior to listing | 16.9% | 12.5% | 38.2% | 30.8% | 33.0% |
all values significantly different between groups (p < 0.001)
Recipient Demographics
The number of isolated deceased donor kidney transplants performed each year has increased between 1995 and 2007. There has been a 450 percent increase for KA1 (1,954 to 8,815 total), a 548 percent increase for KA2 (184 to 1,008), a 400 percent for KALu (1 to 4); 760 percent increase for KAH (5 to 38); and 2,150 percent for KALi (2 to 43). 13.4% of KA2 and 10.2% of KALi recipients were listed for renal transplantation within one year of the first organ, compared to 1.4% and 3.6% for KALu and KAH respectively. The mean number of years from the time of first transplant to the kidney transplant varied among the recipient groups: KA2 7.8 (4.4), KALu 7.6 (2.9), KAH 6.7 (4.7), KALi 4.3 (3.2), p < 0.001. At the time of transplant, greater than 80% of KA1 and KA2 recipients were receiving dialysis as compared to 58.3, 64.4, and 69.3 percent of KALu, KAH, and KALi respectively (Table 2). Recipients of a previous non-renal transplant had shorter kidney waiting times. 17.2% of KA1, 10.5% KA2, 13.9% KALu, 17.2% KAH and 14.0% of KALi received an expanded criteria donor organ. Hepatitis C was most common among previous liver recipients.
Table 2.
Subject characteristics of deceased donor kidney transplant recipients*.
| Kidney alone KA1 |
Repeat kidney KA2 |
Kidney after lung KALu |
Kidney after heart KAH |
Kidney after liver KALi |
|
|---|---|---|---|---|---|
| Number | 93,789 | 10,694 | 72 | 360 | 557 |
| Age at Transplant - Years (SD) | 50.7 (13.0) | 44.6 (12.2) | 52.9 (10.6) | 59.2 (9.8) | 55.0 (9.5) |
| Gender | |||||
| Male | 61.1% | 59.8% | 43.1% | 84.4% | 68.6% |
| Female | 38.9% | 40.2% | 56.9% | 15.6% | 31.4% |
| Race | |||||
| White | 50.3% | 62.2% | 94.4% | 81.4% | 74.1% |
| Black | 29.5% | 23.6% | 4.2% | 11.9% | 8.3% |
| Other | 20.2% | 14.2% | 1.4% | 6.7% | 17.6% |
| Diabetes | 31.3% | 17.4% | 27.8% | 31.4% | 39.1% |
| Dialysis at Time of Transplant | 85.1% | 81.1% | 58.3% | 64.4% | 69.3% |
| Hepatitis C Positive | 6.8% | 9.7% | 1.8% | 5.7% | 41.3% |
| Missing | 12.1% | 13.4% | 20.8% | 16.9% | 14.7% |
