Abstract
Intensity modulated radiation therapy (IMRT) allows optimization of radiation dose delivery to complex tumor volumes with rapid dose drop-off to surrounding normal tissues. A prospective study was performed to evaluate the concept of conformal avoidance using IMRT in canine sinonasal cancer. The potential of IMRT to improve clinical outcome with respect to acute and late ocular toxicity was evaluated. Thirty-one dogs with sinonasal cancer were treated definitively with IMRT using helical tomotherapy and/or dynamic multileaf collimator (DMLC) delivery. Ocular toxicity was evaluated prospectively and compared to a comparable group of historical controls treated with conventional two-dimensional radiotherapy (2D-RT) techniques. Treatment plans were devised for each dog using helical tomotherapy and DMLC that achieved the target dose to the planning treatment volume and limited critical normal tissues to the prescribed dose-volume constraints. Overall acute and late toxicities were limited and minor, detectable by an experienced observer. This was in contrast to the profound ocular morbidity observed in the historical control group treated with 2D-RT. Overall median survival for IMRT treated and 2D treated dogs was 420 days and 411 days, respectively. Compared with conventional techniques, IMRT reduced dose delivered to eyes and resulted in bilateral ocular sparing in the dogs reported herein. These data provide proof-of-principle that conformal avoidance radiotherapy can be delivered through high conformity IMRT, resulting in decreased normal tissue toxicity as compared to historical controls treated with 2D-RT.
Introduction
Intensity-modulated radiation therapy (IMRT) exploits the concept of conformal avoidance where the objective of treatment planning becomes avoidance of critical normal structures. Rather than demarcating the treatment area precisely, critical structures can be defined specifically such that radiation to these areas is avoided. 1,2 Conformal avoidance is essentially an “everything-but” treatment approach and as such creates rapid reduction in radiation dose near sensitive structures.2
Helical tomotherapy is an advanced form of conformal IMRT that also uses image-verification to deliver radiation precisely to the desired target. Image-guided radiation therapy seeks to remove uncertainties associated with anatomic positioning at each treatment by acquiring images of the patient immediately prior to radiation beam delivery.1–3 Tomotherapy merges a 6 MV linear accelerator, equipped with a binary MLC, and an integrated helical computed tomography subsystem. The tomotherapy unit delivers rotational fan-beam RT and provides megavoltage CT (MV CT) capabilities for patient set-up verification and volumetric image guidance. The strategy of targeting sensitive normal structures for avoidance while effectively treating arbitrarily shaped treatment volumes was not possible before the emergence of IMRT,2 and was applied in this study.
Radiation therapy, with a tumor prescription of 42–54 Gy in 10–15 fractions, is considered the most effective means of achieving local control of canine sinonasal tumors, with median survival ranging from 8–19.7 months.4–10 The overall median survival in 139 dogs with untreated nasal carcinomas was 3.1 months, although in a subset of 32 dogs that did not have epistaxis the median survival was 7.4 months..11 As in humans, dose to tumor within the nasal cavity is limited by the tolerance of the eyes and brain.12 Radiation-induced ocular complications in dogs mimic those observed in humans.13–16 Chronic keratoconjunctivitis sicca, corneal ulceration and secondary uveitis, chronic conjunctivitis, cataract formation, radiation retinopathy and optic neuropathy are potential consequences of radiation for doses above 40 Gy.17–19 Manifestation of late ocular changes in the dog occur at a typical time-course 6–9 months following RT and include keratoconjunctivitis sicca and cataract formation. 17,18
To assess the impact of the improved conformity of IMRT and the concept of conformal avoidance, we evaluated the acute and late ocular complications in dogs with naturally occurring sinonasal cancer treated with tomotherapy. Results were compared to the ocular toxicity observed in a comparable group of historical controls treated with conventional two-dimensional (2-D) megavoltage external beam RT using the same dose and fractionation.5,20
Materials And Methods
Between January 2003 and September 2006, 31 client-owned dogs with a biopsy-confirmed sinonasal tumor were treated with IMRT in the context of a phase I/II fixed-dose clinical trial of conformal avoidance. Inclusion criteria included lack of clinical evidence of metastatic disease (N0M0), and no intracranial tumor extension at the time of diagnosis, cataracts, or prior treatment. Concurrent medical therapy was not permitted while dogs participated in the study. Additional therapy was permitted at the time of tumor progression. A modified WHO staging system was used for all dogs including historical controls (Table 1).5
Table 1.
World Health Organization Staging protocol for canine sinonasal tumors.
| Modified WHO Stage |
Tumor Characteristics |
|---|---|
| T1 | Confined to one nasal passage or paranasal sinus, with no bony involvement |
| T2 | Bony involvement, without evidence of orbital, subcutaneous, or submucosal mass |
| T3 | Presence of orbital, subcutaneous, or submucosal mass |
| T4 | Tumor extension into nasopharynx or through cribriform plate |
Various mixed breed dogs and purebreds were represented, with a median age and weight of 10 years (range 5–13 years) and 24 kg (8.0–60.0 kg), respectively. Thirteen dogs had T1 tumors, 9 had T2, 5 dogs had T3, and the remaining 4 dogs had T4 tumors. Clinical signs were characteristic of nasal disease. Three dogs had facial deformity (3). Histopathologic diagnoses were carcinoma (25) or sarcoma (6), which were more specifically identified as carcinoma (6), adenocarcinoma (14), transitional carcinoma (2), papillary adenocarcinoma (1), undifferentiated carcinoma (1), squamous cell carcinoma (1), spindle cell sarcoma (2), and chondrosarcoma (4).
Planning CT scans were performed on anesthetized dogs in treatment position using a GE Highlight advantage scanner (General Electric Medical Systems, Milwaukee, WI). With the dog in sternal recumbency, the head and thorax were immobilized in a deflatable mattress (Vac-Lok™, Med-Tec, Orange City, IA) conformed to the body contour of the dog. CT images of the head were acquired in 3–5 mm-thick, contiguous slices to include the nasal cavity and nasal sinuses with a 3-cm margin beyond the cranial and caudal limits of image-detectable gross tumor. Additional 1–3 mm transverse images were acquired through the region of the cribriform plate with high-frequency reconstruction algorithms to rule out cribriform plate involvement. CT images were obtained before and immediately after administration of intravenous contrast medium (2 ml/kg, Hypaque-76; Amersham Health Inc, Princeton NJ).
