Abstract
We have shown that CD39 and CD73 are co-expressed on the surface of murine CD4+Foxp3+ regulatory T cells (Treg) and generate extracellular adenosine, contributing to Treg immunosuppressive activity. We now describe that CD39, independently of CD73, is expressed by a subset of blood derived human CD4+CD25+CD127lo T regulatory cells (Treg), defined by robust expression of Foxp3. A further distinct population of CD4+CD39+ T lymphocytes can be identified, which do not express CD25 and FoxP3 and exhibit the memory effector cellular phenotype. Differential expression of CD25 and CD39 on circulating CD4+ T cells distinguishes between Treg and pathogenic cellular populations that secrete pro-inflammatory cytokines such as IFNγ and IL-17. These latter cell populations are increased, with a concomitant decrease in the CD4+CD25+CD39+ Tregs, in the peripheral blood of patients with renal allograft rejection. We conclude that the ectonucleotidase CD39 is a useful and dynamic lymphocytes surface marker that can be used to identify different peripheral blood T cell populations to allow tracking of these in health and disease, as in renal allograft rejection.
INTRODUCTION
CD39 is an ectonucleotidase that is co-expressed with CD73 in the mouse by a subset of CD4+ regulatory T cells (Treg) (1). Extracellular nucleotides e.g. ATP and ADP are hydrolyzed by CD39 to AMP (2); which is subsequently converted to adenosine by CD73 (3). The identification of both CD39 (1) and CD73 (1, 4) on murine Treg suggests that adenosine could serve as an important immunomodulatory component of the Treg suppressive repertoire (1).
In mice, two subpopulations of CD4+CD39+ T cells can be identified. One subset is Foxp3+CD73+ comprising of bona fide Treg (1). The other subset is Foxp3−CD73−, is non-suppressive and has a memory phenotype (5). This latter group expresses higher levels of mRNA for T-helper (Th) lineage specific cytokines, typically encompassing all Th1, Th2 and Th17 subtypes. Upon activation these cells rapidly secrete pro-inflammatory cytokines. Many murine Treg manifest an unstable phenotype with transient or unstable Foxp3 expression and as such exhibit phenotype plasticity. These “exFoxp3” T cells exhibit an activated memory phenotype and produce inflammatory cytokines such as IFNγ and IL-17A (6).
In humans, the Treg molecular signature is still evolving. The expression of CD39 by human Treg (7) is restricted to a subset of T regulatory effector memory cells (8) capable of suppressing IL-17 production (9). In some systems, the mechanism by which immunoregulation suppression is exerted is contact dependent (9). Moreover, CD39+ Treg abrogate ATP – dependent effects such as cellular toxicity and maturation of dendritic cells (8).
In contrast to the mouse, Foxp3+CD4+ T cells in human peripheral blood encompass both Treg and non-Treg cells (10). The latter are characterized by the absence of cell surface expression of CD39 (9) and the ability to secrete IFNγ, IL-2, and IL-17, and thereby to contain cells with Th17 potential.
We show that within the human CD4+ T cell population the differential expression of CD25 and CD39 can be used to identify four distinct CD4+ T cell populations. CD4+CD25+CD39+ expression identifies a Treg subset while CD4+CD25+CD39− expression denotes a population of T cells with Th17 potential, in accordance with recently published data (8–9). In contrast to the phenotype observed in mice, CD73 is not substantially co-expressed with CD39 in these Treg populations. Moreover, CD39+ expression in the absence of CD25 expression further identifies a memory phenotype, which differentiates pathogenic effector memory cells (11) from regulatory memory cells. Such CD4+CD25−CD39+ T cells may represent pro-inflammatory “exFoxp3” effector memory cells, recently defined in mice (6), which are increased in peripheral blood of patients with antibody mediated renal allograft rejection.
MATERIALS AND METHODS
Human peripheral blood mononuclear cell preparation and Treg isolation
Peripheral blood mononuclear cells (PBMC) from controls were prepared by density gradient centrifugation on Ficoll-Paque (GE Healthcare, Uppsala, Sweden). The protocol to obtain volunteer human blood samples was approved by the Beth Israel Deaconess Medical Center Institutional Review Committee. CD4+ T cells were isolated by negative selection using CD4+ no-touch T cell isolation kit (Miltenyi Biotec, Auburn, CA). For some experiments, leukofilters were collected (Blood Donor Center at Children’s Hospital, Boston, MA), and CD4+ T cells were isolated using Rosette-sep Human CD4+ T cell isolation kit (Stemcell technologies, Vancouver, Canada) and by density gradient centrifugation on Lymphoprep (Nycomed, Oslo, Norway). Treg were positively selected by staining for CD25 and CD39 using PE or FITC selection kits. Flow cytometry cell sorting (FACS Aria, BD Biosciences, San Jose, CA) was used to obtain highly pure (>98%) Treg (CD4+CD25+CD127low/−).
Antibodies and cytokines
Antibodies used: anti-human FITC-, APC-CD39 (Ancell, Bayport, MN); PE-CD3; PE-Cy5-, PerCP-Cy5.5-, PE-Cy7-CD4;-CD8;-CD19; APC-Cy7-, PE-CD25; PE-IL-17A; Pacific Blue IFNγ; Alexa Fluor 647-CD127 (eBioscience, San Diego, CA); PE-CD127, -CD45RO, and -CD73 (BD Pharmingen, Franklin Lakes, NJ); PE-CD44;-CD62; Alexa Fluor 488-, APC-FOXP3 (Biolegend, San Diego, CA). Human recombinant IL-1β, IL-2, IL-23 and TGFβ1 from R&D systems (Minneapolis, MN).