| Panel Reactive Antibody (SD) | 11.5 (24.4) | 42.0 (39.1) | 7.4 (17.1) | 8.1 (18.3) | 14.2 (26.6) |
| Missing | 5.4% | 4.4% | 5.6% | 10.8% | 15.4% |
| Days on Waitlist (SD) | 651 (590) | 737 (681) | 499 (461) | 488 (523) | 401 (466) |
| Years btw. Previous Tx. and Kidney Tx. (SD) | *** | 7.8 (4.4) | 7.6 (2.9) | 6.7 (4.7) | 4.3 (3.2) |
| 1st transplant to kidney wait list - Years (SD) | *** | 5.7 (4.2) | 6.2 (2.7) | 5.4 (4.4) | 3.9 (3.8) |
| 1st transplant to dialysis - Years (SD) | *** | 3.9 (5.2) | 6.6 (3.0) | 5.2 (4.9) | 3.8 (3.5) |
| Dialysis to renal transplant - Years (SD) | 3.2 (3.0) | 3.9 (3.8) | 1.7 (1.2) | 2.0 (2.0) | 1.7 (1.9) |
| 1st transplant to death - Years (SD) | *** | 9.5 (4.5) | 8.8 (3.9) | 9.9 (4.9) | 7.6 (3.0) |
| Dialysis at listing | *** | 9.4 (4.5) | 9.3 (4.5) | 11.0 (4.2) | 7.7 (3.0) |
| No dialysis at listing | *** | 10.3 (4.1) | 8.1 (3.3) | 9.4 (5.0) | 6.8 (2.9) |
| Median Adjusted Survival Following Kidney Transplantation - Years | |||||
| Graft | 8.4 (8.3–8.5) | 8.3 (7.9–8.7) | 4.2 (3.6–4.8) | 7.9 (6.2–9.6) | 8.1 (7.2–9.0) |
| Patient | 13.3 (13.1–13.5 | 11.6 (11.3–11.9) | 4.9 (2.9–6.9) | 11.6 (10.2–13.0) | 11.6 (11.2–12.1) |
all values significantly different between groups (p < 0.001)
Wait List Survival
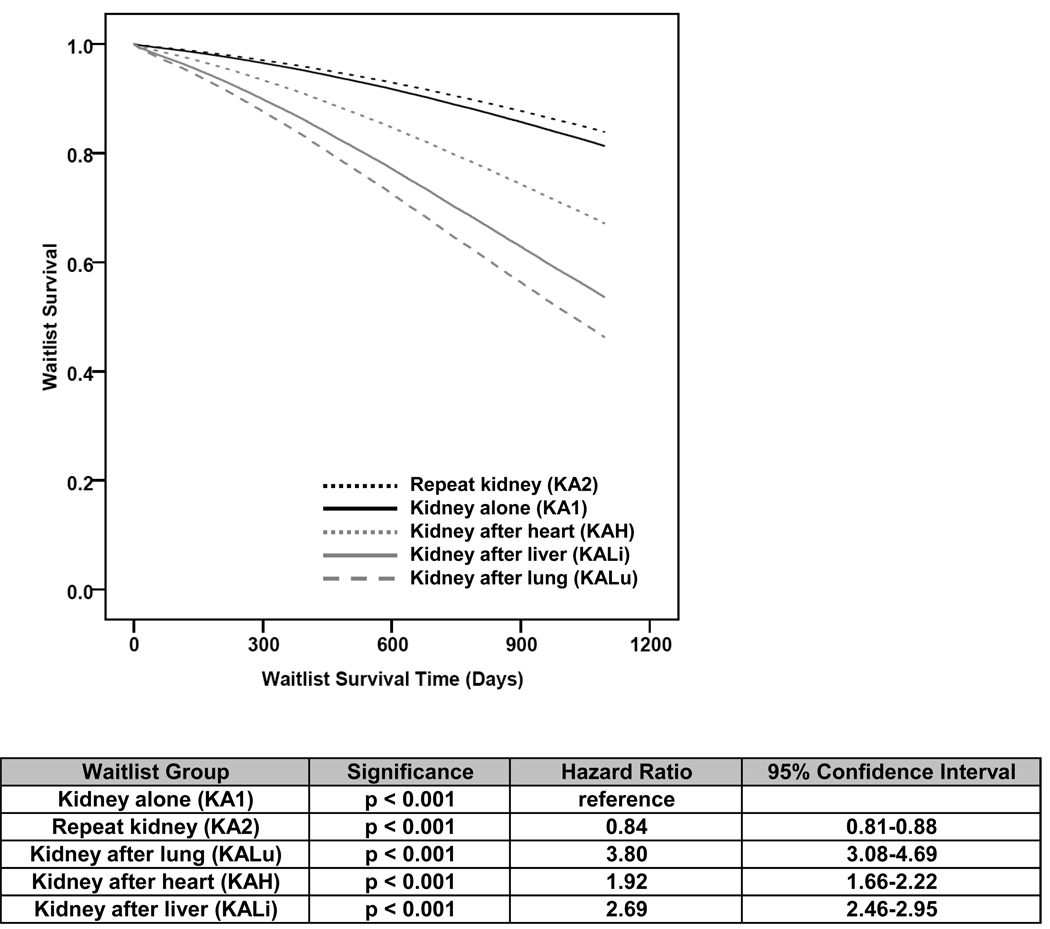
In examining the number of wait list removals due to death or illness per 100 wait list years, KA2 had the least with 5.4 for those not on dialysis at the time of listing and 7.3 deaths per 100 wait list years for those on dialysis at the time of listing. This was followed by KA1 with 7.5 and 8.6, KAH with 15.8 and 20.3, KALi with 22.6 and 24.9, and KALu with 18.7 and 30.7 deaths per 100 wait list years for those patients not on dialysis and those on dialysis at the time of listing, respectively. A 3-year adjusted analysis of wait list survival (Figure 2) using KA1 as the reference demonstrates that the KA2 population (HR = 0.84, CI = 0.81–0.88, p<0.001) had the lowest risk of wait list removal. KAH (HR = 1.92, CI = 1.66–2.22, p<0.001), followed by KALi (HR = 2.69, CI = 2.46–2.95, p<0.001) and KALu (HR = 3.80, CI = 3.08–4.69, p<0.001) demonstrated increasing risk of wait list removal due to death or illness.
Figure 2.
Adjusted 3 year kidney wait list survival.