Magnetic resonance imaging (MRI) was also performed in all dogs prior to starting radiation treatments, which served to ensure lack of intracranial extenstion of tumor. Dogs were imaged in dorsal recumbency at 1.0 T (GE Signa Advantage) with a transmitting and receiving quadrature extremity or head coil. Protocols included T2-weighted and T1-weighted precontrast and postcontrast spin echo images in transverse plane and T1-weighted sagittal and dorsal plane spine echo images postcontrast.21 The CT data were transferred to a Pinnacle ™ Radiation Therapy Planning computer (Philips Electronics, Andover, MA, USA) for localization and contouring of treatment volumes and critical structures. The primary intent of the planning process was to identify, contour and exclude the ocular globes, lacrimal apparatus and intervening brain from the high radiotherapy dose volume. The clinical tumor volume (CTV) included the gross tumor volume (GTV), subcutaneous tissues where the confines of the sinonasal cavity had been breached and a 1 cm cranio-caudal margin. The planning tumor volume (PTV) included all nasal sinuses where tumor extension was apparent on CT, both right and left nasal cavities and any subcutaneous tissues considered at risk. The PTV included the CTV and an additional 1-cm cranial-caudal margin. This was done to mimic a similar PTV used to treat the historical controls treated with 2-D planning and Cobalt external beam therapy. Overall, the PTV represented a large and irregularly shaped target carved out between and around the ocular globes and brain, thus exploiting the concept of conformal avoidance.
Based on prior studies 5,20,22 the prescribed dose was 42 Gy to 95% of the PTV delivered in 10 daily fractions over 2 weeks. The dose-volume constraint for each eye was set at 15 Gy to no more than 60% of ocular volume; dose-volume constraint to brain was set at 20 Gy to no more than 50% of brain volume. The optic chiasm was not in the radiation treatment field. The olfactory bulbs were the only portion of the brain irradiated.
Treatment plans were generated using two different inverse-planning IMRT software systems. Delivery method was determined on a fraction-by-fraction basis and was dependent on equipment availability. The tomotherapy unit was the first clinical unit; therefore dual tomotherapy and DMLC plans were performed for each dog. In the event that the tomotherapy unit was in need of repair, dogs would be treated with DMLC technique to avoid treatment interruptions. Tomotherapy software (TomoTherapy Inc., Madison, WI, USA) was used to optimize plans to be delivered on the tomotherapy unit. The Pinnacle P3IMRT (release 6.2) treatment planning system was used to design plans to be delivered by dynamic MLC (120-leaf DMLC) on a Varian 6 MV linear accelerator (Clinac 600C/D, Varian Medical Systems, Palo Alto, CA, USA). DMLC plans were performed on the basis of a seven-beam arrangement. Treatment plans were optimized to produce the best dose-volume histogram (DVH) based on the tumor prescription and normal tissue constraints.
For treatment delivery, dogs were anesthetized and positioned in their customized immobilization mattress. For tomotherapy treatments, MV CT images were obtained with the tomotherapy unit using a 2 to 3 cGy imaging dose just prior to each daily treatment in real time.23 Images were then aligned with the planning CT to verify accuracy of set-up. Based on image alignment, dogs were repositioned as necessary, in the lateral, longitudinal, vertical, pitch, roll and yaw dimensions after final registration of the images (Figure 1). Later upgrades to the software automatically applied roll shifts. Shifts of 1mm or greater were applied without re-imaging, however significant shifts in the pitch, roll or yaw dimensions (>5 degrees) required a second MVCT after repositioning to ensure accurate delivery. For DMLC treatments, orthogonal dorsal-ventral and lateral port films were used to verify isocenter position on the first day of DMLC-based treatment and then as needed to verify positioning. For the dogs that were treated sporadically, orthogonal port films were obtained at each substituted treatment.
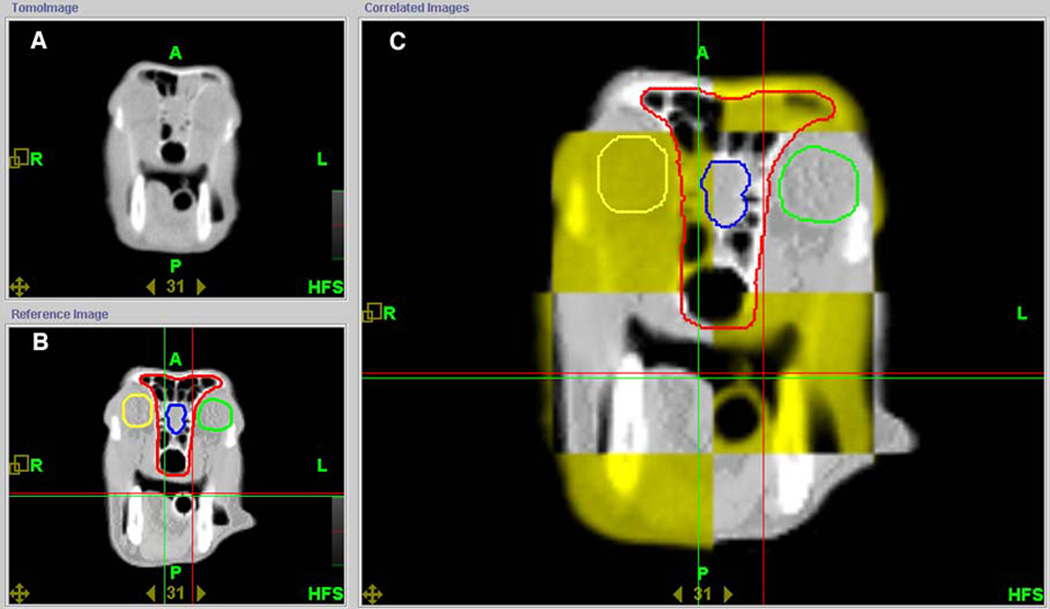
Figure 1.
Typical image of the tomotherapy operator station showing alignment of the MVCT (top left) with the original planning kVCT (bottom left) of a dog treated with helical tomotherapy. In the correlated image (large image, right) the yellow squares represent the MVCT image obtained prior to treatment and the grey areas represent the planning kVCT. The round yellow and green contours represent the right and left eyes, respectively. The blue contour is the rostral brain and the red contour is the PTV.
Standardized ophthalmic examinations were performed on IMRT-treated dogs prior to RT, at completion of therapy, 2 and 4 weeks post therapy, and then every 3 months thereafter. An American College of Veterinary Ophthalmologist Board-certified veterinary ophthalmologist (P.E.M.) performed the examinations, which included slit-lamp biomicroscopy, indirect ophthalmoscopy, Schirmer tear testing, fluorescein and Rose Bengal staining, and applanation tonometry. Changes were classified as acute if they occurred within 1 month of completion of radiotherapy and late if they occurred after this time.17 Lesions were qualitatively graded on a 0 – 4 scale. A score of 0 indicates no abnormality; Grade 1 represents trace changes perceptible only to an experienced observer using magnification and bright focal illumination; Grades 2, 3, and 4 refer to mild, moderate, and maximally severe changes, respectively. Ophthalmic complications were classified further into seven clinical syndromes in accordance with clinical standards of practice19: 1) conjunctivitis and/or blepharitis characterized by conjunctival hyperemia and/or blepharospasm; 2) keratitis, characterized by the presence of lipid corneal degeneration, corneal edema, scarring, pigmentation, and/or corneal vascularization (considered keratoconjunctivitis if associated with conjunctivitis); 3) ulcerative keratoconjunctivitis with or without blepharitis and characterized by fluorescein stain retention; 4) keratoconjunctivitis sicca characterized by a Schirmer tear test less than 10 mm/min and compatible clinical signs; 5) anterior uveitis characterized by ocular vascular injection, aqueous flare/cell, miosis or an intraocular pressure below normal; 6) presumed radiation-induced cataracts characterized as progressive opacification of the lens from that noted prior to radiation therapy; 7) radiation retinopathy/optic neuropathy characterized by retinal hemorrhages, detachment, degeneration or radiation induced damage to the optic nerve. Categories 1 to 3 were mutually exclusive, and the score that was assigned was the most severe complication manifested in either eye during the entire period of follow-up.
