Abstract
Purpose
The objective of the investigation was to study possible inhibition of oxidative stress and cataract formation by caffeine in vivo.
Methods
Oxidative stress and consequent cataract formation was induced by intraperitoneal administration of a single dose of sodium selenite (1.16µmoles) to Sprague Dawley rat pups on day 9 postnatally. In experiments designed to inhibit such cataract formation, the pups were pretreated intraperitoneally with caffeine (5.15 µmoles), starting two days prior to the administration of selenite and continuing such treatment till day 21, when the experiments were terminated. The extent of tissue damage caused by the selenite was assessed biochemically by measurements of the levels of GSH and ATP in the isolated lenses. Cataract formation and its prevention were monitored by examining the eye with pen light illumination and subsequent photography of the isolated lenses.
Results
Injection of selenite led to a significant loss of lens clarity due to cataract formation. In the group treated with caffeine, the formation of cataract was significantly prevented. In the caffeine untreated group, the levels of lens GSH and ATP were substantially lower than in the caffeine treated group. The levels of GSH decreased from a value of ~ 8.2 µmoles to ~ 2 µmoles/g wet weight of the lens. The content of ATP decreased form~ 2.5 µmoles to about ~1 µmoles. In the case of caffeine treated group these decreases were significantly prevented from taking place, the corresponding values of GSH and ATP being ~5.8 and ~1.6 µmoles/g, respectively.
Conclusion
Over all, the results suggest that caffeine can exert a significant preventive effect against cataract formation induced by agents generating reactive oxygen species such as sodium selenite.
Keywords: Selenite cataract, anti-cataractogenic effect of caffeine, oxidative stress, antioxidant
Introduction
Oxidative stress has been implicated as one of the major contributors of cataract formation associated with aging and certain genetic diseases such as diabetes and galactosemia. While oxidative stress has been suggested to be involved in the pathophysiology of many age dependent disabling manifestations including the extra-ocular ones, its role is considered particularly significant in lens and cataract formation because of a continued intra-ocular production of reactive oxygen species (ROS) due to the continued light penetration in the eye during the long periods of photopic vision and consequently an incessant generation of ROS. The reactions are driven pseudo-catalytically by absorption of light by the endogenous chromophores (Varma et al 1977, 1984). The significance of light penetration in the eye and cataract development has also been corroborated by epidemiological data showing that about 20% of cataract blindness or visual impairment is globally related to intraocular UV penetration (Taylor et al, www.who.int/mediacentre/factsheet/fs227/en). Among the many antioxidants previously studied (Varma 1987; Vinson et al. 1992; Creighton & Trevithick 1979, Robertson et al. 1989, Varma et al. 1977, Hegde & Varma 2004, Devamanoharan et al. 1991), recent studies suggest that caffeine can also inhibit such photochemical damage and cataract formation induced by UV irradiation (Varma et al. 2008, Varma & Hegde 2010). Although caffeine has many modes of its physiological effects (Serafin 1996), the observed inhibition of UV damage to the lens has been found to coincide with its ability to scavenge ROS (Shi et al. 1991, Stadler & Fay 1995, Devasagayam et al 1996, Stadler et al 1996, Chung & Chay 1997, Dalmazio et al. 2005, Telo & Vieira 1997), particularly the hydroxyl radical as shown by ESR studies. This was also apparent by parallel effectiveness of tempol in protecting against the lens against UV damage (Varma & Hegde In press). More recently, we have shown that caffeine administration also inhibit the development of cataracts in vivo using the galactosemic rat model (Varma et al. 2010). Since it does not inhibit aldose reductase, it has been suggested that its effectiveness against the sugar cataract formation, demonstrated for the first time in vivo, is attributable to its antioxidant effect, exerted via its property of scavenging ROS. This is conformity with several previous studies that also suggest the involvement of ROS and consequent oxidative stress in the pathogenesis of sugar cataracts (Vinson et al. 1992; Creighton & Trevithick 1979, Chung & Chay 1997, Dische et al. 1956, Creighton et al. 1985, Kubo et al. 1999, Unakar & Tsui 1980). However, further studies examining the in vivo effectiveness of caffeine against cataract formation were deemed desirable using an animal model where cataract formation is initiated by oxidative stress, concomitant to a more direct generation of oxyradicals as opposed to the case of sugar cataracts where ROS generation takes place indirectly due to initial metabolic alterations induced by the high sugar levels (Kubo et al. 1999). This communication therefore reports further studies aimed at investigating in vivo the anti-cataractogenic/ antioxidant effects of caffeine using the selenite model of cataracts (Ostadalova 1977, Painter 1941, Devamanoharan et al. 1991) where the ROS generation is more direct, depending directly on the redox properties of the compound itself. As expected, the formation of cataracts in rat pups given sodium selenite was substantially inhibited by administration of caffeine. The inhibition was associated also with better maintenance of the levels of GSH and ATP. The results therefore further demonstrate the possible effectiveness of caffeine in attenuating cataract formation induced by oxidative stress in vivo. The findings may therefore be found useful from the pharmacological points of view.