Transplant Survival Benefit
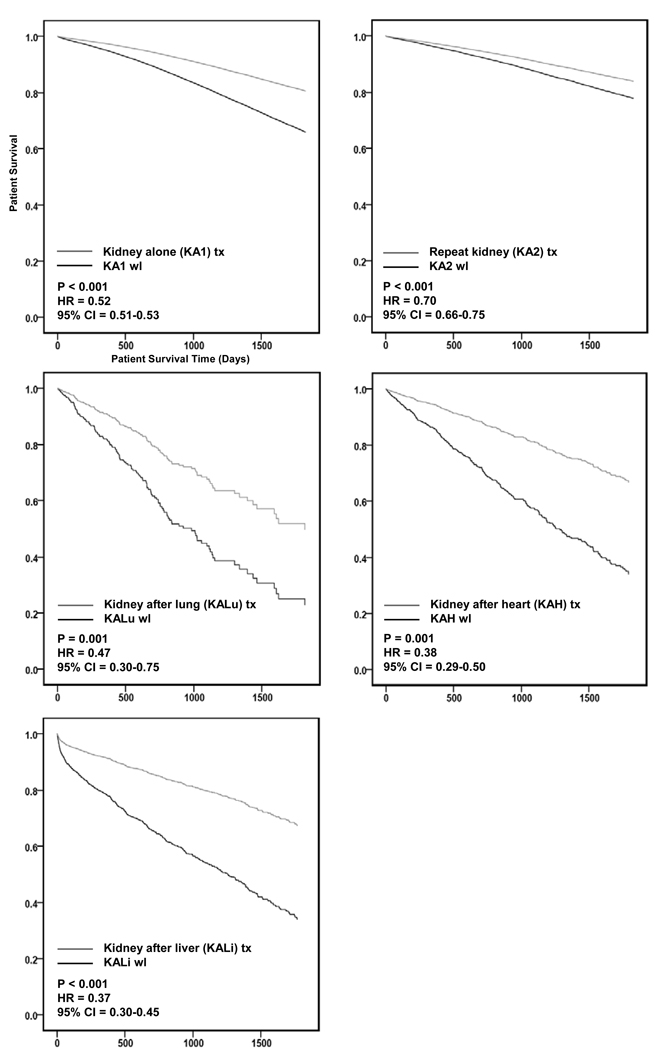
A significant survival benefit from renal transplantation with a deceased donor kidney compared to remaining on the kidney wait list was observed for all clinical settings: KALu (HR = 0.47, CI = 0.30–0.75, p = 0.001), KAH (HR = 0.38, CI = 0.29–0.50, p = 0.001), KALi (HR = 0.37, CI = 0.30–0.45, p < 0.001), KA1 (HR = 0.52, CI = 0.51–0.53, p < 0.001), and KA2 (HR = 0.70, CI = 0.66–0.75, p < 0.001) (Figure 3).
Figure 3.
5 year adjusted survival benefit of transplantation compared to remaining on the wait list.
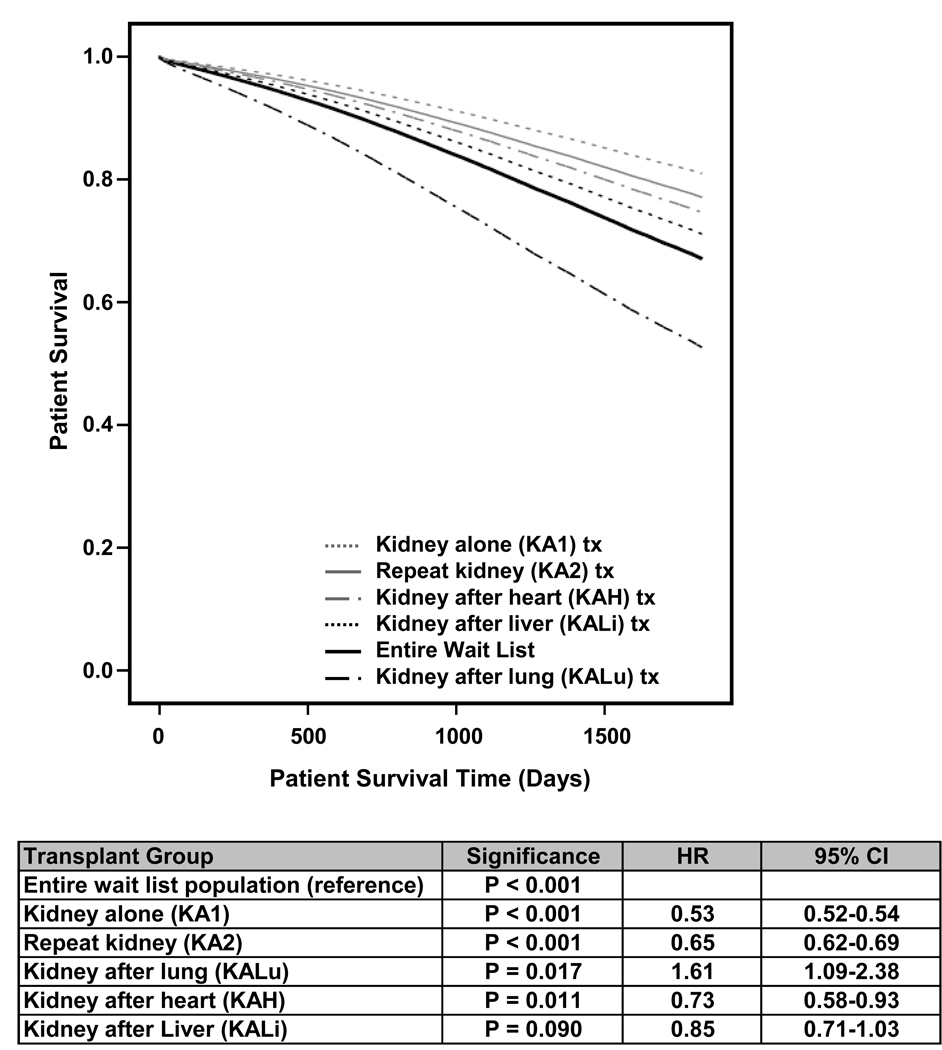
In order to estimate the relative survival benefit from a deceased donor kidney transplant across the different transplant settings, we performed an adjusted analysis comparing the risk of wait list removal due to death or illness using the entire wait list population as the reference (Figure 4) to outcomes of post-kidney transplant survival among KA1, KA2, KAH, KALi, and KALu. In this analysis, KA1 had the lowest hazard ratio (HR = 0.53, CI = 0.52–0.54, p<0.001). The relative risk of death after renal transplant was significantly decreased compared to the risk of death of the wait list population for sequential transplantation in KA2 (HR=0.65, CI = 0.62–0.69, p < 0.001) and KAH (HR=0.73, CI = 0.58–0.93, p = 0.011), and approached significance for KALi (HR=0.85, CI = 0.71–1.03, p = 0.09). Among KALu the risk of death after a kidney transplant (HR = 1.61, CI = 1.09–2.38, p = 0.017) was greater than the risk of death or removal from the entire wait list population.
Figure 4.
5 year adjusted transplant survival compared to survival of the entire wait list
The impact of living donation on survival benefit was compared to that of deceased donation. KALu had the greatest percentage of living donation, 57.6% (N=98) of all KALu transplants, with 29.3% (N=39,067) for KA1, 24.9% for KA2 (N=3,548), 29.6% for KAH (N=152), and 19.1% for KALi (N=132). The survival benefit of receiving a living donor kidney or a deceased donor kidney as compared to remaining on the wait list was evaluated in a separate model for each clinical scenario. Compared to receiving a deceased donor kidney, receipt of a living donor kidney was associated with an approximately two-fold increase in survival benefit as compared to deceased donor kidney transplantation for KA1, KA2, and KALu, while KAH and KALi living donation was associated with a 1.3 fold or greater increase in survival benefit compared to a deceased donor (Table 4).
Table 4.
Five year adjusted survival benefit of living (LD) or deceased (DD) donor kidney transplantation compared to remaining on the wait list.