Tumor response and recurrence in dogs treated with IMRT was determined objectively via CT imaging and/or gross tumor measurements. Gross tumor measurements were performed only in dogs with lymph node metastases or with extra-nasal extension according to response criteria guidelines.24 Tumor response was documented per RECIST criteria and all images were evaluated by an American College of Veterinary Radiology Board-certified radiologist (LJF).24 As a component of this prospective clinical investigation, serial CT scans were scheduled for baseline (prior to radiation therapy) and then at 6 weeks, 3 months, 6 months, 9 months, 12 months, and 18 months following completion of therapy.
Historical data documenting extent of ocular toxicity was available for assessment from a comparable group of client-owned dogs with sinonasal cancer that received conventional two-dimensional (2D) megavoltage external beam radiation therapy and follow-up ophthalmic assessments.5,20 Dogs in this group were had no treatment prior to radiation therapy and one or both eyes were included in the radiation field in all dogs.
Thirty-six dogs of various breeds were evaluated in the historical control group.5,20 Median age and weight were 11 years (range 2.7–14.2 years) and 23.6 kg (range 6.0–50.4 kg), respectively. Four dogs had T1 tumors, 14 dogs had T2 tumors, 4 dogs had T3 tumors and 14 dogs had T4 tumors. Eight dogs had cribriform plate involvement. The median tumor stage was T2 and dogs presented with clinical signs typical of nasal disease. Tumor histologies consisted of 37 carcinomas, 5 sarcomas and included 7 carcinomas, 10 adenocarcinomas, 2 squamous cell carcinomas, 11 anaplastic carcinomas, 5 sarcomas, and one neoplasia of unknown histology.
Computer generated radiotherapy plans based on CT images were utilized for all dogs using a 2D multi-slice software program (Prowess 3000 Radiotherapy Treatment Planning System, SSGI, Chico, CA) and performed as an adaptive forward procedure. Treatment plans were non-uniform in terms of defining the PTV, although the PTV typically included the GTV and a 1 to 3-cm margin from the cranial and caudal limits of image detectable tumor. The eyes and brain were considered sensitive structures at risk. A single isocenter technique was employed using 60Cobalt γ-ray beams and two orthogonal, or three field arrangements. Beam weights and wedge angle were optimized manually. Using beam’s eye view technique, lead block arrangements were added to the fields to limit dose to the contralateral eye and brain without significantly compromising dose to the PTV. Dose heterogeneity at any structure was limited to +15% of the target dose. Similar to the IMRT dogs, the goal was to deliver a prescribed dose of 42 Gy to the PTV in 4.2 Gy daily fractions, 5 days a week. Irradiation was delivered using a Theratron 780 external beam 60Cobalt unit (Atomic Energy of Canada Ltd., Kanata, Ont., Canada). Orthogonal dorsal-ventral and lateral port films were used to verify treatment field position once weekly or more frequently if needed for verification of position.
After careful review of medical records of these dogs, ocular changes were classified but grading could not be retrospectively applied (Table 3). Detailed ophthalmic examinations by a veterinary ophthalmologist were not performed on a standardized and systematic basis; rather examinations were conducted as complications were encountered. Computer generated RT plans were reviewed for the historical control group and dose to the center of each lens was recorded; for the IMRT treated dogs, the mean dose to the eyes were either estimated from the dose volume histogram (DVH) or directly obtained from the treatment plan, when available. Mean dose to the eyes from the two groups were compared by a two-sample t-test. Survival curves were calculated by the Kaplan-Meier method, with confidence intervals obtained from Greenwood’s formula inverted to produce confidence intervals for the median.25 The log-rank test was used to compare survival curves.25 Dogs were censored from survival analysis if they were alive at the time that follow-up was completed (for the survival endpoint) or if they were progression-free at the time follow-up was completed (for the progression-free survival endpoint). The continuity adjusted chi-square test was used to compare groups. A p-value <0.05 was considered statistically significant.
Table 3.
Ocular toxicity classification for dogs treated with IMRT versus 2D radiotherapy.
| Category | Grade | IMRT n=31 # affected dogs (%) |
Category | 2D n=36 |
|---|---|---|---|---|
| Conjunctivitis | 0 | 24 (77%) | Conjunctivitis | 1 (3%) |
| Blepharitis | 1 | 4 | Blepharitis | * |
| 2 | 2 | Keratitis | * | |
| 3 | 1 | Keratoconjunctivitis | * | |
| 4 | 0 | * | ||
| Total | 7 (23%) | 35 (97%) | ||
| Keratitis | 0 | 28 (90%) | ||
| Keratoconjunctivitis | 1 | 3 | ||
| 2 | 0 | |||
| 3 | 0 | |||
| 4 | 0 | |||
| Total | 3 (10%) | |||
| Ulcerative | 0 | 31 (100%) | 28 (78%) | |
| keratoconjunctivitis | 1 | 0 | * | |
| 2 | 0 | * | ||
| 3 | 0 | * | ||
| 4 | 0 | * | ||
| Total | 0 (0%) | 8 (22%) | ||
| Keratoconjunctivitis | 0 | 28 (90%) | 26 (69%) | |
| sicca | 1 | 2 | * | |
| 2 | 0 | * | ||
| 3 | 1 | * | ||
| 4 | 0 | * | ||
| Total | 3 (10%) | 11 (31%) | ||
| Anterior Uveitis | 0 | 31 (100%) | 31 (86%) | |
| 1 | 0 | * | ||
| 2 | 0 | * | ||
| 3 | 0 | * | ||
| 4 | 0 | * | ||
| Total | 0 (0%) | 5 (14%) | ||
| Cataracts | 0 | 26 (84%) | 17 (47%) | |
| 1 | 3 | * | ||
| 2 | 0 | * | ||
| 3 | 0 | * | ||
| 4 | 2 | * | ||
| Total | 5 (16%) | 19 (53%) | ||
| Retinopathy | 0 | 27 (87%) | 24 (67%) | |
| Optic neuropathy | 1 | 4 | * | |
| 2 | 0 | * | ||
| 3 | 0 | * | ||
| 4 | 0 | * | ||
| Total | 4 (13%) | 12 (33%) | ||
| Blind eye(s) | Total | 0 | 20 (56%) | |
| Any Acute Effect | Total | 7 (23%) | 35 (97%) | |
| Any Chronic Effects | Total | 8 (26%) | 23 (64%) |
Due to the retrospective nature of the analysis, assignment of grade was not possible for ocular lesions in the 2D cohort; lesions were noted by veterinarians and/or pet-owners. More detailed ophthalmic examinations were performed by a veterinary ophthalmologist as complications were encountered. Therefore, lesions would likely be graded as 2, 3, or 4. Additionally many acute ocular effects in the 2D group were not separated into as many categories as the IMRT group.
