Abstract
Surgery has become the standard of care for patients with intractable temporal lobe epilepsy with anterior temporal lobe resection the most common operation performed for adults with hippocampal sclerosis. This procedure leads to significant improvement in the lives of the overwhelming majority of patients. Despite improved techniques in neuroimaging that have facilitated the identification of potential surgical candidates, the short and long term success of epilepsy surgery has not changed substantially in recent decades. The basic surgical goal, removal of the amygdala, hippocampus, and parahippocampal gyrus, is based on the hypothesis that these structures represent a uniform and contiguous source of seizures in the mesial temporal lobe epilepsy syndrome. Recent observations from the histopathology of resected tissue, preoperative neuroimaging and the basic science laboratory suggest that the syndrome is not always a uniform entity. Despite clinical similarity, not all patients become seizure free. Improving surgical outcomes requires a re-examination of why patients fail surgery. This review will examine recent findings from the clinic and laboratory. Historically, we have considered MTLE a single disorder, but it may be time to view it as a group of closely related syndromes with variable type and extent of histopathology. That recognition may lead to identifying the appropriate subgroups that will require different diagnostic and surgical approaches to improve surgical outcomes.
Insanity: doing the same thing over and over again and expecting different results.
Albert Einstein
Surgery has become the standard of care for patients with intractable focal epilepsy, especially those with positive neuroimaging. Surgical candidacy is determined by the identification of the presumed seizure focus, with our concept of the focus based on a correlation of preoperative clinical and imaging findings, abnormal histopathology and surgical outcomes. In the case of mesial temporal lobe epilepsy (MTLE), the results from the last half century have pointed to the mesial temporal structures with a sclerotic hippocampus as the likely site of seizure onset for most patients. Despite clinical and apparent neuropathological similarity, not all patients become seizure free following anterior temporal lobectomy. Reanalysis of the associated histopathology, advances in neuroimaging, and data from animal models of MTLE raise questions about the structural substrate and the changes in the seizure onset zone, particularly its extent and variability. These new observations call for a reassessment of our understanding of the pathophysiology and, perhaps, the optimal surgical approach for the MTLE syndrome.
At present, we work from the hypothesis that successful surgery occurs when all of the tissue responsible for seizure generation is removed. This review evaluates this hypothesis by determining factors that may contribute to surgical success or failure. We will first review clinical predictors of seizure freedom after surgery for MTLE. This overview will be followed by an examination of how variations in the histopathology and the patterns in neuroimaging may relate to outcome. We will then use data from animal models to understand the functional significance of these variations. We will conclude with a discussion about the implications of these findings for surgical planning with the goal of improving long term outcomes.
Seizure Freedom after MTLE surgery
Results of meta-analyses surveying the literature from 1985 to 2003 indicate that about two-thirds of patients are seizure-free in the first two to three years after surgery for MTLE. Surgical risks include a 0.24% chance of death, a 2% chance of serious permanent complications, and a 6% chance of transient complications.1, 2 In comparison, best medical therapies over a similar period yield a 5% chance of becoming seizure free and a 0.5 to 1.0% chance of death per year from the epilepsy3. Hence, surgery is a highly effective treatment for patients with medically refractory MTLE. However, about one third of patients continue with seizures after surgical therapy. At present it is not clear why some patients fail to become or remain seizure-free after surgery or what factors may predict seizure freedom, but emerging studies are beginning to define these variables (Table 1).
Table 1.