Materials and Methods
Most of the chemicals used in these investigations were obtained from Sigma Chemical Company (St. Louis, MO, USA). Sprague Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN) and used in accordance with the ARVO guidelines and as approved by the institutional animal care and use committee (IACUC). Most of the methods used were similar to those we have used previously (Devamanoharan 1991).
Induction of selenite cataracts
Seven day old rat pups were divided into three groups and labeled as A,B and C and treated as follows.
Group A was maintained as normal controls. Groups B and C were treated as follows. Group B: Postnatal days 7 and 8; 0.2ml of normal saline injected intraperitoneally. Day 9: 0.2 ml of normal saline containing 1.16 micromoles of sodium selenite per animal. . No further treatment. Group C: Day 7 and 8; 0.2 ml of normal saline containing 5.15 micromoles of caffeine. On day 9: injected in addition, sodium selenite as in group B. In addition caffeine injection remained continued every day till day 20. Eyes were examined daily for noting the day of opening and monitoring the appearance of cataracts. Final assessment of lens clarity or cataract was made after sacrificing the rats and photographing the isolated lenses after placing them on a Millipore grid The lenses in various groups were then biochemically analyzed for the contents of ATP and GSH along with that in the basal controls.
Measurement of ATP
Lenses were homogenized in 0.5 ml dH2O and centrifuged to obtain a clear supernatant. ATP was then determined by mixing 50 µl of this supernatant with 200 µl of firefly lantern extract containing luciferin-luciferase (Sigma FLE 50) and measuring the luminescence using a Turner Designs photometer. ATP standards were run simultaneously.
Measurement of GSH
The remaining aqueous supernatant was deproteinized by mixing it with 100% trichloracetic acid to the final concentration of 5% followed by centrifugation. 100 µl of this acid extract was neutralized with 300 µl of 0.6M Na2HPO4. This was followed by the addition of 100µl DTNB (5,5’dithio-bis-2-nitrobenzoic acid) reagent. The resulting yellow color was read spectrophotometrically at 412nm. DTNB reagent was prepared by dissolving 4mg DTNB and 100 mg trisodium citrate in 10 ml dH2O. GSH standards were also run simultaneously.
Statistical analysis was done by determination of p value based on t-test.
Results
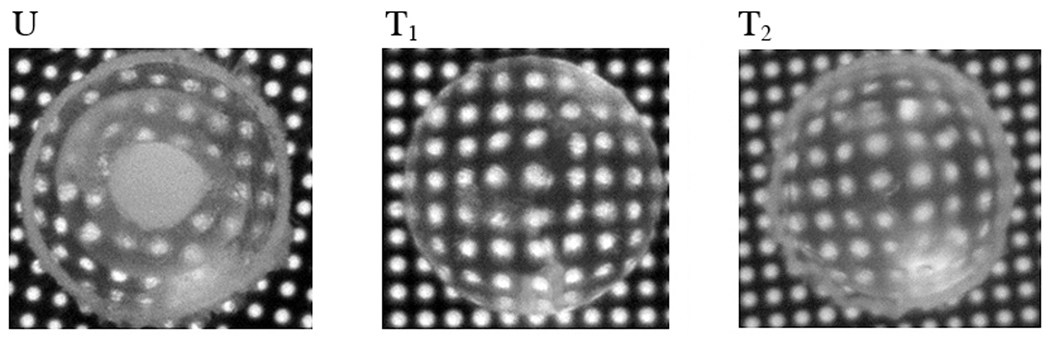
The primary aim of this study was to evaluate the possible protective effect of caffeine against oxidative stress related cataract formation induced by injection of sodium selenite to Sprague Dawley rat pups. Figure 1 shows representative trans-illumination photographs of the lenses of the selenite injected pups, untreated (U) and treated (T) belonging to groups B and C. As shown, lenses of the pups isolated from the caffeine untreated group developed highly advanced opacities by day 20, the nuclear opacity being denser than in the cortical region. The denser opacity in the nucleus corresponds to the lower levels of antioxidant defenses therein, such as of the levels of GSH (Reddy VN 1971, Paul H, Graf P and Sies H.1990) and the antioxidant enzymes such as superoxide dismutase and glutathione peroxidase (Ohrloff and Hockwin et al 1984). In the group treated with caffeine, cataractogenesis is highly attenuated in majority of the lenses. As apparent in the photograph labeled T1 