Cytokine analysis
CD4+ T cells were sorted into CD4+CD25+CD39+/− populations and stimulated with 1μg/mL PMA and 0.5μm/mL PHA for 72 hours. Supernatant was analyzed by BD Cytometric Bead Array kit (BD Biosciences, San Jose, California).
Treg culture methods
FACS-sorted Tregs were activated with anti-CD3/CD28 coated microbeads (Invitrogen Dynal AS, Oslo, Norway) in a 96-well U-bottom plate. Two x105 cells/well were cultured in a 1:1 cell:bead ratio, in a final volume of 250 μL of complete medium (RPMI-1640 containing L-Glutamine (Cellgro, Mediatech Inc, Manassas, VA), 1% non-essential amino acids (BioWhittaker, Walkersville MD), 10% FBS (Gemini Bio-Products, West Sacramento, CA), 50 U/ml penicillin and 50 μg/ml streptomycin (Gemini Bio-Products)). Tregs were cultured in triplicate in the absence or presence of cytokines to induce Th17-polarizing conditions(12).
Intracellular cytokine staining and phenotypic analysis
During the last 4 hours of culture, cells were stimulated with PMA (100 ng/ml) and ionomycin (1 μg/ml) in the presence of Brefeldin A (10 μg/ml, all from Sigma-Aldrich, St. Louis, MO). Intracellular cytokine staining was performed according to manufacturer’s instructions using eBioscience Foxp3 staining Kit. The labeled cells were analyzed by flow cytometry on LSRII (Becton Dickinson) using FACSDiva (BD) and FlowJo software (Tree Star, Ashland, OR).
End Stage Renal Failure and transplant patient studies
Patients with end stage renal failure (ESRF) were recruited from the nephrology unit at St. Vincent’s Hospital (Melbourne), Australia. Some have received a renal transplant and have been studied in the early post-operative period (post operative day 1–6) or at least 5 years post transplantation with stable allograft function. Written informed consent was obtained. The study was approved by the St. Vincent’s Hospital (Melbourne) Human Ethics Committee.
Leucocytes were obtained by Ficoll gradient and CD4+ T cells isolated using MACS microbeads (Miltenyi Biotec). CD4+ T cells were stained with anti-human PE-Cy7-CD4, APC- CD39, APC-Cy7-CD25, PE -CD73, followed by fixation, permeabilization and intracellular staining with Alexa Fluoro 488- Foxp3. Samples were analyzed on SRII Fortessa (BD) using FlowJo software. Statistical analysis was performed using Prism 4.0 software (GraphPad). A one-way ANOVA followed by a Newman-Keuls multiple comparison test was used to determine significance.
RESULTS
CD4+CD25+/−CD39+/− expression identifies four distinct T cell populations
The use of CD4 and CD25 expression to define Treg is inherently flawed as CD25 is also expressed on conventional activated CD4+ T cells. Moreover, resting Treg express lower levels of CD25 expression than activated conventional Treg (10).
The greater proportion of CD25highCD4+ T cells express Foxp3 (Fig 1A). These CD25+ and Foxp3+CD4+ T cell populations contain both CD39+ (1.12±0.85% and 1.56±1.13% respectively, n=20) and the very minor CD39− fraction (Fig 1B, C). In addition, CD39 can be shown to be expressed by distinct CD25− and Foxp3− T cell subpopulations (1.37±1.04% and 1.07±0.92% respectively, n=20) (Fig 1B, C). The size of these populations are similar to that of CD4+CD25+(Foxp3+)CD39+ T cells and parallel our findings in mice (5).
Figure 1. CD4+ T cell populations are defined by differential CD4 CD25 and CD39 expression.
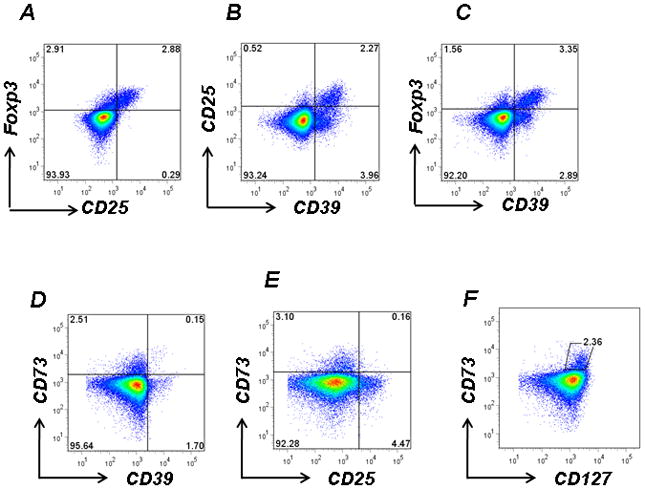
A. Human CD4+ T cells were isolated by Ficoll gradients, stained with anti-human CD25 and intracellularly labeled with Foxp3 then analyzed by FACS. B, C. Human CD4+ T cells were stained with CD25, Foxp3 and CD39. The additional use of CD39 as a phenotypic marker identifies four distinct CD4+ T cell populations.
