Abstract
Pulmonary arterial hypertension is a common and fatal complication of scleroderma that may involve inflammatory and autoimmune mechanisms. Alterations in the gene expression of peripheral blood mononuclear cells have been previously described in patients with pulmonary arterial hypertension. Our goal is to identify differentially expressed genes in peripheral blood mononuclear cells in scleroderma patients with and without pulmonary hypertension as biomarkers of disease.
Gene expression analysis was performed on a Microarray Cohort of scleroderma patients with (n= 10) and without (n= 10) pulmonary hypertension. Differentially expressed genes were confirmed in the Microarray Cohort and validated in a Validation Cohort of scleroderma patients with (n= 15) and without (n= 19) pulmonary hypertension by RT‐qPCR. We identified inflammatory and immune‐related genes including interleukin‐7 receptor (IL‐7R) and chemokine receptor 7 as differentially expressed in patients with scleroderma‐associated pulmonary hypertension. Flow cytometry confirmed decreased expression of IL‐7R on circulating CD4+ T‐cells from scleroderma patients with pulmonary hypertension.
Differences exist in the expression of inflammatory and immune‐related genes in peripheral blood cells from patients with scleroderma‐related pulmonary hypertension compared to those with normal pulmonary artery pressures. These findings may have implications as biomarkers to screen at‐risk populations for early diagnosis and provide insight into mechanisms of scleroderma‐related pulmonary hypertension. Clin Trans Sci 2010; Volume 3: 210–218
Keywords: pulmonary hypertension, gene array, gene expression, interleukins, inflammation
Introduction
Pulmonary arterial hypertension (PAH) is a poorly understood, clinically devastating disease characterized by the proliferation of endothelial and smooth muscle cells within the precapillary pulmonary vasculature. These changes result in a progressive increase in pulmonary vascular resistance and, if untreated, right ventricular failure and death. The etiology of these pulmonary vascular changes is not known; however, inflammation and autoimmunity may play a role.
Systemic sclerosis is a multisystem autoimmune disease that can affect a variety of organ systems, including the pulmonary vasculature. Up to 10–15% of scleroderma (SSc) patients develop PAH. 1 Many of these patients die as a direct result of pulmonary hypertension. 2 The systemic sclerosis population has a predictable risk for developing this devastating disease and represents a group that may benefit from early diagnosis and treatment. 3 However, identification of PAH frequently occurs after the patient has progressed to advanced disease. 4 Early pathologic diagnosis of pulmonary hypertension is difficult as direct investigation of the vascular pathology is prohibitive due to risk associated with lung biopsy. Current noninvasive means of identifying PAH such as transthoracic echocardiography, 6‐minute walk testing and measurement of diffusing capacity for carbon monoxide (DLco) are suboptimal screening tests as they become abnormal only after significant pulmonary vasculopathy has occurred.
Previous studies have supported a role for inflammation in various forms of pulmonary hypertension. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Mononuclear cells, T and B lymphocytes, and dendritic cells have been described in close proximity to the vasculopathy associated with idiopathic PAH (IPAH) and scleroderma‐related PAH (SSc‐PAH). 13 , 14 , 15 Differences in percentage of T and B lymphocytes in peripheral blood have also been noted in patients with IPAH compared to normal controls. 16 , 17 Furthermore, PAH is a known complication of a number of systemic autoimmune inflammatory conditions such as scleroderma, rheumatoid arthritis, lupus, sarcoidosis and mixed connective tissue disease. 18 , 19
In concert with chronic inflammation, immunologic mechanisms may contribute to the progression of PAH. 20 , 21 , 22 , 23 , 24 SSc‐PAH patients can have positive antinuclear and anticentromere antibodies, compared to SSc patients with pulmonary fibrosis and no evidence of PAH. 25 , 26
Thus, we sought to identify relevant differences in inflammatory and immune‐related gene expression and T‐cell lymphocyte surface markers in peripheral blood mononuclear cells (PBMCs) that could distinguish SSc‐PAH patients from SSc without PAH. We then examined for cell surface protein changes related to the changes noted in gene expression. These differences in immune phenotype may potentially aid in early diagnosis and provide insight into the inflammatory and autoimmune pathogenesis of SSc‐PAH.
Methods
Subjects and recruitment
All subjects were recruited and prospectively enrolled from the Pulmonary Hypertension and Scleroderma clinics at the University of Colorado Denver and at the Pulmonary Hypertension Association biennial meeting. All recruited subjects provided written consent to participate in this study, and the protocol was approved by the institutional human‐subjects review board (Colorado Multiple Institutional Review Board [COMIRB] protocol number 06–0247) at the University of Colorado Denver.
Scleroderma patients were identified using American College of Rheumatology diagnostic criteria. 27 Patients with SSc but without PAH were clinically identified by a normal 2nd heart sound, lack of tricuspid regurgitation murmur and an estimated RVSP <35 mmHg on a transthoracic echocardiogram when available. The SSc without PAH subjects in the Microarray and Validation Cohorts were prospectively followed for a 2‐year period to confirm the absence of PAH by these criteria. Right heart catheterization (RHC) was performed on all patients with SSc‐PAH to confirm the diagnosis. Pulmonary arterial hypertension was defined as a resting mean pulmonary artery pressure (mPAP) ≥25 mmHg with pulmonary artery occlusion pressure of ≤ 15 mmHg.