the lenses in this group remained much more transparent than that in the caffeine untreated group. The grid holes are highly much visible than in the untreated group. As summarized in table 1, sodium selenite was effective in inducing advanced cataracts in all the untreated animals (22 eyes, 100%). On the contrary, cataract development was significantly inhibited in the majority of pups treated with caffeine. No advanced cataract developed at least in the majority (64%) of this group. There was however a general haze in about 36% of the eyes (photograph labed as T2) despite treatment with caffeine. This was also much attenuated in comparison to the caffeine untreated group. The preventive effect of caffeine against cataract development was hence highly convincing.
Figure 1.
Transillumination pictures of representative lenses showing the state of lens transparency in different groups. As apparent, the lenses of the animals given sodium selenite are severely cataractous (U), the opacity being more dense in the nuclear than in the surrounding areas. In the majority (64%) of the caffeine treated pups (T1), the lenses remained largely transparent. T2 represents 36% of the caffeine treated animals where cataract prevention remained incomplete in comparison to T1, but the severity of transparency loss was significantly less that that in group U. .
Table 1.
Incidence of cataracts and the levels of ATP and GSH in the lenses of rat pups injected with sodium selenite (B) and sodium selenite plus caffeine (C). Experimental details are described in the text. The division of group C between two groups was done after looking at the extent of opacity in the animals treated with caffeine, the ones having less clarity were groups under C2. In the first row, the numbers without parentheses indicate the number of eyes. Numbers in parentheses indicate the corresponding number of animals. The # of animals in the selenite only and Selenite + caffeine were 11 in each. P values between A& B, B&C1 and C2&B are <0.001, except in the case of ATP between C2 & B, which was not significant.
| A: Normal (no cataract) |
B: Sel (advanced cataract) |
C1:Sel + caff: no cataract |
C2: Sel + caff: moderate cataract |
|
|---|---|---|---|---|
| # of eyes (# of animals) | 8 (4) | 22 (11) | 14 (7) | 8 (4) |
| ATP µmoles/g | 2.5 ± 0.04 | 1.0 ± 0.2 | 1.6 ± 0.2 | 1.05 ± 0.07 |
| GSH µmoles/g | 8.2 ± 0.4 | 2.0 ± 0.86 | 5.8 ±1.4 | 3.6 ± 0.8 |
The protective effect of caffeine was apparent also biochemically, in terms of the levels of ATP and GSH as summarized in table 1. The level of ATP in the basal controls (group A) was 2.5 ± 0.04 µmoles/g wet weight of the tissue. This was reduced to 1.0± 0.2 in the selenite group (B) not given caffeine. In the caffeine treated animals (group C1) where the lenses were largely clear, the level was 1.6±0.2. Although it was lower than that in the basal controls, it was significantly higher than in the caffeine untreated group. The effectiveness of caffeine is hence apparently related to its ability to prevent oxidative stress to the tissue and consequent maintenance of tissue metabolism as reflected by maintenance of ATP level. The role of ATP maintenance through the inhibition of oxidative stress by caffeine is also apparent by the relation between the ATP levels and maintenance of lens transparency. In group C2 where it was lower than 1.6 micromoles /g, the effectiveness was decreased, although not abolished.