Presurgical Predictors of Seizure Control for MTLE
| Positive Predictors | Negative Predictors |
|---|---|
| Circumscribed hippocampal sclerosis | No histopathology in surgical specimen |
| Circumscribed low grade tumors | Lesion outside of resection |
| Greater volume resected | Decreased hippocampal asymmetry |
| Atrophy ipsilateral to EEG abnormality | History of secondary generalization |
| Only hippocampal atrophy on MRI | Bilateral atrophy on quantitative MRI |
| Concordant memory asymmetry on WADA | Increased regional heterogeneity on MRI surface modeling |
| Incomplete mesial resection including parahippocampal | |
| Controversial Predictors | Wider FDG PET abnormality |
| Duration of epilepsy history | Atypical Ictal SPECT changes outside resection mesial temporal lobe |
| Amygdala and hippocampal atrophy on MRI |
Recurrence of seizures in the first year following surgery is a predictor of poor outcome and favors the notion that the epileptogenic zone has not been completely removed.4,5 Early seizure recurrence is seen in over 60% of TLE patients without evidence of histopathology in the surgical specimen, in those with lesions outside the area of resection (distant lesion)6 or in those with incomplete resections of mesial temporal structures.7 However, even patients with evidence of adequate removal of well-circumscribed temporal lesions have a 10% risk of recurrent seizures in the first year after surgery. In some studies this number increases to 25% to 30% with follow-up of three years or more. However, another study suggests that after several years of postsurgical seizure freedom, the risk for seizure recurrence is low.8 Although the duration of epilepsy prior to surgery has been associated with poorer long term outcomes,9,10 this association does not appear to be true for all MTLE patients.7,8 A history of secondarily generalized tonic-clonic seizures is associated with recurrent seizures two years after surgery,11,12 as well as over the longer term. Although seizure recurrence following surgery is usually considered evidence for remnants of a seizure onset zone, it has been well recognized that some patients with recurrent seizures have developed psychogenic seizures, and this issue should be taken into consideration in the evaluation of long-term outcomes.
In addition to the clinical history there is an association between the volume of tissue removed and seizure freedom following surgery.7,13 Reports of patients who failed the first mesial temporal resection have shown significant improvement in outcome with an extension of the amount of mesial temporal tissue removed.14,15 The extent of the hippocampal resection itself has been shown to influence seizure free outcomes with resections that extend more posteriorly having a significantly greater proportion of patients who become seizure free.16 These observations suggest that, despite early seizure control after surgery, tissue beyond the hippocampus and parahippocampal complex or remnants of mesial structures may be capable of generating seizures in some MTLE patients and that it may be important to remove the hippocampus completely as well as the other mesial temporal structures to achieve seizure freedom. Overall these observations suggest that the seizure onset zone may involve the entire mesial temporal region. As will be discussed later there may also be a second independent seizure focus.
Ideally the goal of surgery is to cure patients, which implies seizure freedom without medication. A cure also implies that critical components of the seizure circuit have been completely removed, and residual tissue is not capable of independent seizure generation. For MTLE patients who stop taking medications following successful surgery, about 75% will have a recurrence of seizures.17 Hence, while surgery often stops seizures, it is uncommon that MTLE patients can remain seizure free off medications. This finding suggests that in patients who have undergone temporal lobe resections, residual tissue and circuitsremain that are capable of generating seizures if medications are discontinued. Whether these regions represent an independent focus arising from a second pathology or are remnants of the primary focus is generally unknown. There is evidence in the literature of patients who have bilateral hippocampal atrophy or second and potentially seizure causing pathologies.18,19
Imaging the Seizure Focus
Preoperative neuroimaging in MTLE is used to lateralize the abnormality to the right or left temporal lobe. Neuroimaging is generally not used to determine the planned extent of resection in many centers. In this section we will review how imaging is used to localize the focus, how the findings may predict surgical outcome and how we may better define the areas likely to generate seizures.
Magnetic resonance imaging (MRI) reliably detects HS preoperatively20 with features such as unilateral hippocampal atrophy, decreased signal intensity seen on T1 and increased signal intensity on T2.21–23 Hippocampal atrophy (by qualitative visual review) ipsilateral to EEG abnormalities is the most reliable predictor of seizure control following surgery with a specificity of 93% and a sensitivity of 83%.24 Although associated MRI features are helpful in diagnosing the underlying pathology, so far they have not added significantly to predicting surgical outcome. However, it is not clear that information about a more extensive histopathology has affected the extent of the resection.
At present we focus on qualitative asymmetry between the left and right hippocampi25, but the presence of atrophy on both sides (suggesting bilateral hippocampal disease) is associated with poorer surgical outcomes.26 Anatomic surface modeling of hippocampal atrophy has shown that individuals who are not seizure-free following surgery have a greater regional variation along the medial and lateral surface of the ipsilateral hippocampus as well as a lesser degree of asymmetry between the ipsilateral and contralateral sides.27 What these findings mean with regard to the underlying histopathology or pathophysiology is not clear, but they emphasize a possible variation in the structural basis of MTLE. At present it is unclear whether these quantitative techniques, which are very resource intensive, will lead to improved outcomes or whether they can be used prospectively to identify the extent of the seizure onset zone.