An initial cohort composed of scleroderma patients with and without pulmonary hypertension, referred to as the Microarray Cohort, underwent microarray gene expression analysis. The microarray cohort was composed of 10 SSc‐PAH and 10 SSc without PAH subjects ( Table 1 ). A second larger cohort referred to as the Validation Cohort contained 15 SSc‐PAH and 19 SSc without PAH subjects ( Table 1 ). The microarray cohort was matched by gender, disease severity (mean PAP, cardiac output, and pulmonary vascular resistance) and medication. Exclusion criteria included pulmonary venous hypertension (pulmonary artery occlusion pressure >15 mmHg), significant obstructive or restrictive lung disease on pulmonary function testing (FEV1 < 70% or FVC < 70%, respectively). Due to concern for the impact on peripheral blood gene expression, patients with continuously infused medications such as intravenous prostacyclin therapy were excluded, as were patients on high doses of methotrexate or corticosteroids. Additionally, patients with evidence of other active disease processes unrelated to PAH within the preceding 30 days (e.g., systemic infection, bleeding diathesis or hospitalization for other processes) were excluded.
Table 1.
Microarray and Validation Cohort subject demographics. SSc‐PAH and SSc without PAH have a statistically significant difference in age (p= 0.03) in the Microarray and Validation Cohorts. There was no statistical difference in hemodynamic values between the two cohorts. NHW= Non‐Hispanic White; AA= African American; HL= Hispanic Latino; AS= Asian. TTE= transthoracic echo; RAP= right atrial pressure; RVSP= right ventricular systolic pressure; TR Jet= tricuspid regurgitant jet. mPAP= mean pulmonary artery pressure, PVR= pulmonary vascular resistance, CO= cardiac output; CI= cardiac index. All values are expressed as mean ± standard deviation.
| Cohort | Microarray | Validation | ||||
|---|---|---|---|---|---|---|
| Subjects | SSc‐PAH | SSc without PAH | p value | SSc‐PAH | SSc without PAH | p value |
| n | 10 | 10 | 15 | 19 | ||
| Age (years) | 61.7 ± 9.4 | 51.2 ± 11.0 | 0.03 | 59.3 ± 10.1 | 51.0 ± 8.5 | 0.014 |
| Ethnicity (number) | ||||||
| NHW | 10 | 6 | 12 | 14 | ||
| AA | 0 | 1 | 2 | 2 | ||
| HL | 0 | 1 | 1 | 3 | ||
| AS | 0 | 1 | 0 | 0 | ||
| Unknown | 0 | 1 | 0 | 0 | ||
| Gender (% female) | 100 | 100 | 86.7 | 84.2 | ||
| Oral Pulmonary hypertension therapy (%) | 100 | n/a | 100 | n/a | ||
| WHO functional class | 2.7 ± 0.5 | 2.5 ± 0.8 | 0.44 | |||
| 6 MWD (m) | 301.5 ± 100.0 | 300.9 ± 91.9 | 0.99 | |||
| Pulmonary function tests | ||||||
| Dlco (%) | 39.6 ± 15.7 | 70.6± 29.4 | 0.02 | 35.6± 18.8 | 78.6 ± 33.8 | 0.001 |
| FVC/DLco | 2.3 ± 0.9 | 1.4 ± 0.5 | 0.03 | 2.1± 0.8 | 1.2 ± 0.3 | 0.001 |
| FVC (%) | 79.3 ± 13.7 | 86.6 ± 13.9 | 0.28 | 63.4 ± 11.1 | 89.1 ± 16.1 | <0.0001 |
| Transthoracic echo data | ||||||
| RAP (mmHg) | 7.2 ± 3.3 | 7.3 ± 3.7 | 0.97 | |||
| RVSP (mmHg) | 80.2 ± 20.3 | 67.6 ± 29.8 | 0.28 | |||
| TR Jet(m/s2) | 4.2 ± 0.6 | 3.8 ± 0.8 | 0.17 | |||
| Right heart catheterization data | ||||||
| RAP (mmHg) | 5.4 ± 3.7 | 4.8 ± 3.1 | 0.67 | |||
| mPAP (mmHg) | 42.9 ± 9.8 | 39.1 ± 8.0 | 0.29 | |||
| PVR (WU) | 8.0 ± 4.4 | 7.0 ± 2.8 | 0.51 | |||
| CO (L/min) | 4.8 ± 1.6 | 4.9 ± 1.7 | 0.90 | |||
| CI (L/min/m2) | 2.5 ± 0.9 | 2.7 ± 0.8 | 0.75 | |||
Blood collection and isolation of PBMCs
Whole blood was drawn from each subject via peripheral venipuncture into BD Vacutainer® CPT tubes with sodium citrate (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Care was taken to standardize blood sample collection and preparation. Blood was collected through a 21‐gauge needle. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll–Hypaque density gradient separation, and cells were stored in RNALater (Qiagen Inc., Valencia, CA, USA) at –80°C. The cellular composition of the Microarray Cohort PBMC samples was assessed and samples utilized were determined to be greater than 87% mononuclear cells.
Microarray preparation
Total RNA was isolated from PBMCs using Trizol (Invitrogen Corporation, Carlsbad, CA, USA) extraction and isopropyl alcohol precipitation, followed by clean‐up with a Qiagen RNeasy mini‐kit (Qiagen). RNA was quantified by spectrophotometry, and RNA integrity was confirmed with the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Microarray targets were prepared from 5 μg of total RNA using the Affymetrix (Santa Clara, CA, USA) kit for reverse transcription and a single round of in vitro transcription. HG‐U133+2.0 were hybridized with 10 μg of cRNA and processed per the manufacturer’s protocol.