The protective effect of caffeine is due to its antioxidant effect and consequent maintenance of tissue metabolism was also apparent by its effect against GSH depletion triggered by sodium selenite administration. Previous studies have shown that the level of this tri-peptide in the lens undergoes a precipitous drop following such treatment (Devamanoharan et.al 1991). This is attributable to the selenite induced conversion of GSH to GSSG caused directly by its reaction with sodium selenite ( Sankui et al 2007) as or through an initial formation of seleno-diglutathione (GSSeSG; Tsen & Tappel 1958). The latter is rapidly converted to GSSG with the generation of selenium dioxide. This in turn restarts the thiol depleting reaction. The GSSG so formed is extruded out the lens. As summarized in table 1, the level of GSH was 8.2±0.4µmoles/g in the lenses of normal rat pups. In the selenite group, it decreased to 2±0.86µmoles/g. As expected, treatment with caffeine offered substantial protection against this decrease, its levels in the cataract free group (group C) and in group where cataract prevention was only moderate (group D) were 5.8 ±1.4 and 3.6±0.8, significantly higher that that in caffeine untreated group.
Discussion
The incidence of cataracts is very high on a global scale. Yet, a pharmacological treatment against this disease has so far not been achieved. Although vision can be restored to a satisfactory level in the majority of patients with cataract by surgically removing the natural lens and substituting it with a lens made of synthetic polymers, the incidence is so large that surgery alone has been found unable to cope up with the problem. Hence it remains a consistent public health problem because of shortage of surgical facilities as well as the continuous replacement of old cases with new ones due to continued increase in longevity as well as the continued population growth. In addition, other vision-impairing surgical and post-surgical complications such as vitreous loss, macular edema and retinal detachment are known to occur in a significant number of people (~5%) (Stark et al. 1984, Fine et al. 1981, Clayman et al. 1981, Smith et al. 1987). Hence development of pharmacological means of cataract prevention is very desirable.
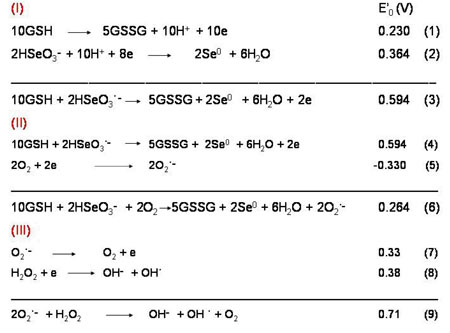
Several studies suggest that oxyradical induced oxidative stress on the lens plays a central role in its pathogenesis. It is therefore highly feasible that the loss in visual acuity due to cataract can be delayed by use of certain scavengers of oxygen free radicals with relatively high potency to inhibit oxidative stress. The present studies have been conducted with caffeine, a compound much more robust and well tolerated by humans. While previous studies have shown that it is an effective ROS scavenger and that it can even prevent oxidative damage to lens in vitro, studies on its effectiveness against oxidative stress and cataract development in vivo are yet very limited, except a previous study wherein we have shown that it can attenuate cataract formation induced by feeding high galactose diet to young rats. Since it does not inhibit aldose reductase, the observed effect was suggested to be in conformity with the view that ROS generation induced by metabolic effects of galactose or high levels of other structurally similar sugars is one of the important causal factors involved. However, increase in the formation of ROS has also been suggested to be involved in cataract formation induced by UV irradiation, as well as by age related sluggishness in utilization of oxygen through normal pathways and its consequent diversion towards initiating direct oxidation of susceptible substrates which in most cases is accompanied by ROS generation. It was hence desired to study the in vivo efficacy of caffeine in preventing oxidative stress inflicted by a more direct generation of ROS. This was accomplished by using the selenite model of cataract formation. As noted below, the reduction potentials of various redox reactions are highly favorable for the reduction of Se (+4) in selenite to eventually Se (0) (reaction 2, Sankui et al. 2007) with simultaneous generation of superoxide (reaction 6, Segel 1976). The latter eventually generates hydroxyl radical (reaction 9, Yamazaki 1993) effective in converting back Se (0) to Se (+4), which restarts the reaction series.
Hence only a small amount of selenite can lead to a significant depletion of the tissue GSH. Other redox couples can similarly be affected. Hence the compound can exert an adverse physiological effect by upsetting the tissue redox status as well as by generation of superoxide and its derivatives, viz. hydrogen peroxide and hydroxyl radicals.