Other quantitative techniques have shown unilateral high T2 relaxometry values to be a useful identifier of hippocampal histopathology28,29 and correlate well with the severity of atrophy25, although normal T2 values can be observed. Abnormal T2 signal is seen in the contralateral hippocampus in 30% to 40% of the cases, similar to the rate in neuropathology studies.17 T2 relaxometry is also a sensitive measure of amygdala pathology.30
Single voxel proton magnetic resonance spectroscopy31 as well as magnetic resonance spectroscopic imaging (MRSI)32 have demonstrated a reduction in temporal lobe N-acetylaspartate to choline plus creatine ratios in up to 75% of MTLE patients, correctly lateralizing in 55%. Bilateral abnormalities, however, have also been reported using MRSI techniques in 45% of MTLE patients. It is unclear whether spectroscopy aids in the prediction of seizure freedom following surgery.33,34
Functional imaging may also reveal wider histopathology. Studies using 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) have shown abnormal areas outside the resected regions, particularly in those not seizure free (Fig. 1).35,36 Ictal SPECT studies with perfusion patterns outside the resected regions are also associated with poor postsurgery seizure outcomes.37,38 The results from these studies suggest that wider areas of dysfunction may be present in those who fail surgery for MTLE. At present functional imaging is not considered sufficiently accurate to define the seizure focus well enough to determine the site and extent of a resection. For now these modalities raise the potential for areas to have histopathology that may be causing the seizure as well as identifying patients that may be less likely to benefit from a resection. To determine whether these techniques can be used to identify the seizure onset zone more exactly it will be necessary to evaluate the current techniques (e.g. PET, SPECT) critically and correlate the extent of histopathology, type of lesion and extent of the resection in relation to the imaging with the long term seizure control outcome. We may find that the current techniques, including the radioligands that we now use, may be insufficient for this goal and that we may need to develop ligands that are seizure focus specific.
Figure 1. FDG-PET/MRI co-registration of two MTLE patients.
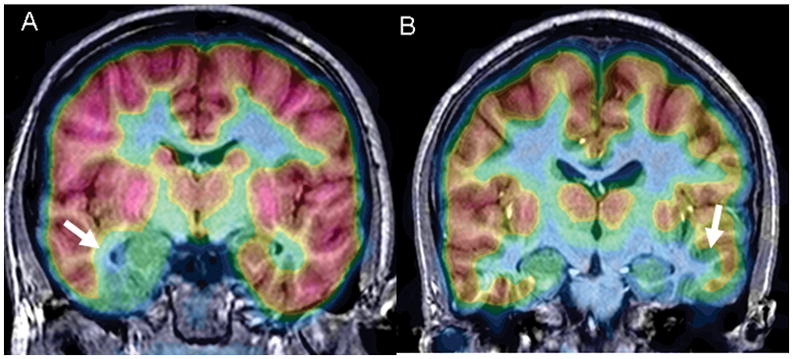
A: This 14 year old had clinical features of left MTLE associated with an area of Type II cortical dysplasia in the mesial temporal lobe (white arrow and blue color). There was associated hypometabolism in the adjoining hippocampus which by histopathology showed hippocampal sclerosis. B: This 9 year old had right MTLE from hippocampal sclerosis. Notice that the area of hypometabolism extends outside the hippocampus (white arrow; blue color). The lateral temporal neocortex showed Type IA (mild) cortical dysplasia at histopathology. Both patients became seizure free after an extended temporal resection that included the areas of FDG-PET hypometabolism.