Mutually exclusive expression of CD73 and CD39 or CD25 by CD4+ T cells
D, E. PBMCs obtained from healthy controls were stained with anti-human CD73, CD39 and CD25. Expression of CD73 appeared largely independent of either CD39 or CD25 expression.
F. CD73 was expressed at high levels on CD127high T cells
Data denote representative findings for ≥ 20 independent experiments
In mice, CD73 is expressed in tandem with CD39 on the same Treg population (1). However, CD73 is not co-expressed on human CD4+ T cells with either CD39 or CD25 (Fig 1D, E). Indeed, CD4+CD127high T cells express CD73 (Fig 1F) in the resting state. CD73 is upregulated on IFNα stimulated endothelial cells (13); however CD73 expression on naïve CD4+CD25− T cells was not altered following stimulation with IFNα (10 U/ml or 100 U/ml) or IFNγ (10 U/ml or 100 U/ml) as determined by flow-cytometry at 96 hrs (data not shown). This data highlights an important difference between the phenotype of murine and human blood Treg ex-vivo.
In summary, four distinct T cell populations may be discriminated via differential expression patterns of CD4, CD25 and CD39: CD4+CD25+CD39+; CD4+CD25+CD39−; CD4+CD25−CD39+ and CD4+CD25−CD39− T cells.
CD4+CD25+CD39+ T cells express an activated Treg molecular signature
CD4+CD25+CD39+ T cells comprise ~2% of all peripheral blood human CD4+ T cells (data not shown). The majority of these cells (80–100% depending on gating) express Foxp3 (Fig 2A) in abundance (Fig 2B).
Figure 2. CD4+CD25+CD39+ T cells have an activated Treg molecular signature.
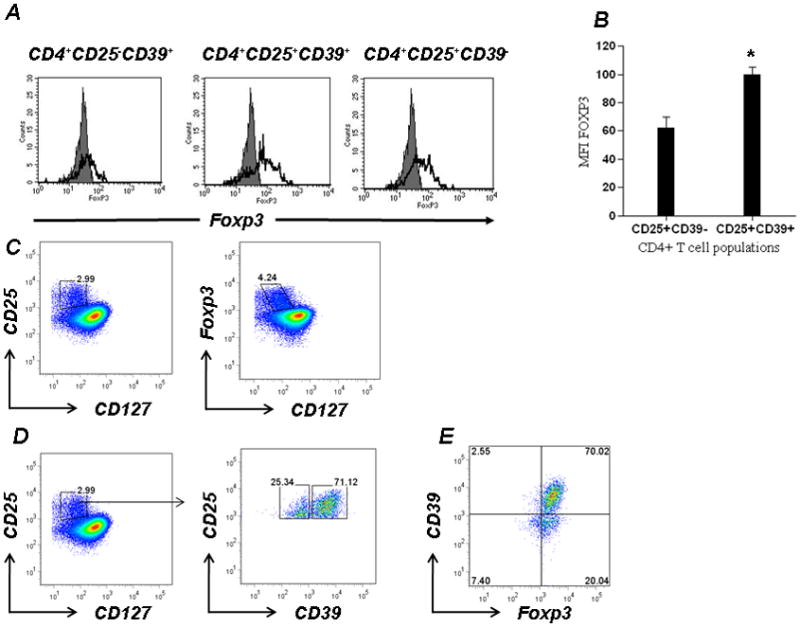
A. CD4+ T cells were stained with CD39-FITC and CD25-PE and analyzed for intracellular Foxp3 staining (open histogram).
B. The mean fluorescence intensity (MFI) for Foxp3 staining in CD4+CD25+CD39+/− T cells populations is shown (*p<0.05).
C. PBMCs obtained from peripheral blood were freshly stained without previous in vitro stimulation and stained with anti-human CD25 and anti-human CD127 and labeled with Foxp3. Expression of CD25 is restricted to CD127low population, while expression of Foxp3 extends to the CD127int population.
D. Treg cells identified based on low expression of CD127 and high expression of CD25 were stained with anti-human CD39. CD4+CD25+CD127low T cells contain 2 distinct populations comprising cells that are either CD39high or CD39low.
E. CD4+CD25+CD127low T cells were stained with anti-human CD39 and labeled with Foxp3. CD39high T cells are largely Foxp3+ while CD39low cells contain an admixture of Foxp3+ and Foxp3− T cells.
Data denote representative findings for ≥ 3 independent experiments.
To further validate these results, we incorporated staining for CD127 into this study. CD127 (IL-7R) is known to be down-regulated on Tregs in human peripheral blood and when used in combination with traditional biomarkers, identifies a highly purified Treg population (14). Although CD25 is restricted to CD127low T cells, Foxp3 expression extends into the CD127int population (Fig 2C). Within the CD4+CD25+CD127low T-cell population, 2 subsets that differentially express CD39 can be identified. In the human PBMCs (n=20), CD39 is expressed by 70±5% of CD4+CD25+CD127low Treg (Fig 2D). CD4+CD25+CD127lowCD39+ T cells are in general Foxp3+, while CD4+CD25+CD127lowCD39− T cells contain a minor Foxp3− population (Fig 2E).