Results
There were more dogs with higher stage tumors in the 2D-RT group compared to the tumors in the IMRT group (p=0.011). There were no statistically significant differences between age (p = 0.19), weight (p =0.88) or tumor histology (carcinoma versus sarcoma, p = 0.58). Table 2 provides a comparison between groups.
Table 2.
Patient, tumor characteristics and outcome of dogs with primary sinonasal tumors treated with conventional 2D-RT (n=36) or IMRT (n = 31).
| Characteristic | 2D-RT (n=36) |
IMRT (n=31) |
Significance (*p<0.05) |
|---|---|---|---|
| Age | |||
| Mean | 10.1 years | 9.3 years | p = 0.19 |
| Median | 11.0 years | 10.0 years | |
| Weight | |||
| Mean | 24.6 kg | 24.9 kg | p = 0.88 |
| Median | 23.6 kg | 24.0 kg | |
| Tumor Histology | |||
| Sarcomas | 5 | 6 | p = 0.58 |
| Carcinoma | 37 | 25 | |
| Tumor Histology Subtype | |||
| Carcinoma (CA) | 7 | 6 | |
| Adenocarcinoma (ACA) | 10 | 14 | |
| Transitional carcinoma | 2 | ||
| Papillary ACA | 1 | ||
| Undifferentiated or anaplastic CA | 11 | 1 | NA |
| Squamous cell CA | 2 | 1 | |
| Sarcoma | 5 | 2 | |
| Chondrosarcoma | 4 | ||
| Unknown | 1 | ||
| Tumor Stage | |||
| 1 | 4 | 13 | |
| 2 | 14 | 9 | *p = 0.011 |
| 3 | 4 | 5 | |
| 4 | 14 | 4 | |
| Mean Dose to Globes | 33.6 Gy (n=30) | 12.5 Gy (n = 27) | *p = 0.0001 |
| Incidence of Late Ocular Toxicity |
64% | 26% | *p = 0.0041 |
| Number of Blind Dogs | 20 (56%) | 0 (0%) | NA |
| Progression-free survival (PFS) | |||
| Overall | 194 days | ||
| Carcinoma | 144 days | NA | |
| Sarcoma | 418 days | ||
| Median survival time (MST) | |||
| Overall | 411 days | 420 days | p = 0.71 |
| Carcinoma | 393 days | 392 days | |
| Sarcoma | 721 days | 804 days | |
Thirty-one dogs qualified for entry into the prospective IMRT study; twenty-five dogs treated with only tomotherapy and 6 dogs with a combination of tomotherapy and DMLC. The median number of treatments with the linear accelerator for the 6 dogs was 3
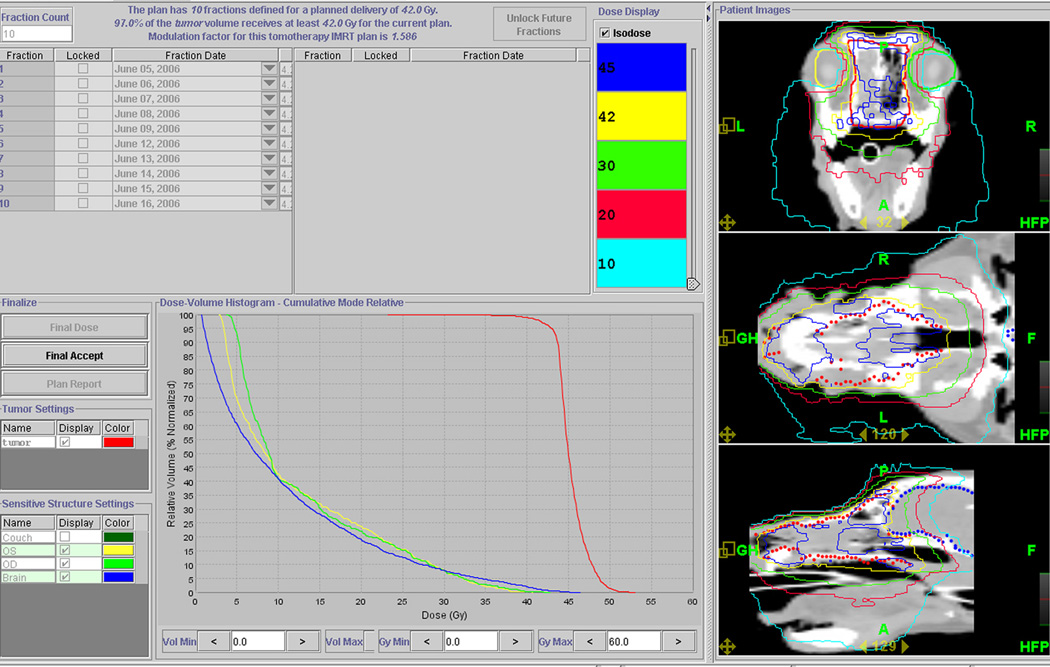
Based on dosimetric comparisons, IMRT plans were superior to the conventional 2D plans, both in terms of homogeneous dose delivery to the target, and reduced dose to the eyes (Figure 2). A steep dose gradient existed around the globes using IMRT, and based on DVH evaluation IMRT planning reduced the dose to ocular structures to within the dose-volume constraints (Figure 3).
Figure 2.
Typical IMRT isodose distributions and dose volume histogram are shown. The IMRT plan shown was designed using helical tomotherapy treatment planning software. The red line represents the PTV, and yellow, lime green and blue lines represent the left eye, right eye and brain, respectively.
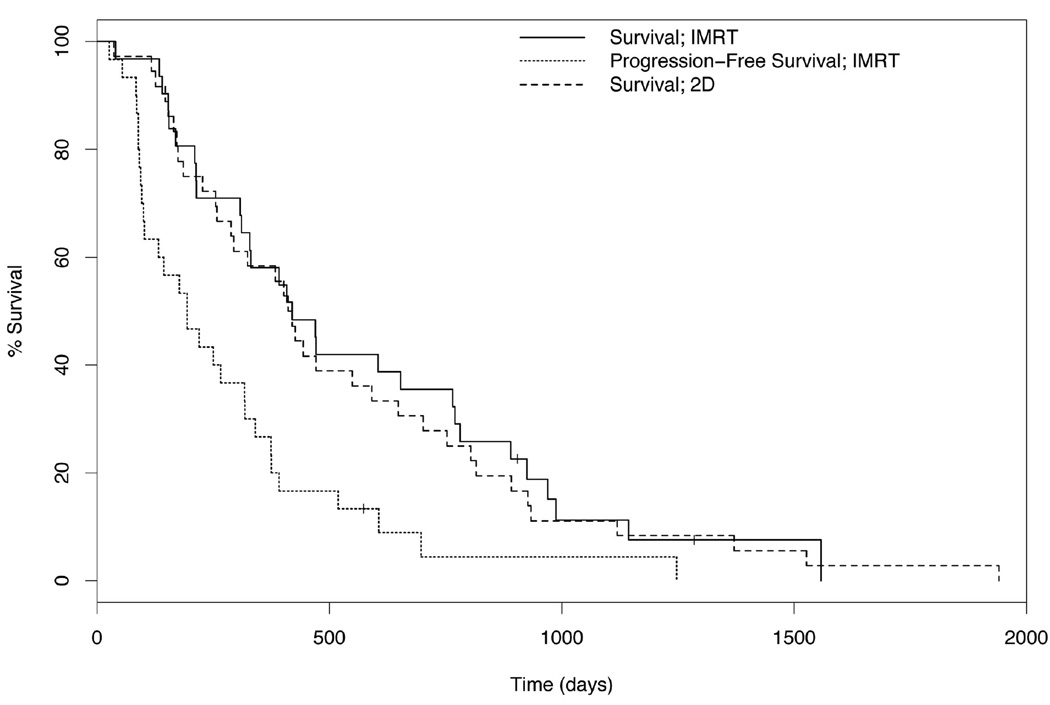
Figure 3.