Data analysis
Patient demographics
Statistical analysis of subject demographic data was performed with Prism version 4.0 for windows (GraphPad Software, San Diego, CA, USA). Unpaired t‐tests were used to compare hemodynamic data. The unpaired student t‐test was used to evaluate demographic data and RT‐qPCR data between SSc‐PAH Microarray and Validation Cohorts. Categorical data were evaluated with a chi‐square test. Continuous data are presented as mean ± standard deviation. Categorical data are represented as numbers and percentages. A p value of < 0.05 was considered statistically significant for demographic and RT‐qPCR data.
Microarray analysis
All microarray data met the quality control criteria established by the Tumor Analysis Best Practices Working Group 28 and is available in the Gene Expression Omnibus repository (GSE22356).
Microarray expression data were analyzed using analysis of variance (ANOVA) methods implemented in Partek (St. Louis, MO, USA) and prediction analysis of microarrays (PAM) 29 implemented in BRB ArrayTools v3.3 developed by Drs. Richard Simon and Amy Peng Lam. 30 We identified PAH associated differential expression by comparing the gene expression profiles of the 10 SSc‐PAH and 10 SSc without PAH PBMC samples using a one‐way ANOVA. Of the 54,675 transcripts measured on the microarray, 2,303 were differentially abundant between these two groups with a False Discovery Rate (FDR) threshold of 5%, and 1,517 transcripts meet a stringent unadjusted p value < 0.001.
Transcripts that were invariant across the microarray cohort were filtered out, and the remaining 17,412 transcripts were evaluated. Unsupervised hierarchical clustering was performed using Euclidian distance and average linkage ( Figure 1A ). In order to identify the subset of these differentially expressed transcripts that may be the most useful for the prediction of PAH in the second SSc cohort, we employed the PAM method of nearest shrunken centroids. 28
Figure 1.
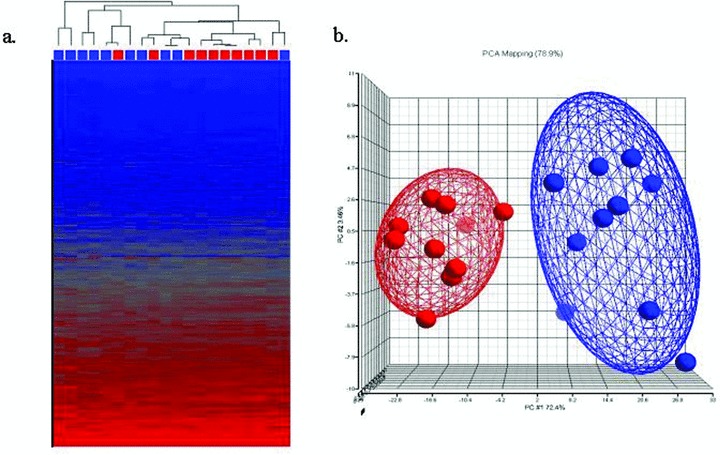
(A) Unsupervised dendrogram and Heatmap. Unsupervised hierarchical clustering was performed using the 17,412 probesets. The red boxes below the dendrogram indicate SSc without PAH; blue boxes indicate SSc‐PAH. Blue color in the Heat map indicates relatively low transcript expression, red color indicates relatively high transcript expression. (B) Supervised principal component analysis of the Microarray Cohort. SSc without PAH (n= 10, red) and SSc‐PAH (n= 10, blue). At p < 0.0001, 459 genes were differentially expressed between the two groups.
Reverse transcription qPCR
Selection of differentially expressed transcripts for confirmation and validation were based upon their ability to discriminate between the SSc‐PAH and SSc without PAH and potential role in inflammatory or autoimmune pathogenesis ( Table 2 ). Confirmation and validation of candidate genes for all cohorts was performed by real time quantitative polymerase chain reaction (RT‐qPCR) after reverse transcription of RNA using the iScript cDNA Synthesis Kit (Bio‐Rad, Laboratories, Hercules, CA, USA). RT‐qPCR was performed retrospectively on the Microarray Cohort, then prospectively in the Validation Cohort. 31 Two subjects from the Microarray Cohort (one SSc‐PAH and one SSc without PAH) were excluded from RT‐qPCR analysis due to degradation of RNA ( Table 1 ).
Table 2.
Genes selected from microarray analysis. Gene symbol and Affymetrix ID are listed. The first 8 transcripts were selected by PAM analysis. *= transcripts not included in PAM analysis, but selected for potential interest. Fold change represents the change in microarray gene expression comparing SSc‐PAH gene expression to SSc without PAH gene expression. p value is the statistically significant difference in the microarray analysis gene expression comparing SSc‐PAH to SSc without PAH. LRRN3 is counted as two transcripts.