The process of depleting the tissue of its reducing equivalents and ROS generation can continue cyclically, inflicting oxidative stress in a continued manner. Such a continued oxidative stress can lead to membrane as well as cytosolic damage. The membrane damage is known to alter the ionic balance, including an increase in cell calcium known to induce proteolysis by calpain activation and consequent formation of abnormal protein aggregates of lower solubility and functions (Bhuyan et al. 1981, Shearer et al. 1997, Hightower et al. 1987, Duncan et al. 1993). Hence the use of sodium selenite model is considered highly suitable to test the efficacy of caffeine or other radical scavengers in preventing the initiation of cataractogenesis by ROS. As summarized in the ‘Results’ section, injecting a very small amount of this substance leads to a substantial depletion of glutathione. These reactions hence offer a reasonable explanation of the mechanism by which caffeine seems to exert a physiologically significant antioxidant and anti-cataractogenic effect. Its property of scavenging ROS is also highly efficient, as judged by the rate constants (Shi et al. 1991, Stadler & Fay 1995, Devasagayam et al 1996, Stadler et al 1996, Chung & Chay 1997, Dalmazio et al. 2005, Telo & Vieira 1997). These reactions of caffeine when combined with the observations on its effectiveness in preventing lens damage induced by UV irradiation in vitro and the current observations showing its effectiveness against selenite and sugar induced cataractogenesis in vivo therefore strongly suggest that caffeine could have a generalized anti-cataractogenic effect when used pharmacologically. This would be specially so when administered topically, avoiding any possible unwanted effect of its systemic administration. Additionally it could favorably influence the lens metabolism by inhibiting cyclic adenosyl monophosphate phosphodiesterase (Beavo et al. 1970) and therefore maintaining adequate levels of cAMP (Creighton & Trevithick 1974).
Acknowledgements
The authors are thankful to NEI (NIH) and Research to Prevent Blindness (RPB) Inc. New York, for financial assistance. SDV is also a recipient of the Senior Scientific Investigator awards by RPB. Current affiliation of Kavita R. Hegde: Department of Natural Sciences, Coppin State University, Baltimore, MD 21216
References
- Beavo TA, Rogers NL, Crofford OB, Hardman JG, Sutherland EW, Newman EV. Effect of xanthine derivatives on lipolysis and on adenosine 3’, 5’-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970;6:597–603. [PubMed] [Google Scholar]
- Bhuyan KC, Bhuyan DK, Podos SM. In: Selenium in Biology and Medicine. Spallholz JE, Martin JL, Ganther HE, editors. Westport: AVI; 1981. pp. 403–412. [Google Scholar]
- Chung WG, Chay N. Oxidation of caffeine to theobromine and theophylline is catalyzed primarily by flavins containing monooxygenase in liver microsomes. Biochem Biophys Res Commun. 1997;235:685–688. doi: 10.1006/bbrc.1997.6866. [DOI] [PubMed] [Google Scholar]
- Clayman HM, Jaffe NS, Light DS, Jaffe MS, Cassady JC. Intraocular lenses, axial length, and retinal detachment. Am J Ophthalmol. 1981;92:778–780. doi: 10.1016/s0002-9394(14)75629-6. [DOI] [PubMed] [Google Scholar]
- Creighton MO, Trevithick J. Effect of c-AMP, caffeine and theophylline on differentiation of lens epithelial cells. Nature. 1974;249:767–768. doi: 10.1038/249767a0. [DOI] [PubMed] [Google Scholar]
- Creighton MO, Trevithick JR. Cortical cataract formation prevented by vitamin E and glutathione. Exp Eye Res. 1979;6:689–693. doi: 10.1016/0014-4835(79)90025-3. [DOI] [PubMed] [Google Scholar]