Lack of CD39 on CD4+CD25+ T cells predicts cells with Th17 potential
Human peripheral blood and lymphoid tissue contain a subpopulation of CD4+Foxp3+ T cells that have the capacity to produce IL-17 (15) and are noted to be CD4+CD25++CD45RA− (10). CD4+CD25+CD39− T cells comprise ~3% of peripheral CD4+ T cells (not shown) of which 50% express Foxp3 (Fig 2A) albeit at lower mean fluorescence intensity (MFI) compared to CD4+CD25+CD39+ T cells (MFI: 100 vs. 65) (Fig 2B).
CD4+CD25+ T cells isolated from peripheral blood were cultured for 72 hours with anti-CD3/CD28-coated microbeads in presence of IL-2, or Th17 promoting conditions (IL-1β, IL-2, IL-23 and TGFβ1). Following culture with IL-2 alone minimal IL-17A expression was detected by CD4+CD39− T cells, which contained equivalent of Foxp3+ and Foxp3− Tcells (Fig 3A).
Figure 3. Lack of CD39 expression by CD4+CD25+ cells with Th17 potential.
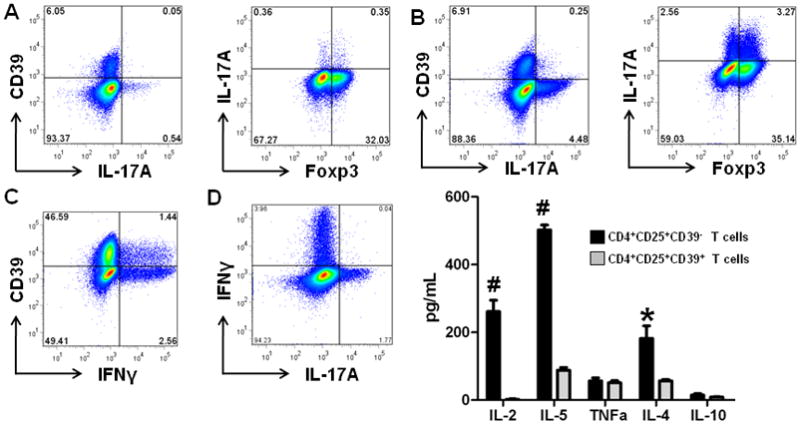
A. CD4+CD25+ T cells were stimulated by anti-CD3/CD28 and analyzed for CD39, Foxp3 and IL-17A expression.
CD4+CD25+ T cells were cultured in the presence of IL-1β, IL-2, IL-23 and TGFβ1 and then analyzed for CD39, Foxp3 and IL-17A (B); CD39 and IFNγ (C) and IFNγ and IL-17 (D) expression.
E.CD4+CD25+CD39+/− T cells were cultured with PMA for 72 hours and the supernatant analyzed for cytokine secretion. CD4+CD25+CD39− T cells (black bars); CD4+CD25+CD39+ T cells (grey bars). Data represents average ± SD (n=3); *p<0.05; #p<0.01
Data are representative of more than 3 experiments.
Under Th17 promoting conditions, the number of IL-17A expressing CD4+CD25+CD39− T cells increased 6–8 fold, which was confirmed in three separate experiments. These IL-17A expressing T cells are recruited directly from the CD4+CD25+CD39− T cell compartment and are equally divided between Foxp3+ and Foxp3− T cells (Fig 3B). No IL-17A production is detected in the CD39+ T cells. In the Th17-promoting culture conditions, two thirds (64%) of IFNγ producing cells are CD39− (Fig 3C). Under these culture conditions, there are distinct populations of IFNγ and IL-17A producing cells (Fig 3D).
To examine the impact of CD39 expression on the cytokine profile of CD4+CD25+ T cells, CD4+ T cells were then sorted on the basis of CD25 and CD39 expression into CD4+CD25+CD39+ and CD4+CD25+CD39− T cell populations, cultured for 72 hours, and the supernatant was analyzed for various cytokines following stimulation. The putative Treg pool characterized by CD4+CD25+CD39+ expression did not proliferate or produce cytokines In contrast, CD4+CD25+CD39− T cells proliferated and secreted large amounts of IL-2, IL-4 and IL-5, and lesser amounts of IL-10 and TNFα (Fig 3E). Together, these data highlight the heterogeneous nature of this small T cell population, which encompasses cells with Th1, Th2 and Th17 potential.
CD39 expression denotes memory CD4+ T cell phenotypes and impacts cytokine production
In mice, CD4+CD39+ T cells are subdivided into equivalent proportions of Foxp3+ Treg (1) and Foxp3− effector memory T cells (5). The vast majority of human CD4+CD39+ T cells are CD45RO+ and CD45RA− (Fig 4A) and can be divided into CD25+(Foxp3+) and CD25−(Foxp3−) T cell subsets (Fig 1B,C). Over 80% of CD4+CD25+CD39+ T cells are CD45RO+ denoting a memory Treg phenotype. Moreover, CD45RO is expressed (near universally) by CD4+CD25−CD39+ T cells; a pattern consistent with the effector memory T cell phenotype (Fig 4B). A minor fraction (5–33%) of CD4+CD25+CD39− and CD4+CD25−CD39− T cells are CD45RO+.
Figure 4. CD4+ CD39+ T cells are memory T cells.