| Gene of interest | Gene symbol | Affymetrix ID | Fold Change | p value |
|---|---|---|---|---|
| Interleukin 7 Receptor | IL7R | 205798_at | ↓1.95 | 3.94 × 10−6 |
| Leucine Rich Repeat Neuronal 3 | LRRN3 | 209840_s_at 209841_s_at | ↓3.91 ↓4.84 | 4.59 × 10−5 1.21 × 10−6 |
| Noggin | NOG | 231798_at | ↓3.04 | 2.12 × 10−5 |
| N‐myristoyltransferase 2 | NMT2 | 215743_at | ↓2.22 | 6.92 × 10−7 |
| Tubulin, epsilon 1 | TUBE1 | 226181_at | ↓1.93 | 2.11 × 10−5 |
| Microtubule‐associated protein 9 | MAP9 | 228423_at | ↓2.06 | 3.19 × 10−6 |
| Full length insert cDNA clone ZD45C02 | — | 1556054_at | ↓2.42 | 3.19 × 10−6 |
| Chemokine Receptor 7* | CCR7 | 206337_at | ↓2.20 | 6.82 × 10−5 |
| Transforming Growth Factor β Receptor 2* | TGFBR 2 | 208944_at | ↓1.33 | 1.12 × 10−4 |
Primers and probes for the following genes were obtained from Applied Biosystems (ABI) Assays on Demand using their recommended conditions (Foster City, CA, USA): Leucine Rich Repeat Neuronal 3 (LRRN3, Hs00539582_s1), Noggin (NOG, Hs00271362_s1), Transforming Growth Factor Beta Receptor 2 (TGFBR2, Hs00234253_m1), Interleukin 7 Receptor (IL‐7R, Hs00904814_m1), and Chemokine Receptor 7 (CCR7, Hs99999080_m1). The Affymetrix probe ID for these genes was utilized to select ABI primer/probe sets on the ABI website (http://www4.appliedbiosystems.com/tools/umapit/). This provided primer/probe sets near the 39 end of the gene adjacent to the Affymetrix probe and within the coding region of the gene when possible. TaqMan® MGB probes and primers were FAM™ and VIC® dye‐labeled; TaqMan® MGB Universal PCR Master Mix was obtained from ABI. The BioRad iCyclerTM iQ Multicolor Real‐Time PCR Detection System (Bio‐Rad) and Applied Biosystems 7300 Real Time PCR System were used to evaluate the 5 genes of interest. All experimental samples and standards were run in duplicate and averaged. Results were standardized to the expression of the control gene human β‐2 microglobulin (B2M). B2M was utilized as a control due to its high and consistent gene expression on microarray analysis among all microarray subject samples.
Immunofluorescence staining of PBMCs
The Flow Cytometry cohort data are listed in Table 3 . PBMCs were isolated from potassium/EDTA anticoagulated blood by Ficoll–Hypaque density gradient separation after 1:1 dilution with Dulbecco’s PBS (Mediatech, Inc., Manassas, VA, USA). Cells were washed in a FACS buffer consisting of 1% bovine serum albumin (Sigma‐Aldrich, St. Louis, MO, USA) in DPBS and surfaced stained with unlabeled anti‐CCR7 (BD Pharmingen, San Diego, CA, USA) for 25 minutes at 4°C, washed with FACS buffer, and stained with a secondary biotinylated antimouse IgM for 20 minutes at 4°C. Cells were washed and stained with anti‐CD4 or CD8 (PerCP; BD Pharmingen), anti‐CD127 (FITC; eBioscience, Inc., San Diego, CA, USA), anti‐CD45RA (APC; BD Pharmingen), and streptavidin‐PE (BD Pharmingen) for 20 minutes at 4°C. Cells were washed and resuspended in 1% formaldehyde (Polyscience, Inc., Warrington, PA, USA) in DPBS. Fluorescence minus one (FMO) was used on representative samples from both controls and patients for establishing appropriate gates in the fully stained samples. 32
Table 3.
Flow Cytometry Cohort subject demographics. SSc‐PAH and SSc without PAH do not have a statistically signifi cant difference in age (p > 0.05). NHW= Non‐Hispanic White; AA= African American; HL= Hispanic Latino; AS= Asian; AI= American Indian. TTE= transthoracic echo; RAP= right atrial pressure; RVSP= right ventricular systolic pressure; TR Jet= tricuspid regurgitant jet. mPAP= mean pulmonary artery pressure, PVR= pulmonary vascular resistance, CO= cardiac output; CI= cardiac index. All values are expressed as mean ± standard deviation.
| Flow cytometry cohort | SSc‐PAH | SSc without PAH | p value |
|---|---|---|---|
| n | 10 | 11 | |
| Age (years) | 59.8 ± 8.0 | 60.1 ± 12.5 | 0.95 |
| Ethnicity (number) | |||
| NHW | 6 | 10 | |
| AA | 2 | 0 | |
| HL | 1 | 0 | |
| AS | 0 | 1 | |
| AI | 1 | 0 | |
| Gender (% female) | 80 | 63.6 | |
| Oral pulmonary hypertension therapy (%) | 100 | n/a | |
| 6 MWD (m) | 304.6 ± 75.5 | ||
| Pulmonary function tests | |||
| FVC (%) | 85.5 ± 20.7 | 90.1 ± 13.9 | 0.57 |
| Dlco (%) | 40.7± 13.9 | 77.3 ± 21.5 | 0.0003 |
| FVC/DLco | 2.2 ± 0.5 | 1.2 ± 0.3 | 6.2 10−5 |
| Transthoracic echo data | |||
| TTE RAP (mmHg) | 7.8 ± 17.7 | 5.3 ± 2.4 | 0.25 |
| TTE RVSP (mmHg) | 68.6 ± 17.7 | 29.1 ± 8.3 | 8.7 × 10−5 |
| TTE TR jet (m/s2) | 3.7 ± 0.3 | 2.4 ± 0.4 | 4.6 × 10−5 |
| Right heart catheterization data | |||
| RAP (mmHg) | 4.9 ± 4.0 | ||
| mPAP (mmHg) | 41.7 ± 9.7 | ||
| PVR (WU) | 8.6 ± 3.6 | ||
| CO (L/min) | 4.4 ± 0.9 | ||
| CI (L/min/m2) | 2.6 ± 0.3 | ||
Flow cytometry
Cells were analyzed using a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA), as previously described. 33 , 34 Data analysis was performed using FlowJo software (Tree Star Inc., Ashland, OR, USA). The number of events collected ranged between 1 and 3 million. For data analysis, an initial lymphocyte gate was set based on forward and 90° light scatter patterns. Results are presented as the percentage, or mean fluorescent intensity (MFI) of positively stained cells within the gated population. Expression of CD4 and CD8 on T cells was analyzed in a bivariate dot plot. Because CD127 expression was not bimodal, expression levels on CD4+ T cells were determined by subtracting the mean fluorescence intensity (MFI) of the FMO control from the CD127 MFI on the population of interest.