- Creighton MO, Ross WM, Stewart-DeHaan PJ, Sanwal M, Trevithick JR. Modelling cortical cataractogenesis VII: Effects of vitamin E treatment on galactose-induced cataracts. Exp Eye Res. 1985;40:213–222. doi: 10.1016/0014-4835(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Dalmazio I, Santos LS, Lopes RP, Eberlin MN, Augusti R. Advanced oxidation of caffeine in water: On-line and real-time monitoring by electrospray ionization mass spectrometry. Environ Sci Technol. 2005;39:5982–5988. doi: 10.1021/es047985v. [DOI] [PubMed] [Google Scholar]
- Devamanoharan PS, Henein M, Morris S, Ramachandran R, Richards RD, Varma SD. Prevention of selenite cataract by vitamin C. Exp. Eye Res. 1991;52:563–568. doi: 10.1016/0014-4835(91)90057-l. [DOI] [PubMed] [Google Scholar]
- Devasagayam TPA, Kamat JP, Mohan H, Kesavan PC. Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim Biophys Acta –Biomembranes. 1996;1282:63–70. doi: 10.1016/0005-2736(96)00040-5. [DOI] [PubMed] [Google Scholar]
- Dische Z, Borenfreund E, Zelmenis G. Proteins and protein synthesis in rat lenses with galactose cataract. 1956;55:633–641. doi: 10.1001/archopht.1956.00930030637003. [DOI] [PubMed] [Google Scholar]
- Duncan G, Webb SF, Dawson AP, Bootman MD, Elliott AJ. Calcium regulation in tissue cultured human and bovine lens epithelial cells. Invest Ophthalmol Vis Sci. 1993;34:2835–2842. [PubMed] [Google Scholar]
- Fine BS, Brucker AJ. Macular edema and Cystoid macular edema. Am J Ophthalmol. 1981;92:466–481. doi: 10.1016/0002-9394(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Hegde KR, Varma SD. Effect of alpha-ketoglutarate against selenite cataract formation. Exp Eye Res. 2004;79:913–918. doi: 10.1016/j.exer.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Hightower KR, David LL, Shearer TR. Regional distribution of free calcium in selenite cataract: relation to calpain II. Invest Ophthalmol Vis Sci. 1987;28:1702–1706. [PubMed] [Google Scholar]
- Kubo E, Miyoshi R, Fakuda M, Akagi Y. Cataract formation though polyol pathway is associated with free radical production. Exp Eye Res. 1999;68:457–464. doi: 10.1006/exer.1998.0624. [DOI] [PubMed] [Google Scholar]
- Ohrloff C, Hockwin O, Olson R, Dickman S. Glutathione peroxidase, glutathione reductase and superoxide dismutase in the aging lens. Curr Eye Res. 1984;3:1089–1115. doi: 10.3109/02713688408997191. [DOI] [PubMed] [Google Scholar]
- Ostadalova I, Babicky A, Obenberger J. Cataract induced by administration of a singly dose of sodium selenite to suckling rats. Experientia. 1977;34:222–223. doi: 10.1007/BF01944690. [DOI] [PubMed] [Google Scholar]
- Painter EP. The chemistry and toxicity of selenium compounds, with special reference to the selenium problem. Chem Rev. 1941;28:179–213. [Google Scholar]
- Paul H, Graf P, Sies H. Glutathione levels in human lens: regional distribution in different forms of cataract. Exp Eye Res. 1990;50:17–20. doi: 10.1016/0014-4835(90)90005-f. [DOI] [PubMed] [Google Scholar]
- Reddy VN. Metabolism of glutathione in lens. Exp Eye Res. 1971;11:310–328. doi: 10.1016/s0014-4835(71)80043-x. [DOI] [PubMed] [Google Scholar]
- Robertson JMCD, Donner AP, Trevithick JR. Vitamin E intake and risk of cataracts in humans. Ann NY Acad Sci. 1989;570:373–382. doi: 10.1111/j.1749-6632.1989.tb14936.x. [DOI] [PubMed] [Google Scholar]
- Sankui S, Arai K, Kojima T, Nagaoka S, Majima H. Photocatalytic reduction of selenate and selenite solutions using TiO2 powders. Metallurgical Material Trans. 2007;30:15–20. [Google Scholar]
- Segel IH. Appendix IX Standard reduction potentials of some oxidation-reduction half-reactions. In: Segel IH, editor. Biochemical Calculations. John Wiley and Sons; 1976. pp. 415–416. [Google Scholar]
- Serafin WE. Drugs used in the treatment of asthma. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Goodman Gillman A, editors. Goodman & Gillman’s Pharmacological basis of therapeutics. New York: McGraw Hill; 1996. pp. 659–679. [Google Scholar]