How can we view the current status of imaging as a tool for defining the extent of the seizure generating zones in patients with MTLE? Current technologies are good at demonstrating unilateral hippocampal abnormalities, but may miss contralateral pathology (Fig. 2A) or the extent of pathology on the ipsilateral side. In limited studies, individuals with volumetric hippocampal atrophy alone are more likely to be seizure free than those with amygdalo-hippocampal atrophy.39 This finding suggests that more extensive histopathology is associated with poorer outcomes. Abnormalities outside of the hippocampus such as cortical malformations40,41 not surprisingly are more common amongst those not seizure free (Fig. 2B). Voxel-based MR morphometry showing more subtle grey matter abnormalities beyond the hippocampus42 and grey matter volume reduction are also associated with persistent seizures after surgery.43 However, similar areas of grey matter reduction in children correspond to areas known to receive hippocampal projections (as seen pathologically), so the exact relationship of such extrahippocampal changes to persisting seizures is unknown.44
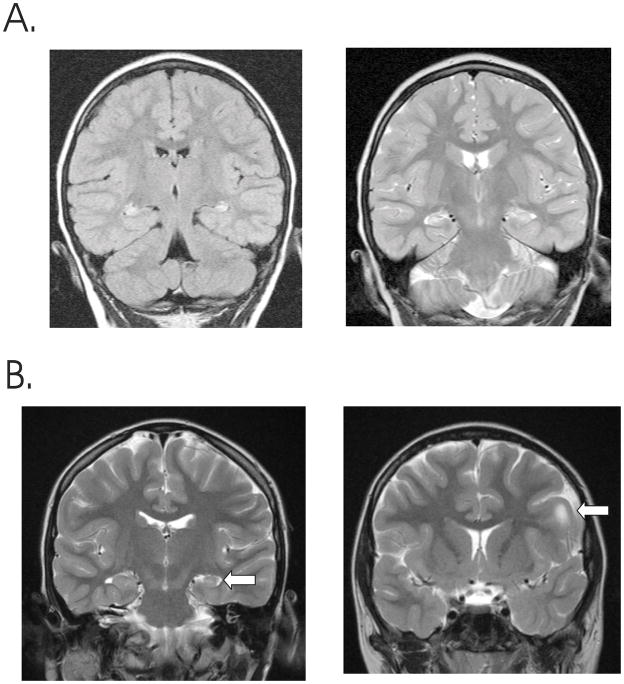
Figure 2. MRI changes associated with MTLE.
A. Bilateral hippocampal abnormalities in a young man with temporal lobe seizures. There is bilateral increased signal from the left and right hippocampus on a FLAIR sequence (left) as well as increased signal and atrophy from both sides on a T2 weighted image (right). B. Unilateral hippocampal atrophy in a T2 weighted image from young woman with MTLE (left image, arrow), who also had a left frontal cortical dysplasia that was confirmed histologically following lesionectomy and temporal lobectomy after invasive EEG monitoring (right image, arrow).
The observations of epilepsy associated imaging changes of uncertain significance raise the question of which alterations are a component of the seizure initiating zone and which are passive associations that have less responsibility for seizure onset. Imaging has provided us with data that are somewhat predictive of surgical outcomes, but we are still not able to determine the full extent of the abnormality, or reliably predict those unlikely to be seizure free following standard anterior temporal lobe resection. Although we can often see pathology beyond the hippocampus, we have not viewed the images as defining a larger or smaller epileptogenic zone. Defining the full area responsible for seizure onset by imaging may be the next major step for improving surgical outcomes for MTLE patients.
Neuropathology
Many view hippocampal sclerosis (HS) as a single entity. However, studies have shown variability in the pattern of HS as well as in the associated histopathology in other limbic sites and other parts of the brain. HS is the most common focal pathology in patients with MTLE undergoing surgery45–47 and is argued to be the cause of the seizures.48 In addition to classical HS other described variations in temporal lobe histopathology include atypical patterns of HS, pathology in adjacent mesial temporal lobe structures, occult second (dual) pathology and bilateral hippocampal damage. There is some evidence to support that these variations may influence surgical outcome.