Results
Study population and hemodynamic characteristics
Table 1 displays the characteristics of the Microarray and Validation Cohorts. The SSc‐PAH and SSc without PAH subjects were matched according to gender, ethnicity, medications, and disease severity. The majority of subjects in both study cohorts were non‐Hispanic white women. The SSc‐PAH subjects in the Microarray and Validation Cohorts were older than those without PAH.
SSc‐PAH subjects in the Microarray and Validation Cohorts had evidence of moderate‐to‐severe PAH with mean pulmonary artery pressures of 42.9 ± 9.8 mmHg and 39.1 ± 8.0 mmHg, respectively. Measured hemodynamic values of SSc‐PAH subjects were not statistically different between the Microarray and Confirmatory Cohorts ( Table 1 ).
Microarray analysis
While there was evidence of clustering within the SSc without PAH and SSc‐PAH groups, unsupervised analysis of the microarray cohort was unable to distinguish SSc‐PAH from SSc without PAH with adequate sensitivity or specificity ( Figure 1A ). We therefore performed a supervised analysis (one‐way ANOVA) identifying 2,303 transcripts differentially expressed between these two groups with a False Discovery Rate threshold of 5%. 1,517 transcripts met a stringent unadjusted p value of < 0.001. A principal component analysis (PCA) plot of the supervised clustering is shown in Figure 1B . The optimal PAM classifier to distinguish SSc‐PAH from SSc without PAH contained eight probe sets representing six well‐described transcripts ( Table 2 ). Cross‐validation of this classifier resulted in the correct classification of 9 of 10 SSc‐PAH and 9 of 10 SSc without PAH samples, and yields positive‐ and negative‐predictive values of 90%.
RT‐qPCR results
Microarray Cohort
Figure 2 demonstrates the RT‐qPCR results performed retrospectively for the five selected genes, NOG, LRRN3, TGFβR2, IL‐7R, and CCR7 which distinguish SSc‐PAH from SSc without PAH in the Microarray groups. SSc‐PAH subjects have statistically significant decreased copy number expression for all genes evaluated.
Figure 2.
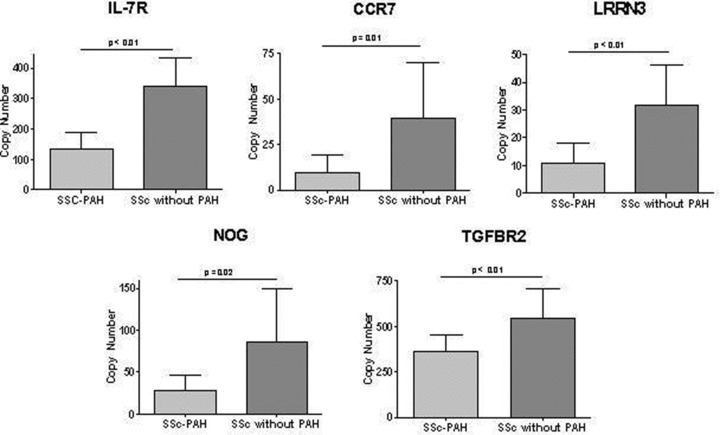
RT‐qPCR of Microarray Cohort. Genes selected for confirmation of the microarray results. Relative expression of SSc‐PAH (n= 9) versus SSc without PAH (n= 9) was significantly different for all 5 genes. IL‐7R (140 ± 53 n= 9 vs. 340 ± 93 n= 9, p < 0.01); CCR7 (9.3 ± 9.9, n= 9 vs. 39 ± 31, n= 9, p= 0.01); LRRN3 (11 ± 7.2, n= 9 vs.32 ± 15, n= 9, p < 0.01); NOG (28 ± 18, n= 9 vs. 86 ± 63, n= 9, p= 0.02), and TGFBR2 (360 ± 92, n= 9 vs. 550 ± 160, n= 9, p < 0.01).
Validation Cohort
RT‐qPCR performed prospectively on a separate Validation Cohort elicited similar results to the Microarray Cohort. Figure 3 shows the relative RT‐qPCR gene expression in the SSc‐PAH subjects and SSc controls. All genes investigated were validated with the exception of TGFβR2, which showed a trend toward statistical significance (p= 0.14).
Figure 3.

RT‐qPCR of Validation Cohort. Genes selected for validation of the microarray results. Relative expression of SSc‐PAH (n= 15) versus SSc without PAH (n= 19) was significantly different for 4 genes. TGFBR2 was not validated in this cohort, but approached statistical significance. IL‐7R (294 ± 158 n= 15 vs. 496.9 ± 248, n= 19, p= 0.01); CCR7 (167 ± 121, n= 15 vs. 333.9 ± 209.5, n= 19, p= 0.01); LRRN3 (18.1 ± 12.7, n= 15 vs. 29.6 ± 15.4, n= 19, p= 0.03); NOG (2.8 ± 2.1, n= 15 vs. 7.9 ± 6.7, n= 19, p < 0.01); TGFBR2 (180 ± 53.5, n= 15, 215.4 ± 76.2, n= 19, p= 0.14).