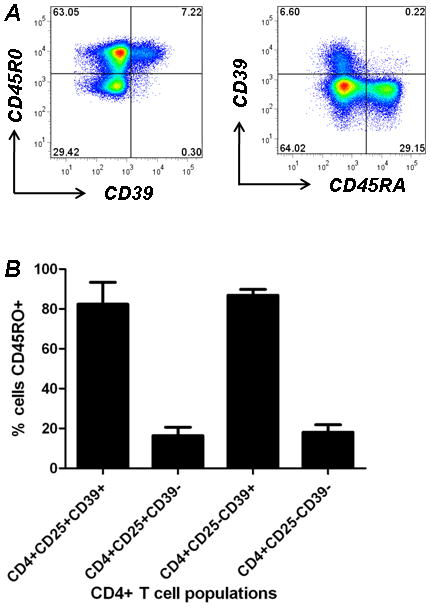
A. Human CD4+ Tcells were stained with anti-human CD39, CD45RO and CD45RA. CD4+CD39+ T cells are CD45RA− and CD45RO+.
B. Percentage of CD45RO+ cells within each CD4+ T cell population.
Data denote findings for ≥ 6 independent experiments
CD45RO+CD39− T cells were then analyzed for expression of IFNγ and IL-17A by FACS. Under Th17 promoting culture conditions, CD39 expression was consistently induced on 5–7% of CD45RO+ T cells; the majority of which are Foxp3- (Fig 5A). Of importance, CD45RO+ T cells that acquire CD39 expression produce only very low levels of IFNγ and no IL-17A (Fig 5B,C). In contrast, CD45RO+CD39+ memory T cells retain the capacity to produce both IFNγ and IL-17A (Fig 5F,G), which in large part arises from the Foxp3−CD39+ T cell population (Fig 5D); the equivalent of murine Foxp3- effector memory T cells (5).
Fig. 5. CD4+CD25−CD39+ T cells produce both IFNγ and IL-17A.
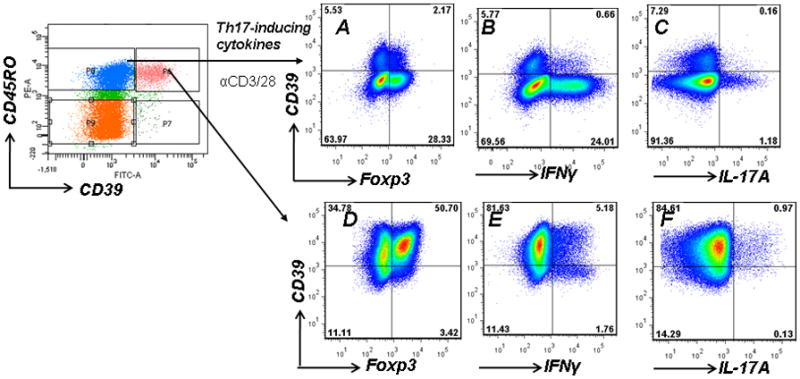
CD4+CD45RO+CD39− T cells were cultured under Th17 polarizing conditions and analyzed for CD39 and Foxp3 (A), IFNγ (B) and IL-17A (C) expression.
CD4+CDRO+CD39+ T cells were cultured under Th17 polarizing conditions and analyzed for CD39 and Foxp3 (D), IFNγ (E) and IL-17A (F) expression.
Data are representative of more than 3 experiments.
Together these data demonstrate that by utilizing three cell surface markers, CD4+CD25+CD39+ Treg cells can be differentiated from two effector T cell populations (CD4+CD25+CD39− and CD4+CD25+CD39+ T cells), which have the capacity to differentially secrete Th1, Th2 and Th17 proinflammatory cytokines.
CD4+CD25+/−CD39+/− co-expressiondefine four T cell populations in patients with end stage renal failure and following transplantation
The number of peripheral Treg cells as defined by CD4+CD25+ expression is thought to be reduced following renal transplantation (16). The proportion of cells in each CD4+ T cell population based on CD25 and CD39 co-expression was prospectively identified in patients with end stage renal failure (ESRF) (n=28) awaiting transplantation, patients in the early kidney transplant period (post-operative day 1–6, n=10) (Early Tx) and long term (more than 5 years) renal-transplant recipients (Late Tx) with stable allograft function (n=5) and compared with healthy controls (n=9). The patterns of expression on CD4+ cells using either CD25/CD39 or Foxp3/CD39 staining patterns were similar in all patient groups (Fig 6A). There was no statistically significant difference between the percentages of cells in these CD4+CD25+/−CD39+/−T cell subsets in controls, patients with ESRF, Early Tx or Late Tx as determined at a time of stable allograft function and in the absence of intercurrent illness.
Figure 6. Definition of T cell CD4+CD25+/−CD39+/− populations in patients with end stage renal failure and following renal transplantation.