Kaplan-Meier survival curves for the IMRT and control group of dogs and progression free survival curve for the IMRT cohort of dogs. The median PFS and median survival time (MST) for dogs receiving IMRT were 194 and 420 days, respectively. The MST for dogs after 2D radiation therapy was 411 days. Vertical hatch marks denote censorings.
Twenty-nine of the 31 dogs enrolled in the trial died during the course of the clinical trial. One dog was lost to follow up at 1285 days while 1 dog was still alive at the time of writing (935 days). For the final 5 dogs enrolled, funding provided only for treatment and one 6-week follow-up evaluation. However, it would be expected that significant ocular effects would be noted by the owner, similar to the historical control dogs, and subsequently evaluated by a veterinarian. Intensity modulated radiation therapy was well tolerated and none of the 31 dogs developed overt vision-impairing complications throughout the follow-up period.
In dogs planned using inverse planning methods and treated with IMRT, 95% of the tumor volume received the planned dose of 42 Gy and less than 60% volume of the globe received more than 15 Gy. The mean dose to the globes was available for 27/31 dogs. In 25 dogs the mean dose was the same for right and left eyes in each dog and was 12 Gy. The mean dose to the eye when all 27 dogs were evaluated was 12.5 Gy, as two dogs received higher dose to the eye ipsilateral to the tumor (14.6 Gy vs. 8.3 Gy; 22 Gy vs. 14 Gy). One dog with periocular tumor extension received 14.6 Gy to that eye and developed keratoconjunctivitis sicca 4 months post treatment. The second dog received a mean dose of 22 Gy to one eye and developed a mature cataract that was noted at necropsy 331 days post therapy. In all dogs that received IMRT, the eyes remained apparently comfortable, visual, and grossly normal in appearance to the pet owner and attending veterinarian throughout the course of the study. Ocular toxicities encountered are summarized in Table 3. Intensity modulated radiation therapy was well tolerated with only 7 dogs (23%) requiring transient topical ocular therapy for mild ocular or periocular surface disease. Although visual acuity is difficult to objectively evaluate in dogs,26 gross impairment of the animals’ visual performance was not detected.
Of the 14 dogs in which globe evaluation were performed post-mortem, presumed radiation-induced ocular changes were described in 15 of the 27 globes (one globe lost to perforation during removal). Changes to the globes included lymphoplasmacytic or lymphoid conjunctivitis (6), atrophy of the gland of the third eyelid (2), subretinal and intraretinal hemorrhage (2), cataract (6), conjunctival hyperplasia (1), keratitis (2), and retinal perivascular fibrosis (1), and Meibomian gland atrophy (1). Of the globes with cataracts identified, only 2 globes had a mature cataract while all others had minimal cortical or subcortical cataracts.
In contrast to the dogs treated with IMRT, the incidence of moderate to severe ocular complications in dogs treated with conventional 2D-RT was markedly higher5,20 (Table 2 and Table 3). Twenty-four of 36 dogs were evaluated by a veterinary ophthalmologist at least once after cessation of therapy. Of these, thirteen were evaluated on multiple occasions at various time intervals. Unlike the IMRT-treated dogs, only major symptomatic complications were identified. Median follow-up time was 8.1 months (range 0.7 – 42 months, mean 11.7 months). The time to development of ocular toxicity was similar to the IMRT treated dogs. Ophthalmic complications resulted in loss of vision in one or both eyes in 56% of dogs; seven dogs were bilaterally blind, 13 dogs were blind in one eye. Twenty-three dogs (64%) experienced symptomatic late ocular effects including chronic KCS, cataracts, retinopathy, or blindness to one or both eyes. Four dogs (11%) eventually required enucleation to alleviate chronic ocular pain or because of corneal perforation. The incidence of late ocular toxicities in the dogs treated with 2D-RT was significantly greater than the incidence of late ocular toxicities in the IMRT group (p = 0.0041).
For the 2D historical control group, the radiation dose to the mid-point of the lens was calculated from the treatment plans for 30 of the 36 dogs and the mean dose to the eyes were 33.6 Gy. Mean dose to 26 blind eyes was 39.06 Gy (range, 16.8–46.8 Gy), and mean dose to 23 sighted eyes was 29.85 Gy (range, 15–48 Gy). Dogs in the 2D historical group received a significantly higher dose to the eyes compared to the dogs treated with IMRT (p = 0.0001).
Maximum tumor response in the IMRT group, as measured by RECIST criteria, included 4 dogs with a complete response (CR), 18 dogs with a partial response (PR), 7 dogs with stable disease (SD), and 2 dogs with progressive disease (PD). Only one dog with CR sustained the response and was lost to follow up at 1285 days. The remaining 3 dogs sustained CR on imaging at 6 weeks, however developed progressive disease over time. Two dogs developed regional lymph node metastases despite lack of disease within the nasal cavity. Twenty-nine dogs died during the course of the study, 86% of them due to disease progression. One dog remains alive over 2.5 years since receiving tomotherapy radiation, however this dog failed local therapy at 317 days and subsequently underwent nasal exenteration. Four dogs died of causes unrelated to progression or treatment of their nasal disease. Post mortem examination was performed on three of the four dogs that died of other diseases. In two dogs, metastatic hemangiosarcoma was confirmed while the third dog died of right heart failure. The remaining dog was euthanized due to severe arthritis; this dog did not have any nasal signs at the time of death. The overall median survival (Fig. 3) was 420 days (95% CI, 311d, 781d). The progression free survival (Fig. 3) was 194 days (95% CI, 102d, 340d). Median survival for dogs with nasal sarcoma was 717 days (95% CI, 470d) with a median progression free survival of 418 days (95% CI, 266d). Outcomes were poorer for dogs with nasal carcinoma, with a median survival of 392 (95% CI, 144d, 770d) and a progression free survival median of 144 days (95% CI, 96d, 318d). This difference was not statistically significant (p = 0.42). Five of the 31 dogs (16%) developed regional lymph node metastasis following radiation therapy. Lymph node extirpation was performed in 3 dogs; one of these dogs was treated subsequently with chemotherapy and died of right heart failure at 1144 days. Of the 14 post mortem evaluations available, 5 dogs had tumor metastasis to other organs, including regional lymph nodes (3), lung (2), liver (2), brain (2), adrenal gland (1), thyroid gland (1), and eye (1).