| Transplant Group | Significance | HR | 95% CI |
|---|---|---|---|
| Kidney alone (KA1) LD | p < 0.001 | 0.27 | 0.27–0.28 |
| KA1 DD | p < 0.001 | 0.52 | 0.51–0.53 |
| Repeat kidney (KA2) LD | p < 0.001 | 0.38 | 0.33–0.43 |
| KA2 DD | p < 0.001 | 0.70 | 0.66–0.75 |
| Kidney after lung (KALu) LD | p < 0.001 | 0.29 | 0.17–0.49 |
| KALu DD | p = 0.002 | 0.50 | 0.32–0.78 |
| Kidney after heart (KAH) LD | p < 0.001 | 0.30 | 0.20–0.45 |
| KAH DD | p < 0.001 | 0.38 | 0.29–0.50 |
| Kidney after liver (KALi) LD | p < 0.001 | 0.23 | 0.15–0.37 |
| KALi DD | p < 0.001 | 0.37 | 0.30–0.45 |
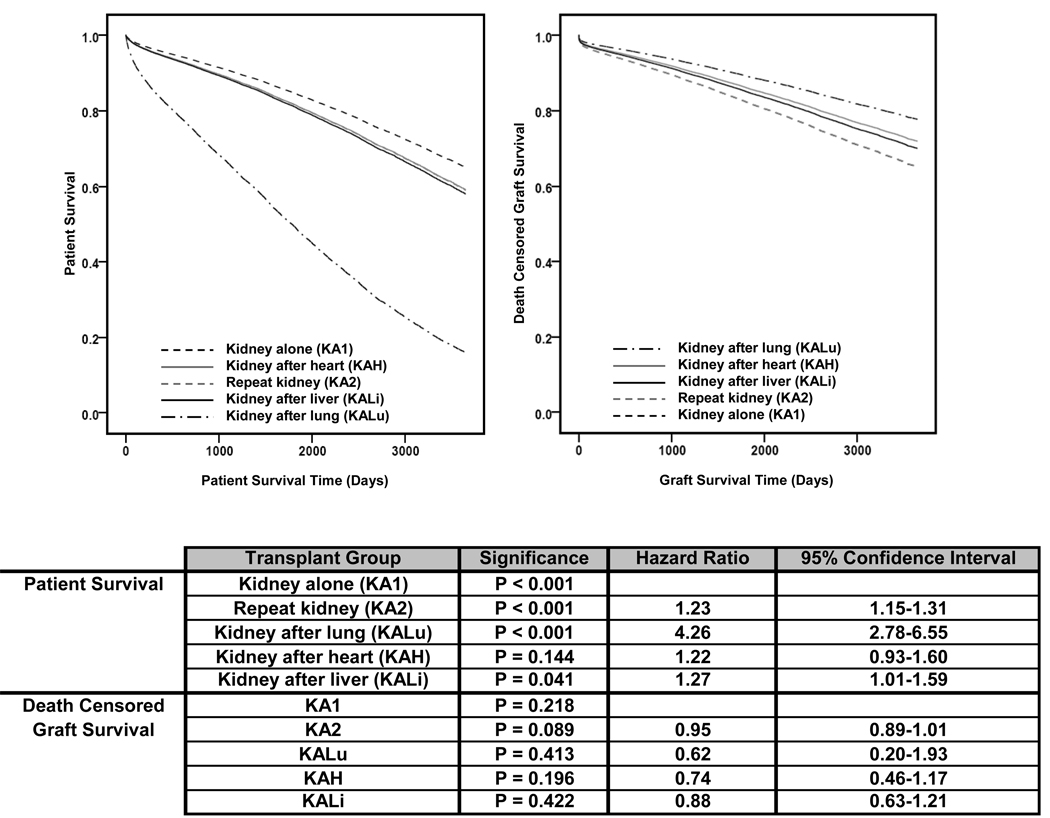
Post Transplant Survival
Ten-year adjusted patient and death-censored graft survival for recipients of a deceased donor kidney are presented in Figure 5. Patient survival in the KA1 population was greatest, while KALu was associated with the poorest survival (HR = 4.26, CI = 2.78–6.55, p<0.001). KA2 (HR = 1.23, CI = 1.15–1.31, p<0.001), KAH (HR = 1.22, CI = 0.93–1.60, p=0.144), and KALi (HR = 1.27, CI = 1.01–1.59, p=0.041) patient survival were similar. No significant differences in death-censored graft survival compared to KA1 were observed. Five years after transplant, death with a functioning graft accounted for 80.0% of graft loss in KALu, 72.0% in KAH, 64.3% in KALi, 47.5% in KA1, and 40.8% in KA2 (p < 0.001).
Figure 5.
10 year adjusted post transplant patient and death censored graft survival.
Discussion
The growing mismatch between the number of kidney transplant candidates and available organs has led to efforts to revise the allocation system for kidney allografts so as to maximize recipient survival benefit.15,23 Allocating kidney transplants to recipients of a prior solid organ, however, poses potential problems for the guiding ethical principles of equity and efficiency.18 Given clinical heterogeneity between the different groups of prior solid organ transplant recipients with chronic kidney disease, we studied the transplant benefit for each group compared to the waiting list population for each group, as well as the relative transplant benefit between the different clinical scenarios compared to the entire waiting list. Our results show that kidney transplantation offers superior survival compared to remaining on the waiting list for all groups examined. However, recipients of a primary isolated kidney transplant have the greatest post-transplant survival and compared to the overall kidney wait list, the greatest survival benefit.