Flow cytometry
There was a significant decrease in the percentage of CD4+ T cells expressing IL‐7R on the cell surface ( Figure 4A ). In addition, Figure 4B shows a representative example of a leftward shift in the MFI of IL‐7R on CD4+ cells. Overall, a significant reduction in the amount of IL‐7R on a per cell basis, as detected by MFI, was seen on CD4+ T cells from the SSc‐PAH subjects as compared to the expression on CD4+ T cells derived from the blood of SSc without PAH ( Figure 4C ). Since IL‐7R is an important survival factor for memory T cells, we evaluated differences in IL‐7R expression on memory CD4+ and CD8+ T‐cell subsets. As previously described 35 , memory compartments were defined as naïve (CCR7+, CD45+), central memory (CCR7+, CD45+), effector memory (CCR7−, CD45−) and terminally differentiated effector memory (TEMRA) (CCR7−, CD45+) T cells. Overall, we identified a statistically significant decrease in the percentage of central memory and effector memory CD4+ T cells expressing IL‐7R in patients with SSc‐PAH as compared to SSc control subjects ( Figure 5 ). Expression of IL‐7R in the remaining memory compartments, naïve, and terminally differentiated memory CD4+ cells approached statistical significance ( Figure 5 ). These findings were not noted when comparing memory compartments of CD8+ T cells expressing IL‐7R in scleroderma patients with and without pulmonary hypertension (data not shown). Differences in expression of CCR7 in CD4+ and CD8+ cells in SSc‐PAH and SSc without PAH were not observed using flow cytometry.
Figure 4.
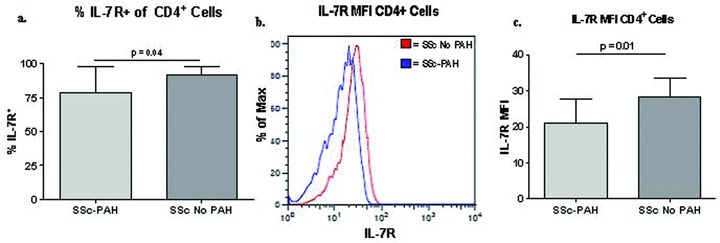
(A) The expression of IL‐7R on CD4+ cells is decreased in scleroderma patients with PAH compared to SSc without PAH, by flow cytometric analysis. (B) Subjects with SSc‐PAH have decreased expression of IL‐7R on CD4+ T cells. Representative histograms of IL‐7R mean fluorescence intensity (MFI) on CD4+ T cells from subjects with SSc, with and without PAH. (C) Comparison of IL‐7R MFI on CD4+ T cells from subjects with SSc, with and without PAH.
Figure 5.
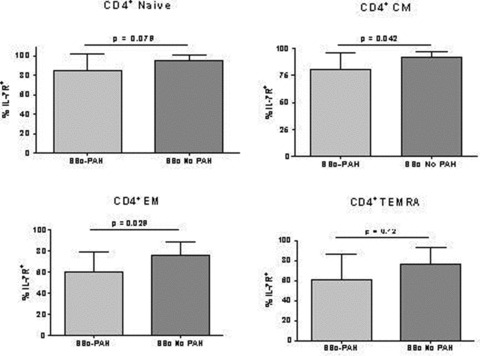
Flow cytometry of PBMCs for IL‐7R expression in all CD4 memory compartments. SSc‐PAH (n= 10); SSc without PAH (n= 11). Differences between PBMCs from SSc‐PAH and SSc without PAH in IL‐7R expression in central memory (CM) and effector memory (EM) memory compartments were statistically significant. IL‐7R expression in naïve and T effector memory RA+ (TEMRA) memory compartments was not statistically different between SSc‐PAH and SSc without PAH. Evaluation of CD8+ cells did not yield statistically significant differences in IL‐7R in any of the memory compartments (data not shown).
Discussion
In this study, we demonstrate significant differences in gene expression of peripheral blood cells between scleroderma patients with and without pulmonary arterial hypertension including a number of genes relevant to inflammation and autoimmunity. We have confirmed these findings in our initial array cohort and in a larger validation cohort. Lastly, we have demonstrated decreased protein expression by flow cytometry for IL‐7R on circulating immune cells and differences in the memory compartments of CD4+ cells between SSc‐PAH and SSc without PAH subjects.
As predicted by our gene expression data, changes in immunologic phenotype are demonstrated by a decrease in the percentage of IL‐7 receptor in central and effector CD4+ T‐cell memory compartments in patients with scleroderma associated PAH. The change in expression of the immune‐related transcript interleukin 7 receptor gene is a notable finding in this study. This transcript has previously been identified in microarray analysis as differentially expressed in PBMCs 6 , 7 in scleroderma subjects.
IL‐7R is a transmembrane protein that is highly expressed in monocytes and lymphocytes, in particular CD4+ and CD8+ T cells. IL‐7R signaling is responsible for maintenance of survival, proliferation, and differentiation of lymphocytes. 36 Decreased expression of IL‐7R results in disruption of immune response in both murine models and human disease. 37 , 38 Interestingly, HIV‐1 infected patients have CD4+ T‐cell depletion that is associated with loss of the IL‐7Rα chain. 39 Polymorphisms in the IL‐7R coding region have been associated with sarcoidosis. 15 Both HIV‐1 infection and sarcoidosis are risk factors for the development of pulmonary hypertension.
Flow cytometry demonstrated a decrease in CD4+ T cells expressing IL‐7R. These findings confirm our gene expression findings in the microarray and RT‐qPCR analyses. Although the exact significance of these findings is unclear, they suggest the possibility of dysregulated immunity in SSc‐PAH patients.