Due to the retrospective nature of the analysis of the control 2D-treated group, and the lack of systematic follow-up of these dogs, the extent and duration of local tumor control after 2D therapy were difficult to assess. Clinical signs, including epistaxis, nasal discharge, sneezing, stertor and facial deformity, resolved with radiotherapy in all control dogs. Median survival time for this cohort was 411 days, (95% CI, 288d, 648d), nearly identical to that observed for the IMRT group (p = 0.71).
Discussion
There has been an ever-spreading worldwide implementation of IMRT and image guided techniques and numerous planning studies that document improved dosimetry over conventional techniques.27–34 Clinical data validating the superiority of IMRT over conventional CRT techniques has been shown, particularly in the treatment of human head and neck cancer.35–39 IMRT clinical studies reported thus far include both acute and late toxicities as data has matured.12,35,36,40–44
This study was predicated on detailed definition of avoidance structures and treatment of a generalized PTV, treating the sinonasal region as a cavity. This is analogous to many human situations, particularly head and neck cancers. From a treatment planning perspective, it is simpler to identify the avoidance regions precisely and define the PTV in broad terms, even to the extent of quantifying it as everything but the avoidance structure”. A conformal avoidance paradigm is commonly used in human radiation therapy practice, but the present study demonstrates its probable benefits with respect to the development of late toxicity.
In the present study, IMRT results in improved ocular sparing and a more favorable ocular side effect profile than with 2D-RT. IMRT was associated with a drastic diminution in the incidence of both acute and late ophthalmic toxicity. Due to the anatomic relationship between the PTV and the eyes in canine and human patients with sinonasal cancer, the eyes represent the dose limiting structures for dogs and humans. In ocular injury studies in the dog, the manifestation and progression of late ophthalmic complications occur by 6 to 9 months after radiation.17 Ocular changes develop along a relatively predictable time course: blepharitis, keratoconjunctivitis and corneal epithelial injury occur at 1 month or less post irradiation; at 3–6 months post RT, retinal hemorrhage and retinal degeneration begin to develop; retinal lesions progress while cataract formation develops at 6 months post irradiation.17 The data obtained in this study correlate with changes identified previously, aided by the frequent ophthalmic examinations.
Most dogs did not experience any significant ocular toxicity secondary to IMRT radiation. In fact, 61% of dogs did not experience any ocular side effects. Seven dogs (23%) developed acute conjunctivitis following radiation therapy, however it is likely that this number is higher than in the control group simply due to frequent, enforced ophthalmic examinations. None of these dogs would have been evaluated by a veterinarian for conjunctivitis had follow up not been provided. Late toxicity occurred in 8/31 (26%) of dogs and consisted of keratoconjunctivitis sicca (3 dogs, 10%), cataracts (5 dogs, 16%), retinopathy (4 dogs, 13%), and keratitis (3 dogs, 10%). While less than half of the dogs underwent post mortem examination, histologic changes to the globe indicate that radiation-induced changes develop, but may not be clinical significant. In stark contrast, toxicity secondary to 2D-RT observed in the historical control dogs caused significant ocular morbidity. Control dogs had higher rates of late toxicity with 64% of dogs experiencing anterior uveitis, keratoconjunctivitis sicca, cataracts, and retinopathies. The two groups of dogs were similar with respect to tumor location and radiation treatment dose and fractionation regimen. While dogs receiving 2D-RT were more likely to have T4 tumors compared to the dogs receiving IMRT, that was not unexpected given our inclusion criteria, which were devised such that long term follow-up would be feasible. Nasal tumor stage T4 includes tumors that have invaded through the cribriform plate or involve the nasopharynx, which would not have altered the dose to the eyes with a 2D-RT treatment plan at our institution. The eyes are located in such close proximity to the nasal cavity that the authors feel this difference in stage, while it may impact overall survival following radiation therapy, does not impact the difference seen in the incidence of late ocular toxicity. Although only the dogs treated with IMRT were evaluated on a prospective basis, the disparity in clinical outcome between the two groups in this study suggests that IMRT was effective at reducing the frequency and severity of ophthalmic complications. These results support the recent observations in which minimal acute ocular toxicity occurs in human patients with sinonasal cancer treated with IMRT.12,41,43
The primary endpoints of this study were to evaluate the benefit of IMRT practices to critical structures adjacent to a complex tumor volume. While tumor response and survival times were documented, these were secondary endpoints in the study. The median progression free survival and median survival times reported herein (194 days and 420 days) are comparable to other reports.4–9 As with many retrospectively collected reports, it is difficult if not impossible to make conclusions regarding differences in survival times between groups, as dogs in both groups were eligible to pursue a variety of treatments following progression of their nasal tumors. This factor makes median survival time a less relevant endpoint for many canine studies. It was not surprising that the dogs in this prospective study had similar outcomes to previous reports, as dose escalation or IMRT boost therapy were not attempted. The lower treatment-related toxicity associated with IMRT provides a theoretical opportunity for tumor dose escalation without increasing the fraction numbers.32,45–47 This should translate into an improvement in likelihood of tumor control. There is a dose-response relationship for nasopharyngeal tumors in people and a typical tumoricidal dose of 60–70 Gy delivered with conventional fractionation yields an overall local control rate near 80%.48–50 Late toxicities in people are dose limiting and efforts have focused on increasing tumor control probability (TCP) via simultaneous boost with stereotactic surgery or IMRT techniques. In two studies, patients that were dose-escalated to a total prescribed dose of at least 10 Gy exceeding the conventional dose (70 Gy) experienced improved local disease control.51,52 It is possible that similar strategies could be employed in canine sino-nasal tumor patients, however acute and late toxicities may increase and be considered unacceptable. In canine nasal tumor radiation studies, conventional doses of definitive radiation therapy have ranged from 42 Gy to 54 Gy without an improvement in outcome at higher overall doses.5–11 Additional fractionation schemes utilizing low-dose per fraction as per curative intent protocols and protocols employing a simultaneous boost dose via IMRT or stereotactic radio-surgery that ultimately deliver 70 Gy or higher may be needed to increase control of canine nasal tumors
In a recently published canine dosimetric study, simultaneously integrated dose boosts delivered with tomotherapy were delivered to increase the estimated TCP without markedly increasing the average dose to the eyes and brain.56 The high conformity of dose distribution with IMRT systems requires great accuracy and reproducibility in target localization and patient set-up to avoid geographic miss of tumor or overdose of sensitive structures. Theoretically, helical tomotherapy effectively addresses this challenge due to its real time tomographic imaging capabilities conferred by the CT component of the tomotherapy unit.2,23,46,53 Future clinical studies will address the use of dynamic planning and escalated doses to areas at risk for persistent or recurrent disease.54 It is important to note that image-guidance is essential if the concept of conformal avoidance is implemented in the clinic. Conformal avoidance implies that the patient must be positioned accurately to maintain the high dose gradient in the appropriate location as opposed to adjacent normal tissue. Conventional techniques of assessing patient positioning, such as weekly orthogonal portal radiographs do not suffice; rather more frequent and careful monitoring is required to avoid geographic miss. Image-guidance seeks to remove uncertainties associated with patient positioning at each treatment, allowing a decrease in the PTV. The PTV as described herein may have been excessive for dogs treated with IMRT, given that both nasal cavities in their entirety were included in the target volume to be treated. In a recent assessment of potential inaccuracies of daily set-up positioning, as evaluated by the translational, longitudinal, vertical, pitch, roll and yaw deviations in 10 of the dogs reported here that were treated with tomotherapy.55 That study supported the use of daily image guidance to ensure accurate delivery of IMRT distributions and avoid a potential geographic miss decreasing the probability of tumor control.55
Overall, use of IMRT drastically reduced the frequency and severity of acute and late ocular toxicity as compared to that seen with conventional 2D-RT delivery. Image guided IMRT techniques may be applied to many canine and feline cancers and future studies will hopefully corroborate the theoretical benefit of tumor control and the patient’s overall quality of life. Future prospective studies may help evaluate the existence of a dose-response relationship for canine tumors, and specifically nasal tumors, and ultimately lead to improved local control and outcome.