With improvements in survival after non-renal transplantation, greater numbers of patients with a prior heart, liver, or lung transplant are developing renal disease and joining the waiting list for a kidney transplant. Although the number of non-renal transplant recipients added to the kidney wait list is a relatively small fraction of the entire wait list, the number of these patients is increasing at a rapid rate. As these patients are competing for a limited resource, policy-makers and clinicians should understand the high risk of death for prior organ recipients while waiting for a kidney transplant as well as the potential survival benefit of a kidney transplant compared to patients with advanced chronic kidney disease who are waiting for a primary or repeat transplant.
This study demonstrates that prior non-renal transplant recipients with advanced chronic kidney disease have a greater risk of dying or being delisted while waiting for a kidney compared to patients awaiting an isolated kidney transplant. Compared to KA1, the risk of death was 192, 269, and 380 percent higher for patients with a previous heart, liver, or lung respectively. The increased risk of death or being delisted occurred despite some conditions generally associated with improved outcome such as earlier referral for kidney listing, lower rates of dialysis, and shorter waiting time for a kidney transplant. Unfortunately, cause of death data are frequently missing in the UNOS dataset, and therefore, we did not analyze cause of death by recipient subgroup. An increased risk of cardiovascular morbidity and mortality is well described and associated with severity and duration of chronic kidney disease among patients who are not transplant recipients.
Thus, it is likely that cardiovascular disease accounts for a substantial portion of patients delisted.24–25 Our findings are consistent with a recent report from Canada showing that the risk of death for dialysis-dependent liver recipients is increased compared to dialysis patients without a prior transplant. In that study, the five year survival was 17 percent, compared to 43 percent among dialysis patients without a liver transplant.26 A similar finding was noted in dialysis dependent heart transplant recipients compared to a non-transplant dialysis cohort.27
Our study also revealed that the relative survival benefit from a kidney allograft varies across recipient groups. In order to compare the survival benefit of a kidney transplant among the different clinical groups, transplant survival hazards for each group were compared to the risk of death or delisting from the wait list population as a whole. A kidney allograft will offer the greatest survival benefit if allocated to a patient without a prior transplant. Kidney transplantation in a patient with a previous heart demonstrated a survival advantage, but the risk reduction was less than if the transplant had been performed in a primary kidney or repeat kidney transplant setting (KA1 or KA2). Kidney transplantation in previous liver recipients improved survival compared to the kidney wait list, however this did not reach statistical significance. This analysis was particularly instructive with regard to KALu recipients. Although KALu recipients had a marked survival advantage with a kidney transplant when compared to the KALu wait list, the risk of death after a kidney transplant for KALu remained quite elevated, such that lung transplant recipients with a kidney transplant had a 61 percent greater risk of death versus all individuals on the waiting list for a kidney.
The results of this study are consistent with previous findings that have addressed graft and patient survival in the setting of retransplantation. Using the Scientific Registry of Transplant Recipients, Magee et. al. demonstrated inferior graft survival among patients retransplanted with the same organ for kidney, liver, lung, and heart compared to first transplants.1 Despite inferior graft survival for recipients of a kidney retransplant compared to a primary transplant, Rao et. al. and Ojo et. al. have shown a patient survival advantage with repeat kidney transplantation compared to remaining on dialysis after primary graft failure, although the survival advantage may not apply to those who received an extended criteria organ.10–11,28 Similar to our results, the survival benefit of a kidney after a non-renal transplant has been demonstrated for heart and lung recipients as well as non-renal transplant recipients as a group, but our study is the first to specifically address this issue among prior liver transplant recipients8,9.
Our results may help inform efforts to change kidney allocation in a way that optimally matches projected patient and allograft survival. We found that KALu post-kidney survival was markedly inferior to KA1 kidney recipients, with a 235 percent increased risk of death, and poorer survival when compared to KALi kidney recipients. Patient survival after kidney transplant was similar among KA2, KAH, and KALi,. Additionally, this analysis demonstrates the high incidence of graft failure due to patient death among non-renal kidney transplant recipients. This high rate of allograft loss due to patient death raises concerns about “wasted” years of potential allograft life that could be realized in an allocation strategy that optimally matched projected patient and allograft survival.
Our findings may also be helpful to healthcare providers of patients with non-renal transplants and advanced CKD. The high risk of death on the waiting list should encourage early listing in order to facilitate a timely kidney transplant. Additionally, in regions where listing for an expanded criteria donor organ shortens waiting time, patients should strongly consider this option. Use of an extended criteria organ may also help to decrease the number of organs which fail due to patient death, which is 80.0, 72.0, and 64.3 percent among KALu, KAH, and KALi respectively. Our findings are also instructive when advising potential living donors considering donating a kidney to a patient with a non-renal transplant. Living donor kidney transplantation improves the survival benefit compared to a deceased donor transplant. However, the shortened patient survival, particularly among previous lung recipients, and the likelihood of death with a functioning graft, may influence a potential donor’s decision.