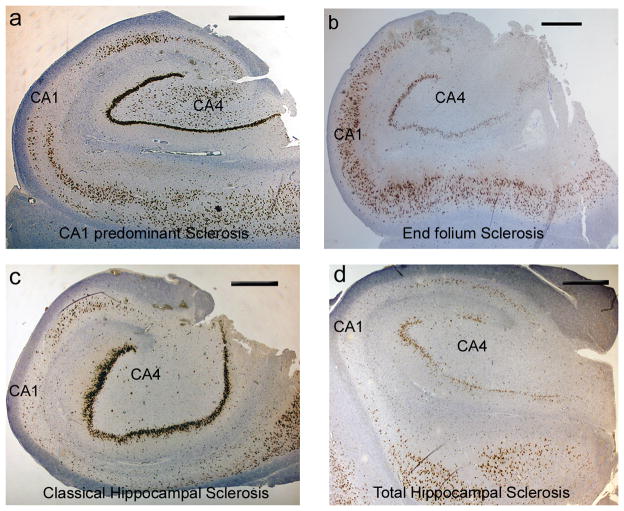
Under the general category of HS, several patterns have been recognized: 1) classical HS (CHS) with neuronal loss in CA1 and the hilar region, 2) end folium sclerosis (EFS) or mesial temporal sclerosis type 3 (MTS type 3) with neuronal loss primarily in the hilar region, and 3) loss restricted to CA1 only (MTS type 2; Fig. 3).49,50 EFS/MTS type 3 is less readily identified by preoperative imaging.51 Non-classical or atypical patterns of HS, which overall account for 4% to 10% of cases47,49,52,53 have been associated with poorer surgical outcomes compared to CHS. Seizure free outcomes for MTS type 3 at 1 year are 25% to 28% compared to 66% to 77% for MTS type 2 and 72% to 84% for CHS.49,51,53 A further group with no significant hippocampal neuronal loss, also fare less well compared to CHS with seizure free outcomes of 44% to 58%49,53 following temporal lobe surgery. These observations, although requiring verification through further series with longer follow-up periods, reinforce the potential value of identifying atypical patterns of HS. In addition to some predictive power in determining outcome, subtypes of HS may point to distinct syndromes with unique pathophysiologies.
Figure 3. Classical and atypical patterns of hippocampal sclerosis (all NeuN immunohistochemistry) .
A. CA1 predominant hippocampal sclerosis. (HS) showing neuronal loss on NeuN staining affecting mainly the CA1 subfield. B. End folium sclerosis has a hippocampus with relative preservation of neurons in all subfields except for loss of neurons from CA4/hilus. There is evidence that this pattern of sclerosis is associated with a poorer postoperative outcome. C. Classical HS. Neuronal loss from CA4 and CA1 with relative preservation of neurons in CA2, the dentate gyrus and subiculum. D. Total HS with loss of neurons in all subfields (CA1-4) including the dentate gyrus.
Knowing the physical extent of the histopathology (and presumed focus) is important in our attempts to remove the area generating the seizures completely. A study of 206 hippocampectomies52 has suggested that the histopathology is fairly uniform along the longitudinal axis of the resected specimen. An earlier study, however, highlighted a greater benefit from surgery in patients showing more severe neuronal loss in the anterior than posterior hippocampus.54 Where MRI studies have shown a uniformity of atrophy along the length of the hippocampus55, post mortem (PM) analysis confirms that sclerosis in some patients may extend to the caudal extremities of the hippocampus, beyond typical surgical resections. Thus, incomplete HS resection may contribute to the persistence of seizures following temporal lobectomy in some cases.
Histopathology at other limbic sites has received less attention as possible contributors to the epileptic focus. Cavanagh and Meyer noted ‘diffuse and disseminated lesions’ in the temporal neocortex, uncus, amygdala and parahippocampal gyrus.46 The term ‘mesial temporal lobe sclerosis’ (MTS) was coined in preference to Ammon’s horn sclerosis (AHS) or HS, to acknowledge the involvement of adjacent structures.
The amygdala is thought to play a role in limbic seizures, but complex anatomy and incomplete surgical specimens of this region limit our understanding of the frequency and extent of amygdalar histopathology and how it may influence surgical outcomes.56 Amygdalar histopathology was bilateral in autopsy studies in TLE and always in association with HS.18 It was identified in 88% of HS patients in one surgical series and correlated with the severity of HS.47 Such histopathology has also been reported in TLE in isolation.57 More recent studies have confirmed preferential neuronal loss and gliosis involving the lateral and basal nuclear groups of the amygdala.58 In addition, the entorhinal cortex58,59 and parahippocampal gyrus also show neuronal loss supported by atrophy of this region.60 Pathology of these regions has been variably reported from 68%44 to more recent estimates of 17–21% of HS patients.58,61
In addition to the variable extent and severity of unilateral mesial temporal pathology, HS is frequently a bilateral, albeit usually asymmetrical, finding. Bilateral HS has been noted in up to 56% of epilepsy postmortems62 in a large series and is frequently asymmetrical.18,63 Bilateral mossy fiber sprouting has also been demonstrated at post mortem.64 It is conceivable that the remaining, less damaged hippocampus could contribute to seizure recurrences following surgery.43,65. Post mortem studies demonstrate atrophy outside the mesial temporal structures in association with HS18, presumably involving hippocampal projection pathways. Regions involved include the ipsilateral mammillary body, fornix, thalamus, cingulate, frontal and temporal neocortex. Whether any of these areas contribute to continued seizures following surgery is as yet unsupported.66,67
As seen in neuroimaging studies, some MTLE patients have another lesion together with HS, such as a tumor, cortical dysplasia or a cavernoma. These dual pathologies have raised the question of whether HS is a ‘secondary’ process induced by the lesion.45 However, other studies have found no evidence for secondary hippocampal neuronal loss from seizures generated by extralimbic abnormalities.68 In addition there are a number of studies that suggest that the majority of the neuronal loss likely precedes the onset of seizures.69,70 Mild dysplasias or cortical malformations in the temporal lobe associated with HS have been observed, but the role of these abnormalities (epiphenomenon or active participant) is uncertain. Well defined criteria for these minor cytoarchitectural abnormalities are required before any definite conclusions can be drawn regarding the relative role of the two separate pathologies in seizure initiation.