Of additional interest, the microarray analysis also demonstrated differential expression of the genes TGFβR2 and Noggin (NOG) in PBMCs. The TGFβ pathway is of recognized importance in the development of some forms of PAH and may be related to the pathogenesis of SSc‐PAH. 40 , 41 , 42 , 43 We were able to validate changes in NOG expression but not TGFβR2 signaling in our Validation Cohort.
Limitations to this study include the small sample size of our array cohorts, which raises the possibility of overfitting our classifier. However, we addressed this issue by confirming a number of identified genes in a separate, larger, and more diverse validation cohort of patients. There was an age difference in the Microarray and Validation Cohorts, and it is therefore possible that age has contributed to the overall gene expression differences. However, it has been previously demonstrated that patients with SSc‐PAH are older than SSc without PAH at the time of diagnosis so the difference in age between cohorts is difficult to avoid and may not be a significant confounder. 44 , 45 Lastly, it is unknown whether the identified gene expression differences between SSc‐PAH and SSc without PAH are a result of pulmonary arterial hypertension or if these genes are related to the pathogenesis of the disease.
Our work demonstrates an altered immune phenotype of peripheral blood cells in SSc‐PAH as compared to SSc without PAH. Patients with scleroderma represent an at‐risk population that would benefit from earlier diagnosis of PAH in hopes of altering the natural history of this disease. The genes and proteins identified could be utilized to assist in the early diagnosis of SSc‐PAH. If these changes in cell phenotype can be related back to the pathobiology of the disease, they may represent future therapeutic targets.
Acknowledgments
This study was funded by NIH K08 HLO72858‐01A2 and the Scleroderma Foundation PN200509‐021 to Dr. Todd Bull.
References
- 1. Steen VD. The lung in systemic sclerosis. J Clin Rheumatol. 2005; 11(1): 40–46. [DOI] [PubMed] [Google Scholar]
- 2. Steen VD, Medsger TA Jr. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007; 66(7): 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galie N, Rubin L, Hoeper M, Jansa P, Al‐Hiti H, Meyer G, Chiossi E, Kusic‐Pajic , A Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double‐blind, randomised controlled trial. Lancet. 2008; 371(9630): 2093–2100. [DOI] [PubMed] [Google Scholar]
- 4. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud‐Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006; 173(9): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 5. Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot‐Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin‐1 and interleukin‐6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995; 151(5): 1628–1631. [DOI] [PubMed] [Google Scholar]
- 6. Bull TM, Coldren CD, Moore M, Sotto‐Santiago SM, Pham DV, Nana‐Sinkam SP, Voelkel NF, Geraci MW. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004; 170(8): 911–919. [DOI] [PubMed] [Google Scholar]
- 7. Grigoryev DN, Mathai SC, Fisher MR, Girgis RE, Zaiman AL, Housten‐Harris T, Cheadle C, Gao L, Hummers LK, Champion HC, Garcia JG, Wigley FM, Tuder RM, Barnes KC, Hassoun PM. Identification of candidate genes in scleroderma‐related pulmonary arterial hypertension. Transl Res. 2008; 151(4): 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009; 54(1 Suppl): S10–S19. [DOI] [PubMed] [Google Scholar]
- 9. Fartoukh M, Emilie D, Le GC, Monti G, Simonneau G, Humbert M. Chemokine macrophage inflammatory protein‐1alpha mRNA expression in lung biopsy specimens of primary pulmonary hypertension. Chest. 1998; 114(1 Suppl): 50S–51S. [DOI] [PubMed] [Google Scholar]
- 10. Dorfmuller P, Zarka V, Durand‐Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb‐Lhermine A, Marfaing‐Koka A, Simonneau G, Emilie D, Humbert M. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002; 165(4): 534–539. [DOI] [PubMed] [Google Scholar]
- 11. Balabanian K, Foussat A, Dorfmuller P, Durand‐Gasselin I, Capel F, Bouchet‐Delbos L, Portier A, Marfaing‐Kok A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002; 165(10): 1419–1425. [DOI] [PubMed] [Google Scholar]
- 12. Chaouat A, Savale L, Chouaid C, Tu L, Sztrymf B, Canuet M, Maitre B, Housset B, Brandt C, Le CP, Weitzenblum E, Eddahibi S, Adnot S. Role for interleukin‐6 in COPD‐related pulmonary hypertension. Chest. 2009; 136(3): 678–687. [DOI] [PubMed] [Google Scholar]
- 13. Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol. 1997; 28(4): 434–442. [DOI] [PubMed] [Google Scholar]
- 14. Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994; 144(2): 275–285. [PMC free article] [PubMed] [Google Scholar]
- 15. Nicolls MR, Taraseviciene‐Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005; 26(6): 1110–1118. [DOI] [PubMed] [Google Scholar]
- 16. Austin ED, Rock MT, Mosse CA, Vnencak‐Jones CL, Yoder SM, Robbins IM, Loyd JE, Meyrick BO. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med. 2009; 104(3): 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ulrich S, Taraseviciene‐Stewart L, Huber LC, Speich R, Voelkel N. Peripheral blood B lymphocytes derived from patients with idiopathic pulmonary arterial hypertension express a different RNA pattern compared with healthy controls: a cross sectional study. Respir Res. 2008; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fagan KA, Badesch DB. Pulmonary hypertension associated with connective tissue disease. Prog Cardiovasc Dis. 2002; 45(3): 225–234. [DOI] [PubMed] [Google Scholar]