This study employed registry data and has limitations. Residual confounding by unmeasured clinical attributes could have influenced the results. These confounders may include differences in how prior organ transplant recipients were evaluated and selected for kidney transplantation. Patients with a prior transplant likely received closer medical attention as demonstrated by the number of preemptive kidney transplants and the greater percentage of living donor transplants in certain groups. Underscoring some of the differences between the wait list groups is the increased risk of wait list removal in non-renal recipients despite evidence of earlier referral for kidney transplantation. The non-renal kidney recipients had significantly shorter waiting times for a deceased donor kidney transplant, suggesting potential unmeasured differences in donor selection and an area for future investigation.
In summary, our analysis demonstrates an elevated risk of death or removal from the kidney wait list among patients with a history of previous liver, lung, or heart transplant. Recipients of a prior heart or liver can achieve similar post kidney transplant survival as a repeat kidney transplant recipient. These data should encourage the timely referral of medically appropriate non-renal transplant recipients for kidney transplantation and also has implications for organ allocation policy that focuses on maximizing patient survival.
Table 3.
Subject characteristics of deceased kidney donors.
| Kidney alone KA1 |
Repeat kidney KA2 |
Kidney after lung KALu |
Kidney after heart KAH |
Kidney after liver KALi |
Significance | |
|---|---|---|---|---|---|---|
| Age - Years (SD) | 37.6 (17.2) | 35.4 (15.9) | 36.3 (19.0) | 37.8 (16.8) | 38.8 (15.5) | p < 0.001 |
| Gender | p = 0.271 | |||||
| Male | 59.2% | 60.1% | 54.2% | 60.8% | 57.8% | |
| Female | 40.8% | 39.9% | 45.8% | 39.2% | 42.2% | |
| Race | ||||||
| White | 72.8% | 75.5% | 84.7% | 79.7% | 71.3% | |
| Black | 11.7% | 10.4% | 8.3% | 10.0% | 10.8% | |
| Other | 15.5% | 14.0% | 6.9% | 10.3% | 18.0% | |
| Diabetes | 5.1% | 4.4% | 4.2% | 6.2% | 6.5% | p = 0.009 |
| Missing | 0.6% | 0.6% | 1.4% | 1.1% | 0.2% | |
| Hepatitis C Status | p < 0.001 | |||||
| Negative | 97.0% | 97.0% | 97.2% | 97.5% | 87.4% | |
| Positive | 2.5% | 2.5% | 1.4% | 2.2% | 11.8% | |
| Unknown / Missing | 0.5% | 0.5% | 1.4% | 0.3% | 0.7% | |
| Expanded Criteria Donor | 17.2% | 10.5% | 13.9% | 17.2% | 14.0% | p < 0.001 |
| Cold Ischemic Time - Hours (SD) | 19.1 (8.8) | 19.2 (8.2) | 18.7 (8.1) | 18.7 (9.5) | 18.1 (9.1) | p = 0.082 |
| Missing | 11.1% | 11.6% | 13.9% | 13.9% | 18.9% | |
Acknowledgements
Funding Sources: James Cassuto was supported by an American Society of Transplantation – Roche Presidential Student Mentor Award. Dr. Reese was supported by NIH Career Development Award, K23 - DK078688-01. This work was supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
The other authors report no financial disclosures. There are no conflicts of interest to disclose.