Acknowledgements
The authors would like to acknowledge the support of NIH P01 CA088960. The authors associated with TomoTherapy Inc. have a financial interest in that company.
Funding support provided by: NIH P01 CA088960
Footnotes
Notification of conflict of interest: T. Rockwell Mackie, PhD, is co-founder of TomoTherapy Inc., chairman of the board, and director of research, Minesh P. Mehta, MD is a paid consultant for TomoTherapy, Inc. Both have a financial interest in that company.
Data presented in part: 48th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Philadelphia PA, November 5–9, 2006.
References
- 1.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Sem Radiat Oncol. 1999;9:108–117. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- 2.Mackie TR, Kapatoes J, Ruchala K, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:89–105. doi: 10.1016/s0360-3016(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 3.Beavis AW. Is tomotherapy the future of IMRT? Brit J Radiol. 2004;77:285–295. doi: 10.1259/bjr/22666727. [DOI] [PubMed] [Google Scholar]
- 4.Adams WM, Bjorling DE, McAnulty JE, et al. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990–2002) J Am Vet Med Assoc. 2005;227 doi: 10.2460/javma.2005.227.936. 396-41. [DOI] [PubMed] [Google Scholar]
- 5.Adams WM, Miller PE, Vail DM, et al. An accelerated technique for irradiation of malignant canine nasal and paranasal sinus tumors. Vet Radiol Ultrasound. 1998;39:475–481. doi: 10.1111/j.1740-8261.1998.tb01637.x. [DOI] [PubMed] [Google Scholar]
- 6.Adams WM, Withrow SJ, Walshaw R, et al. Radiotherapy of malignant nasal tumors in 67 dogs. J Am Vet Med Assoc. 1987;191:311–315. [PubMed] [Google Scholar]
- 7.McEntee MC, Page RL, Heidner GL, et al. A retrospective study of 27 dogs with intranasal neoplasms treated with cobalt radiation. Vet Radiol. 1991;32:135–139. [Google Scholar]
- 8.Theon AP, Madewell BR, Harb MF, et al. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J Am Vet Med Assoc. 1993;202:1469–1475. [PubMed] [Google Scholar]
- 9.Thrall DE, Harvey CE. Radiotherapy of malignant nasal tumors in 21 dogs. J Am Vet Med Assoc. 1983;183:663–666. [PubMed] [Google Scholar]
- 10.Thrall DE, Heidner GL, Novotney CA, et al. Failure patterns following cobalt irradiation in dogs with nasal carcinoma. Vet Radiol Ultrasound. 1993;34:126–133. [Google Scholar]
- 11.Rassnick KM, Goldkamp CE, Erb HN, et al. Evaluation of factors associated with survival in dogs with untreated nasal carcinomas: 139 cases (1993–2003) J Am Vet Med Assoc. 2006;229:401–406. doi: 10.2460/javma.229.3.401. [DOI] [PubMed] [Google Scholar]
- 12.Claus F, Boterberg T, Ost P, et al. Short term toxicity profile for 32 sinonasal cancer patients treated with IMRT. Can we avoid dry eye syndrome? Radiother Oncol. 2002;64:205–208. doi: 10.1016/s0167-8140(02)00172-x. [DOI] [PubMed] [Google Scholar]
- 13.Gordon KB, Char DH, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys. 1995;31:1123–1139. doi: 10.1016/0360-3016(95)00062-4. [DOI] [PubMed] [Google Scholar]
- 14.Hemple M, Hinkelbein W. Eye sequelae following external irradiation. Recent Results Cancer Res. 1993;130:231–236. doi: 10.1007/978-3-642-84892-6_20. [DOI] [PubMed] [Google Scholar]
- 15.Jiang GL, Tucker SL, Guttenberger R, et al. Radiation-induced injury to the visual pathway. Radiother Oncol. 1994;30:17–25. doi: 10.1016/0167-8140(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 16.Parsons JT, Bova FJ, Fitzgerald CR, et al. Severe dry-eye syndrome following external beam irradiation. Int J Radiat Onocol Biol Phys. 1994;30:775–780. doi: 10.1016/0360-3016(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 17.Ching SV, McChesney-Gillette S, Powers BE, et al. Radiation-induced ocular injury in the dog: A histological study. Int J Radiat Oncol Biol Phys. 1990;19:321–328. doi: 10.1016/0360-3016(90)90540-z. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson VE, Davidson MG, Nasisse MP, et al. Ocular complications following Cobalt 60 radiotherapy of neoplasms in the canine head region. J Am Animal Hosp Assoc. 1991;27:51–55. [Google Scholar]
- 19.Roberts SM, Lavach JD, Severin GA, et al. Ophthalmic complications following megavoltage irradiation of the nasal and paranasal cavities in dogs. J Am Vet Med Assoc. 1987;190:43–47. [PubMed] [Google Scholar]
- 20.Green EM, Adams WM, Forrest LJ, et al. Accelerated radiotherapy for canine nasal neoplasia, 2002 Annual Scientific Meeting of the American College of Veterinary Radiology; Chicago, IL: Vet Radiol Ultrasound; 2003. p. 113. pp 113. [Google Scholar]
- 21.Drees R, Forrest LJ, Chappell R. Comparison of computed tomography and magnetic resonance imaging for evaluation of canine intranasal neoplasia. J Small Anim Pract. 2009;50:334–340. doi: 10.1111/j.1748-5827.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest LJ, Lawrence JA, Miller PE, et al. Ocular Sparing Using Image-Guided Helical Tomotherapy (IGHT) in Spontaneous Sino-Nasal Tumors in Dogs #2387. Int J Radiat Oncol Biol Phys. 2006;66:S452. [Google Scholar]
- 23.Forrest LJ, Mackie TR, Ruchala K, et al. The Utility of Megavoltage Computed Tomography Images from a Helical Tomotherapy System for Set-up Verification Purposes. Int J Radiat Oncol Biol Phys. 2004;60:1639–1644. doi: 10.1016/j.ijrobp.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- 26.Miller PE, Murphy C. Vision in dogs. J Am Vet Med Assoc. 1995;207:1623–1634. [PubMed] [Google Scholar]
- 27.Adams EJ, Nutting CM, Convery DJ, et al. Potential role of intensity-modulated radiotherapy in the treatment of tumors of the maxillary sinus. Int J Radiat Oncol Biol Phys. 2001;51:579–588. doi: 10.1016/s0360-3016(01)01655-8. [DOI] [PubMed] [Google Scholar]
- 28.Cheng JC, Chao KS, Low D. Comparison of intensity modulated radiation therapy (IMRT) treatment techniques for nasopharyngeal carcinoma. Int J Cancer. 2001;96:126–131. doi: 10.1002/ijc.1004. [DOI] [PubMed] [Google Scholar]