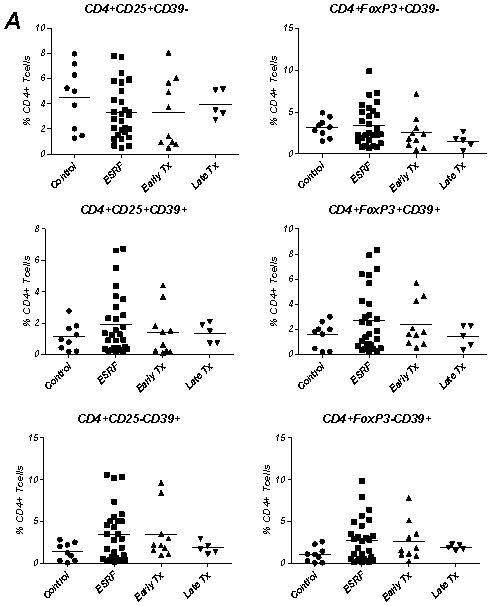

A. PBMCs were obtained and analyzed by flow cytometry from controls (n=9), patients with end stage renal failure (ESRF) (n=28), those in the early transplant period (post-operative day 1–6) (Early Tx) (n=10) and patients with stable allograft function at least 5 years post transplantation (Late Tx) (n=5). The percentage of CD4+CD25+/−(Foxp3+/−)CD39+/− T cells in each patient group was calculated. There were no significant differences noted between the groups.
B. Representative histological sections of allograft rejection (H&E)
i) Marked interstitial hemorrhage and edema with fibrinoid change in peritubular capillary (arrow)
ii) Severe glomerulitis
iii) Mild glomerulitis and inflammatory cells in ecstatic peritubular capillaries
iv) Transmural arteritis with severe intimal arteritis and focal myocyte necrosis (arrows)
C. Peripheral blood CD4+ T cells were obtained from four renal transplant patients during allograft rejection and analyzed for CD4+CD25+/−CD39+/− expression by flow cytometry. The CD4+CD25−CD39+ T cell population was increased during allograft rejection compared with both controls and stable late transplant recipients (*p<0.01).
D. The ratio of CD4+CD25−CD39+ (Tmeff): CD4+CD25+CD39+ (mTreg) was determined at the time of allograft rejection. An increase in the Tmeff and concomitant decrease in mTreg was observed.
In controls, CD4+CD25+CD39+ and CD4+CD25−CD39+ T cells are present in equivalent proportions (Fig 1B, C). A similar scenario is evident in Late Tx recipients. In patients with ESRF and early following transplantation around two thirds of CD4+CD39+ T cells were of the CD25− effector memory T cell phenotype however; this modest increase did not reach statistical significance compared with controls (data not shown).
With the advent of potent immunosuppressive regimens, the incidence of acute renal allograft rejection is low. The pattern of CD4+CD25+/−CD39+/− T cell expression was followed in four patients presenting with allograft rejection. The Banff staging and scores (17), C4d staining and the presence of donor specific antibodies in these patients are presented in Table 1 with representative histopathological sections depicted in Figure 6B. In the peripheral blood of these patients the frequencies of CD4+CD25−CD39+ T cells were increased (Fig 6C) with concomitant decrease in CD4+CD25+CD39+ T cells. This phenomenon is best appreciated when considering the ratio of CD4+CD25+CD39+ to CD4+CD25−CD39+ T cells. In renal allograft rejection, the effector memory pool is increased substantially, when compared to the Treg memory pool, and the magnitude of this increase appeared associated with the severity of rejection. Resolution of rejection biochemically and histologically was associated with more equivalent distributions of CD4+CD39+ T cells (not shown) and in all patients studied, CD73 was not expressed within the peripheral CD4+ T cell population (not shown).
Table 1.
Characteristics of kidney transplant recipients (KTR) presenting with allograft rejection.
| Patient | KTR #1 | KTR #2 | KTR #3 | KTR #4 |
|---|---|---|---|---|
| Diagnosis | Acute rejection with interstitial hemorrhage and edema + necrotising capillaritis C4d0 | Acute antibody-mediated rejection type II with severe glomerulitis + mild intimal arteritis C4d0 | Chronic active antibody-mediated rejection with mild glomerulitis, PTC mononuclear inflammatory cells and chronic allograft arteriopathy C4d1 | Acute antibody-mediated rejection type II + acute T cell-mediated rejection with severe glomerulitis and transmural arteritis type III C4d0 |
| Banff acute rejection score17 | i0 t0 g1 v1 ptc1 | i0 t0 g3 v1 ptc1 | i1 t1 g1 v0 ptc2 | i2 t2 g3 v3 ptc1 |
| Banff chronic changes score17 | ci0 ct0 cg0 cv0 ah0 mm0 | ci0 ct0 cg0 cv1 ah1 mm0 | ci1 ct1 cg0 cv2 ah1 mm0 | ci0 ct0 cg0 cv0 ah0 mm0 |
| DSA | Negative | Cw5 | A32; Cw2 | A29 |
DSA = donor specific antibodies
DISCUSSION
In this study, we show that the expression of CD39 on human peripheral CD4+ T cells is intimately associated with regulatory cell (Foxp3+) signatures and the acquisition of a memory (CD45RO+) phenotype. CD39 may allow tracking of dynamic pathogenic effector T cells, recently described in mice as “exFoxp3” cells (6), which are increased in states of inflammation such as in renal transplant rejection and, as recently described, within the joints of patients with childhood arthritis (11). Crucially, the absence of CD39 on CD4+CD25+ T cells discriminates between Treg and a population of cells with Th17 potential and is in agreement with recent data (9).