References
- 1.Magee JC, Barr ML, Basadonna GP, et al. Repeat organ transplantation in the United States, 1996–2005. Am J Transplant. 2007;7:1424–1433. doi: 10.1111/j.1600-6143.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 2.Chandrakantan A, de Mattos AM, Naftel D, Crosswy A, Kirklin J, Curtis JJ. Increasing referral for renal transplant evaluation in recipients of nonrenal solid-organ transplants: a single-center experience. Clin J Am Soc Nephrol. 2006;1:832–836. doi: 10.2215/CJN.01191005. [DOI] [PubMed] [Google Scholar]
- 3.Cohen DJ, St Martin L, Christensen LL, Bloom RD, Sung RS. Kidney and pancreas transplantation in the United States, 1995–2004. Am J Transplant. 2006;6:1153–1169. doi: 10.1111/j.1600-6143.2006.01272.x. [DOI] [PubMed] [Google Scholar]
- 4.Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 5.Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy. Kidney Int. 1996;50:1089–1100. doi: 10.1038/ki.1996.415. [DOI] [PubMed] [Google Scholar]
- 6.Lindelow B, Bergh CH, Herlitz H, Waagstein F. Predictors and evolution of renal function during 9 years following heart transplantation. J Am Soc Nephrol. 2000;11:951–957. doi: 10.1681/ASN.V115951. [DOI] [PubMed] [Google Scholar]
- 7.Hamour AM, Omar F, Lyster HS, Palmer A, Banner NR. Chronic kidney disease after heart transplantation. Nephrol Dial Transplant. 2009;24:1655–1662. doi: 10.1093/ndt/gfn759. [DOI] [PubMed] [Google Scholar]
- 8.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 9.Lonze BE, Warren DS, Stewart ZA, et al. Kidney transplantation in previous heart or lung recipients. Am J of Transplant. 2009;9:578–585. doi: 10.1111/j.1600-6143.2008.02540.x. [DOI] [PubMed] [Google Scholar]
- 10.Ojo AO, Wolfe RA, Agodoa LY, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System. Transplantation. 1998;66:1651–1659. doi: 10.1097/00007890-199812270-00014. [DOI] [PubMed] [Google Scholar]
- 11.Rao PS, Schaubel D, Wei G, Fenton S. Evaluating the survival benefit of retransplantation. Transplantation. 2006;82:669–674. doi: 10.1097/01.tp.0000235434.13327.11. [DOI] [PubMed] [Google Scholar]
- 12.Gupta J, Amaral S, Mahle WT. Renal transplantation after previous pediatric heart transplantation. J Heart Lung Transplant. 2008;27:217–221. doi: 10.1016/j.healun.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Coopersmith CM, Brennan DC, Miller B, et al. Renal transplantation following previous heart, liver, and lung transplantation; an 8-year single-center experience. Surgery. 2001;130:457–462. doi: 10.1067/msy.2001.115834. [DOI] [PubMed] [Google Scholar]
- 14.Federal Register. 42 CFR Part 121. 1998 April 2;Vol 63(No. 63) [Google Scholar]
- 15.Stock PG. Balancing multiple and conflicting allocation goals: a logical path forward. Am J Transplant. 2009;9:1519–1522. doi: 10.1111/j.1600-6143.2009.02715.x. [DOI] [PubMed] [Google Scholar]
- 16.2007 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997–2006. Rockville, MD: Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation;
- 17.Summary of the Kidney Committee Report to the OPTN Board of Directors. 2007 September 20; www.unos.org/CommitteeReports/board_main_KidneyTransplantationCommittee_9_20_2007_16_41.pdf.
- 18.Ubel PA, Arnold RM, Caplan AL. Rationing failure: the ethical lessons of the retransplantation of scarce vital organs. JAMA. 1993;270:2469–2474. doi: 10.1001/jama.270.20.2469. [DOI] [PubMed] [Google Scholar]
- 19.Hippen B. The kidney allocation score: methodological problems, moral concerns and iunintended consequences. Am J Transplant. 2009;9:1507–1512. doi: 10.1111/j.1600-6143.2009.02594.x. [DOI] [PubMed] [Google Scholar]
- 20.Stegall MD. Developoing a new kidney allocation policy: the rational for including life years from transplant. Am J Transplant. 2009;9:1528–1532. doi: 10.1111/j.1600-6143.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 21.Freeman RB, Mataws AT, Henry M, Segev DL, Kaufman DB, Roberts JP. Moving kidney allocation forward; the ASTS perspective. Am J Transplant. 2009;9:1501–1506. doi: 10.1111/j.1600-6143.2009.02697.x. [DOI] [PubMed] [Google Scholar]
- 22.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 23.Bromberg J, Gill J. Heavy LYFTing: KASting pearls before swine. Am J Transplant. 2009;9:1489–1490. doi: 10.1111/j.1600-6143.2009.02688.x. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol. 2001;38:705–711. doi: 10.1016/s0735-1097(01)01450-4. [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Riyami DA, Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M. Decreased survival in liver transplant patients requiring chronic dialysis: a Canadian experience. Transplantation. 2008;85:1277–1280. doi: 10.1097/TP.0b013e31816c4e6b. [DOI] [PubMed] [Google Scholar]
- 27.Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M. The outcome of heart transplant recipients following the development of end-stage renal disease: analysis of the Canadian Organ Replacement Register. Am J Transplant. 2007;7:461–465. doi: 10.1111/j.1600-6143.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 28.Miles CD, Schaubel DE, Jia X, Ojo AO, Port FK, Rao PS. Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant. 2007;7:1140–1147. doi: 10.1111/j.1600-6143.2007.01742.x. [DOI] [PubMed] [Google Scholar]