- 19. Heron M, Grutters JC, Van Moorsel CH, Ruben HJ, Huizinga TW, Van Der Helm‐van Mil AH, Claessen AM, Van Den Bosch JM. Variation in IL7R predisposes to sarcoid inflammation. Genes Immun. 2009; 10(7): 647–653. [DOI] [PubMed] [Google Scholar]
- 20. Morse JH, Barst RJ, Fotino M, Zhang Y, Flaster E, Gharavi AE, Fritzler MJ, Dominguez M, Angles‐Cano E. Primary pulmonary hypertension, tissue plasminogen activator antibodies, and HLA‐DQ7. Am J Respir Crit Care Med. 1997; 155(1): 274–278. [DOI] [PubMed] [Google Scholar]
- 21. Morse JH, Antohi S, Kasturi K, Saito S, Fotino M, Humbert M, Simonneau G, Basst RJ, Bona CA. Fine specificity of anti‐fibrillin‐1 autoantibodies in primary pulmonary hypertension syndrome. Scand J Immunol. 2000; 51(6): 607–611. [DOI] [PubMed] [Google Scholar]
- 22. Isern RA, Yaneva M, Weiner E, Parke A, Rothfield N, Dantzker D, Rich S, Arnett FC. Autoantibodies in patients with primary pulmonary hypertension: association with anti‐Ku. Am J Med. 1992; 93(3): 307–312. [DOI] [PubMed] [Google Scholar]
- 23. Cosio MG. Autoimmunity, T‐cells and STAT‐4 in the pathogenesis of chronic obstructive pulmonary disease. Eur Respir J. 2004; 24(1): 3–5. [DOI] [PubMed] [Google Scholar]
- 24. Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. 2003; 58(10): 832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steen V, Medsger TA Jr. Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003; 48(2): 516–522. [DOI] [PubMed] [Google Scholar]
- 26. Steen VD, Lucas M, Fertig N, Medsger TA Jr. Pulmonary arterial hypertension and severe pulmonary fibrosis in systemic sclerosis patients with a nucleolar antibody. J Rheumatol. 2007; 34(11): 2230–2235. [PubMed] [Google Scholar]
- 27. Lonzetti LS, Joyal F, Raynauld JP, Roussin A, Goulet JR, Rich E, Choquette D, Raymond Y, Senecal JL. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum. 2001; 44(3): 735–736. [DOI] [PubMed] [Google Scholar]
- 28. Tumor Analysis Best Practices Working Group . Expression profiling–best practices for data generation and interpretation in clinical trials. Nat Rev Genet. 2004; 5(3): 229–237. [DOI] [PubMed] [Google Scholar]
- 29. Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002; 99(10): 6567–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simon R, Radmacher MD, Dobbin K, McShane LM. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J Natl Cancer Inst. 2003; 95(1): 14–18. [DOI] [PubMed] [Google Scholar]
- 31. Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real‐time RT‐PCR. Nat Protoc. 2006; 1(3): 1559–1582. [DOI] [PubMed] [Google Scholar]
- 32. Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001; 45(3): 194–205. [DOI] [PubMed] [Google Scholar]
- 33. Mack DG, Lanham AM, Palmer BE, Maier LA, Fontenot AP. CD27 expression on CD4+ T cells differentiates effector from regulatory T cell subsets in the lung. J Immunol. 2009; 182(11): 7317–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mack DG, Lanham AM, Falta MT, Palmer BE, Maier LA, Fontenot AP. Deficient and dysfunctional regulatory T cells in the lungs of chronic beryllium disease subjects. Am J Respir Crit Care Med. 2010; 181(11): 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999; 401(6754): 708–712. [DOI] [PubMed] [Google Scholar]
- 36. Palmer MJ, Mahajan VS, Trajman LC, Irvine DJ, Lauffenburger DA, Chen J. Interleukin‐7 receptor signaling network: an integrated systems perspective. Cell Mol Immunol. 2008; 5(2): 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corcoran AE, Smart FM, Cowling RJ, Crompton T, Owen MJ, Venkitaraman AR. The interleukin‐7 receptor alpha chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J. 1996; 15(8): 1924–1932. [PMC free article] [PubMed] [Google Scholar]
- 38. Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998; 20(4): 394–397. [DOI] [PubMed] [Google Scholar]
- 39. Rethi B, Fluur C, Atlas A, Krzyzowska M, Mowafi F, Grutzmeier S, De MA, Bellocco R, Falk KI, Rajnavolgyi E, Chiodi F. Loss of IL‐7Ralpha is associated with CD4 T‐cell depletion, high interleukin‐7 levels and CD28 down‐regulation in HIV infected patients. AIDS. 2005; 19(18): 2077–2086. [DOI] [PubMed] [Google Scholar]
- 40. Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, Nichols WC, Morrell NW, Berg J, Manes A, McGaughran J, Pauciulo M, Wheeler L. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001; 345(5): 325–3234. [DOI] [PubMed] [Google Scholar]
- 41. Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor‐beta signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004; 170(12): 1340–1348. [DOI] [PubMed] [Google Scholar]
- 42. Loscalzo J. Genetic clues to the cause of primary pulmonary hypertension. N Engl J Med. 2001; 345(5): 367–371. [DOI] [PubMed] [Google Scholar]
- 43. Zimmerman LB, De Jesus‐Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996; 86(4): 599–606. [DOI] [PubMed] [Google Scholar]
- 44. Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten‐Harris T, Hummers L, Krishnan JA, Wigley F, Hassoun PM. Clinical differences between idiopathic and scleroderma‐related pulmonary hypertension. Arthritis Rheum. 2006; 54(9): 3043–3050. [DOI] [PubMed] [Google Scholar]
- 45. Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, Black CM, Coghlan JG. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003; 62(11): 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]