Treg are not uniquely defined by CD4+CD25+ expression as this phenotype encompasses many recently activated non-regulatory cells. CD39 together with CD73 have previously been identified as dual surface markers of murine Treg (1, 8). Using engineered mice in which GFP expression is driven by Foxp3 gene sequences, we have demonstrated CD39 to be a superior phenotypic marker of Foxp3+ Treg when compared to CD25 in mice (1). Moreover, Foxp3 appears to drive CD39 expression as evidenced by retroviral transduction of CD4+CD25− T cells with Foxp3 (8). Other studies have also demonstrated amplification of the Cd39 gene by Foxp3 (18).
In humans, CD4 and CD25 expression defines a population of T cells with potent suppressive properties (14) that harbors both activated Tregs (CD45RA−Foxp3highCD25+++) and cytokine secreting non-Tregs (CD45RA−Foxp3lowCD25++) (10). From a practical perspective isolating cells on the basis of CD25++ versus CD25+++ (10) carries operation dependent error and has subjective bias. We propose that staining CD4+CD25+ T cells for CD39 expression better distinguishes both the subsets, where CD4+CD25+CD39+ T cells are activated/memory Tregs and CD4+CD25+CD39− T cells are cytokine secreting non- suppressive Tregs. We suggest this approach as an alternative strategy. Indeed, within the highly purified CD4+CD25+CD127low T cell subset, differential levels of expression of CD39 can identify resting from activated Treg, recently defined as CD45RA+Foxp3low(CD25++) and CD45RA−Foxp3high(CD25+++) respectively (10).
CD4+CD25+CD39− T cells have been shown to suppress proliferation and IFNγ, but not IL-17A, production (9). We demonstrate that the 50% of these cells express Foxp3 albeit at lower intensity when compared with Treg. We suggest there is considerable overlap between the “prototype” cell surface signature of Treg and those other cells with Th17 potential. Our data further highlights the spectrum of biological activity of CD4+CD25+CD39− T cells. This population not only has IL-17 secreting capacity, but also has the potential to secrete IFNγ, IL-4, IL-5 and IL-10 and encompass differentiating memory CD4+ T cells, Treg, Th1 and Th2 cells (5, 15).
Extrapolation of data from murine model systems to human Treg is not always valid. Foxp3, for example, is dynamically regulated in humans in much the same way as CD25. We show that in contrast to the phenotypes observed in mice, CD73 is not consistently co-expressed by isolated human Treg as defined by CD4+CD25+CD39+ under basal conditions within the peripheral circulation of controls or patients and following in vitro activation. Moreover, in the acute inflammatory state of transplant rejection, there was no evidence of specific expression of CD73 on T cell subsets of interest.
In mice adenosine has been implicated in the mechanism of immune suppression (1) and regulates the function of both the innate and adaptive immune systems through targeting virtually every cell type that is involved in orchestrating an immune response (19). Treg effects may be also due to cell contact dependent transfer of cyclic adenosine monophosphate (cAMP) (20) the intracellular level of which is increased following signaling through adenosine receptors. Roles for ATP scavenging and metabolic disruptive effects have been also implicated in the anti-proliferative T cell responses (8, 21).
Given these observations in mice, it is intriguing that CD73 is not co-expressed with CD39 on human Treg. This difference raises a question surrounding the regulatory role of adenosine as a cellular immune suppressive factor in humans. It is possible that the transcellular expression of CD73 is required at the target cell location to generate adenosine from AMP produced by CD39 expressed by Treg. Hence immune suppressive pathways involving adenosine may be dependent upon paracrine mechanisms requiring the close proximity of other cells expressing CD73 and other ectoenzymes (22). Other pathways involved in the deviation to the Th17 phenotype could involve scavenging of nucleotides by CD39: for example, ATP has recently been shown to drive the differentiation of gastrointestinal derived Th17 cells (23). Further, tissue derived adenosine, the downstream product of ATP hydrolysis, acting via the adenosine A2A receptor is an important negative regulator of T cell function (24). Extracellular adenosine inhibits the generation of adaptive effector T cells and drives CD4+ T cells away from Th17 differentiation towards Foxp3+ T cell differentiation. It is also possible that CD73 is restricted to tissue derived Treg, which has been confirmed in the skin (25) and gastric mucosa (26). These putative pathways could provide highly regulated and controlled method of immune regulation, which may however be disrupted in states of inflammation.
Practically, CD39 can be used for the isolation of functionally active human Treg from the peripheral blood of healthy donors (27). In addition, we show that CD39 in conjunction with CD4 and CD25 clearly distinguishes four distinct T cell populations in controls as well as patients with ESRF and following transplantation. Moreover, these T cell populations are dynamic and can be followed longitudinally. Human cells are more difficult to track with the same precision as mice where genetic tags have developed, however parallels can be drawn between “exFoxp3” cells (6) and CD4+CD25−CD39+ memory effector T cells (5).
In humans CD4+CD25−CD39+ T cells are CD45RO+, Foxp3− and secrete IFNγ and IL-17A. These cells are increased following autoantigen (11) and alloantigen recognition. In the patients presenting with allograft rejection three of the four had antibody mediated rejection as determined by Banff criteria, C4d staining and/or presence of donor specific antibodies (DSA). Although the histopathological appearances of KTR#1 lacked definitive features of T cell mediated rejection and were more consistent with antibody mediated rejection, both DSA and C4d were negative. The detrimental role of antibody in renal allotransplantation is well established and is dominated by endothelial damage secondary to an inflammatory response encompassing complement activation and leukocytes (28). Frequencies of CD4+CD25−CD39+ T cells were increased in the peripheral blood of patients with antibody mediated allograft rejection, the magnitude of which correlated with the severity of rejection as evidenced histologically. Resolution of rejection was associated with a concomitant reduction in the frequencies of CD4+CD25−CD39+ T cells to levels observed in non-rejecting transplant recipients.