- Shearer TR, Ma H, Fukiage C, Azuma M. Selenite nuclear cataract: review of the model. Mol Vis. 1997;3:8–16. [PubMed] [Google Scholar]
- Shi X, Dalal NS, Jain AC. Antioxidant behaviour of caffeine: efficient scavenging of hydroxyl radicals. Food Chem Toxicol. 1991;29:1–6. doi: 10.1016/0278-6915(91)90056-d. [DOI] [PubMed] [Google Scholar]
- Smith PW, Stark WJ, Maumenee AE, Enger CL, Michels RG, Glaser BM, Bonham RD. Retinal detachment after cataract extraction with posterior chamber intraocular lens. Ophthalmology. 1987;94:495–504. doi: 10.1016/s0161-6420(87)33418-9. [DOI] [PubMed] [Google Scholar]
- Stadler RH, Fay LB. Antioxidative reactions of caffeine: formation of 8-oxocaffeine (1,3,7 trimethyl uric acid) in coffee subjected to oxidative stress. J Agric Food Chem. 1995;43:1332–1338. [Google Scholar]
- Stadler RH, Richoz J, Turesky RJ, Welti DH, Fay LB. Oxidation of caffeine and related methylxanthines in ascorbate and polyphenol driven Fenton-type oxidation. Free Rad Res. 1996;24:225–235. doi: 10.3109/10715769609088020. [DOI] [PubMed] [Google Scholar]
- Stark WJ, Jr, Maumenee AE, Fagadau W, Datiles M, Baker CC, Worthen D, Klein P, Auer C. Cystoid macular edema in pseudophakia. Surv Ophthalmol. 1984;28 suppl:442–451. doi: 10.1016/0039-6257(84)90226-1. [DOI] [PubMed] [Google Scholar]
- Taylor HR, West SJ, Rosenthal FS, Munoz B, Newland HS, Abbey H, Emmett EA. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1998;319:1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- Telo JP, Vieira AJSC. Mechanism of free radical oxidation of caffeine in aqueous solution. J Chem Soc Perkin Trans. 1997;2:1755–1757. [Google Scholar]
- Tsen CC, Tappel AL. Catalytic oxidation of GSH and other sulfhydryl compounds by selenite. J Biol Chem. 1958;233:1230–1232. [PubMed] [Google Scholar]
- Ultraviolet radiation. Solar radiation and human health. Too much sun is dangerous. Available at www.who.int/mediacentre/factsheet/fs227/en.
- Unakar NJ, Tsui J. Sodium-potassium-dependent ATPase II. Cytochemical localization during the reversal of galactose cataracts in rat. Invest Ophthalmol Vis Sci. 1980;19:378–385. [PubMed] [Google Scholar]
- Varma SD, Hegde KR. Prevention of oxidative damage to lens by caffeine. J Ocular Pharmacol Therap. 2010;26:73–77. doi: 10.1089/jop.2009.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Chand D, Sharma YR, Kuck JF, Jr, Richards RD. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res. 1984;3(1):35–57. doi: 10.3109/02713688408997186. [DOI] [PubMed] [Google Scholar]
- Varma SD, Ets TK, Richards RD. Protection against superoxide radicals in rat lens. Ophthalmic Res. 1977;9:421–431. [Google Scholar]
- Varma SD, Hegde K, Kovtun S. Oxidative stress in lens in vivo. Inhibitory effect of caffeine. A preliminary report. Molecular Vision. 2010;16:501–505. [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Hegde KR, Kovtun S. UV-B induced damage to the lens in vitro. Prevention by caffeine. J Ocular Pharm Therap. 2008;24:439–444. doi: 10.1089/jop.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Mikuni I, Kinoshita JH. Diabetic cataracts and flavonoids. Science. 1977;195:205–206. doi: 10.1126/science.401544. [DOI] [PubMed] [Google Scholar]
- Varma SD. Ascorbic acid and the eye with special reference to lens. Ann NY Acad Sci. 1987;498:280–306. doi: 10.1111/j.1749-6632.1987.tb23768.x. [DOI] [PubMed] [Google Scholar]
- Varma SD, Hegde KR. Kynurenine induced photo oxidative damage to lens in vitro. Protective effect of caffeine. Mol Cell Biochem. doi: 10.1007/s11010-010-0399-4. In Press. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Courey BS, Maro NP. Comparison of the two forms of vitamin C on galactose cataract. Nutr Res. 1992;12:915–922. [Google Scholar]
- Yamazaki Y. Hydroxyl radical formation in biological systems. Quica nova. 1993;16:365–369. [Google Scholar]