- 29.Clark CH, Bidmead AM, Mubata CD, et al. Intensity-modulated radiotherapy improves target coverage, spinal cord sparing and allows dose escalation in patients with locally advanced cancer of the larynx. Radiother Oncol. 2004;70:189–198. doi: 10.1016/j.radonc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Cozzi L, Fogliata A, Bolsi A, et al. Three-dimensional conformal vs. intensity modulated radiotherapy in head-and-neck cancer patients: comparative analysis of dosimetric and technical parameters. Int J Radiat Oncol Biol Phys. 2004;58:617–624. doi: 10.1016/j.ijrobp.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 31.Huang D, Xia P, Akazawa P, et al. Comparison of treatment plans using intensity-modulated radiotherapy and three-dimensional conformal radiotherapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:158–168. doi: 10.1016/s0360-3016(03)00080-4. [DOI] [PubMed] [Google Scholar]
- 32.Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys. 2003;56:145–157. doi: 10.1016/s0360-3016(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 33.Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–154. doi: 10.1016/s0360-3016(03)01452-4. [DOI] [PubMed] [Google Scholar]
- 34.Nutting CM, Convery DJ, Cosgrove VP, et al. Improvements in target coverage and reduced spinal cord irradiation using intensity-modulated radiotherapy (IMRT) in patients with carcinoma of the thyroid gland. Radiother Oncol. 2001;60:173–180. doi: 10.1016/s0167-8140(01)00382-6. [DOI] [PubMed] [Google Scholar]
- 35.Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–1069. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 36.Chao KS, Ozyigit G, Blanco AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Jabbari S, Kim HM, Feng M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys. 2005;63:725–731. doi: 10.1016/j.ijrobp.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 38.Munter MW, Karger CP, Hoffner SG, et al. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int J Radiat Oncol Biol Phys. 2004;58:174–184. doi: 10.1016/s0360-3016(03)01437-8. [DOI] [PubMed] [Google Scholar]
- 39.Parliament MB, Scrimger RA, Anderson SG, et al. Preservation of oral health-related quality of life and salivary flow rates after inverse-planned intensity- modulated radiotherapy (IMRT) for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:663–673. doi: 10.1016/S0360-3016(03)01571-2. [DOI] [PubMed] [Google Scholar]
- 40.Butler EB, Teh BS, Grant WHr, et al. Smart (simultaneous modulated accelerated radiation therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:21–32. doi: 10.1016/s0360-3016(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 41.Claus F, Boterberg T, Ost P, et al. Postoperative radiotherapy for adenocarcinoma of the ethmoid sinuses: treatment results for 47 patients. Int J Radiat Oncol Biol Phys. 2002;54:1089–1094. doi: 10.1016/s0360-3016(02)02985-1. [DOI] [PubMed] [Google Scholar]
- 42.Lu TX, Mai WY, Teh BS, et al. Initial experience using intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:682–687. doi: 10.1016/S0360-3016(03)01508-6. [DOI] [PubMed] [Google Scholar]
- 43.Madani I, Bonte K, Vakaet L, et al. Intensity-modulated radiotherapy for sinonasal tumors: Ghent University Hospital update. Int J Radiat Oncol Biol Phys. 2009;73:424–432. doi: 10.1016/j.ijrobp.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 44.Teh BS, Amosson CM, Mai WY, et al. Intensity modulated radiation therapy (IMRT) in the management of prostate cancer. Cancer Invest. 2004;22:913–924. doi: 10.1081/cnv-200039674. [DOI] [PubMed] [Google Scholar]
- 45.Group IMRTCW. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 46.Welsh JS, Patel RR, Ritter MA, et al. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat. 2002;1:311–316. doi: 10.1177/153303460200100413. [DOI] [PubMed] [Google Scholar]
- 47.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 48.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–270. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]
- 49.Teo PM, Yu P, Lee WY, et al. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int J Radiat Oncol Biol Phys. 1996;36:291–304. doi: 10.1016/s0360-3016(96)00323-9. [DOI] [PubMed] [Google Scholar]
- 50.Yan JH, Xu GZ, Hu YH, et al. Management of local residual primary lesion of nasopharyngeal carcinoma: II. Results of prospective randomized trail on booster dose. Int J Radiat Oncol Biol Phys. 1990;18:295–298. doi: 10.1016/0360-3016(90)90092-x. [DOI] [PubMed] [Google Scholar]
- 51.Chang SD, Tate DJ, Gofinet DR, et al. Treatment of nasopharyngeal carcinoma: stereotactic radiosurgical boost following fractionated radiotherapy. Sterotact Funct Neurosurg. 1999;73:64–67. doi: 10.1159/000029753. [DOI] [PubMed] [Google Scholar]
- 52.Chau RM, Teo PM, Choi PH, et al. Three-dimensional dosimetric evaluation of a conventional radiotherapy technique for treatment of nasopharyngeal carcinoma. Radiother Oncol. 2001;58:143–153. doi: 10.1016/s0167-8140(00)00336-4. [DOI] [PubMed] [Google Scholar]
- 53.Welsh JS, Lock M, Harari PM, et al. Clinical implementation of adaptive helical tomotherapy: a unique approach to image-guided intensity modulated radiotherapy. Technol Cancer Res Treat. 2006;5:465–479. doi: 10.1177/153303460600500503. [DOI] [PubMed] [Google Scholar]
- 54.Gutíerrez AN, Deveau MA, Forrest LJ, et al. Radiobiological and treatment planning study of a simultaneously integrated boost for canine nasal tumors using helical tomotherapy. Vet Radiol Ultrasound. 2007;48:594–602. doi: 10.1111/j.1740-8261.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 55.Deveau MA, Gutierrez AN, Mackie TR, et al. Dosimetric impact of daily setup variations during treatment of canine nasal tumors using intensity-modulated radiation therapy. Vet Radiol Ultrasound. 2010;51:90–96. doi: 10.1111/j.1740-8261.2009.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]