Frequencies of CD4+CD25+ Treg, decrease following renal transplantation occurring within 2 weeks of the procedure. These changes are significantly impacted on by immunosuppressive agents (16) the effects of which on Treg biology have been well documented (29). Contrary to this other recent report (16), we did not observe a difference in the frequencies of CD4+CD25+/−CD39+/− T cells in healthy controls, patients with ESRF or renal transplant recipients with stable renal allograft function. Further there was no significant difference observed in patients in the early versus late post transplant phase. The percentage of CD4+CD25+CD39+ T cells in this study was ~1.5%, which was significantly less than the percentage of CD4+CD25+ T cells observed in the healthy controls and patients with ESRF observed by others. Interestingly, in this other referenced study (16) the percent of CD4+CD25+ T cells accounted for ~2% of all CD4+ T cells following transplantation, which is comparable with values we obtained. This phenomenon may reflect elimination of recently activated cells contaminating the CD4+CD25+ Treg population. Similar data have been obtained in patients with immune activation states such as multiple sclerosis (9, 30).
The clinical relevance of Treg tracking in the peripheral blood and the relationships to the equivalent population resident within the graft is not yet established. The differences from peripheral blood sampling to sampling of the graft, the site of allograft rejection and patterns of immune compartmentalization may decrease the sensitivity for detecting changes in lymphocyte populations. As an example, in patients with childhood arthritis only subtle changes are observed in the peripheral blood of patients and controls whereas in contrast within the joint itself, striking changes are evident with a significant increase in the effector memory T cell pool (CD4+CD25−CD39+ T cells) (11). Further definition of CD39 expression patterns and leukocyte trafficking within the graft itself may provide useful additional information.
CONCLUSIONS
The use of CD39 when used in conjunction with CD4 and CD25 surface lymphocyte staining identifies four distinct and readily identifiable populations in both healthy individuals and those with disease states such as end stage renal failure and following renal transplantation. These T cell populations can be defined and classified as CD4+CD25+CD39+ (activated/memory Tregs); CD4+CD25+CD39− (pro-TH subsets); CD4+CD25−CD39+ (memory effector T cells) and CD4+CD25−CD39− T cells (naïve/effector T cells). These cellular populations are dynamic and can be tracked with ease in states of inflammation such as in the setting of renal allograft rejection.
Acknowledgments
Grant support: KMD: NHMRC, Australia; SCR and TBS: NIH.
Abbreviations
- Treg
regulatory T cells
- Foxp3
forkhead box P3 transcription factor
References
- 1.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007 Jun 11;204(6):1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997 Jan 6;185(1):153–63. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992 Jul 15;285( Pt 2):345–65. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006 Nov 15;177(10):6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009 Oct;9(10):2303–11. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009 Sep;10(9):1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer K, Deaglio S, Crikis S, Gao W, Enjyoji K, Strom TB, et al. Salutary roles of CD39 in transplantation. Transplantation Reviews. 2007;21:54–63. [Google Scholar]
- 8.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007 Aug 15;110(4):1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009 Dec 1;183(11):7602–10. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 10.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009 Jun 19;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, Eaton S, et al. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol. 2010 Jul 1;185(1):134–43. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995 Dec 15;155(12):5483–6. [PubMed] [Google Scholar]
- 13.Niemela J, Henttinen T, Yegutkin GG, Airas L, Kujari AM, Rajala P, et al. IFN-alpha induced adenosine production on the endothelium: a mechanism mediated by CD73 (ecto-5′-nucleotidase) up-regulation. J Immunol. 2004 Feb 1;172(3):1646–53. doi: 10.4049/jimmunol.172.3.1646. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006 Jul 10;203(7):1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Presser D, Sester U, Mohrbach J, Janssen M, Kohler H, Sester M. Differential kinetics of effector and regulatory T cells in patients on calcineurin inhibitor-based drug regimens. Kidney Int. 2009 Sep;76(5):557–66. doi: 10.1038/ki.2009.198. [DOI] [PubMed] [Google Scholar]
- 17.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008 Apr;8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 18.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007 Feb 15;445(7129):771–5. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 19.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008 Oct;22(10):3491–9. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007 Jun 11;204(6):1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005 Sep 1;175(5):3075–83. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 22.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007 Apr;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 23.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008 Oct 9;455(7214):808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 24.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008 Jan 1;111(1):251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008 Sep 29;205(10):2221–34. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, et al. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis. 2009 Feb 15;199(4):494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009 Jul 31;346(1–2):55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010 Mar;10(3):464–71. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 29.De Serres SA, Sayegh MH, Najafian N. Immunosuppressive drugs and Tregs: a critical evaluation! Clin J Am Soc Nephrol. 2009 Oct;4(10):1661–9. doi: 10.2215/CJN.03180509. [DOI] [PubMed] [Google Scholar]
- 30.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008 Jan;123(1):79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