In summary, there is significant variability in the histopathology associated with MTLE, with accumulating evidence that “atypical” and possibly widespread pathology is associated with poorer outcomes. However, it is unclear what the role these more widely distributed changes have in seizure initiation. They could be responsible for a more distributed seizure onset zone, a second focus, a focus capable of becoming independently epileptogenic in time, or simply an association with the “real” focus that resides elsewhere.
Insights Into the Seizure Focus of MTLE From Animal Models
The data from surgical outcomes and clinico-histopathological correlations suggest that the responsible changes are not uniform across individuals. Data from animal models of MTLE may provide insight into the clinical significance of the variability. Although the models do not exactly replicate the human condition, they have enough similarities to allow appropriate questions that may help understand the human situation.
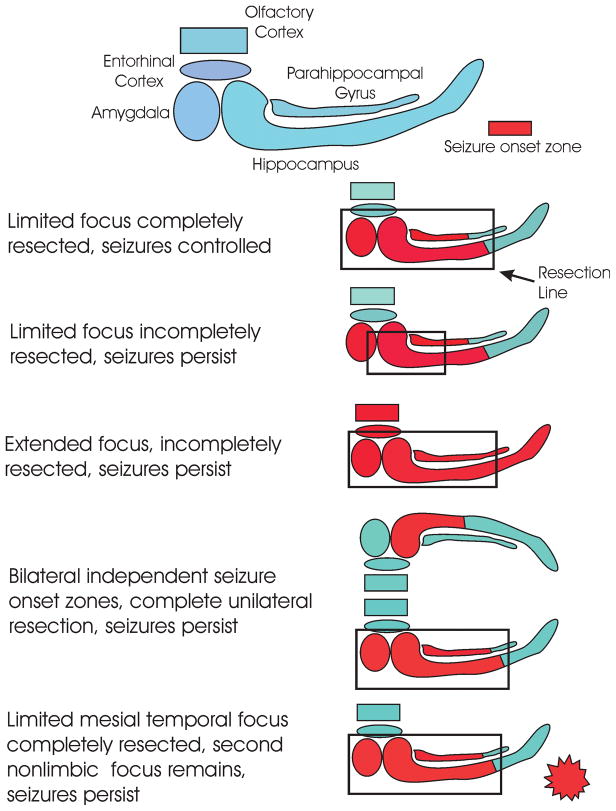
In the post status epilepticus spontaneous recurrent seizure MTLE models, the regions of damage include the amygdala, hippocampus, entorhinal cortex, olfactory cortex as well as a number of subcortical structures.71–75 The severity of the histopathology varies from site to site and animal to animal, ranging from no observable damage to severe neuronal loss and gliosis. Neuronal physiology shows that all of these regions have enhanced excitability76–79, and EEG recordings show that these sites are capable of initiating a seizure.80 The kindling literature shows that it is possible to initiate the same behavioral and electrographic seizure from any of these sites through focal electrical stimulation.81 Hence, the animal data raise the possibility that MTLE seizures may begin multifocally in the broader limbic system. In other words in any one animal, the seizure can sometimes arise out of one region (e.g. the hippocampus), and the next seizure could start in the amygdala. There are potential confounds in drawing parallels between the animal models and patients with temporal lobe epilepsy. Perhaps the most important are the potential effects of medication on the pattern of seizure onset (the animals were not treated with AEDs) and the frequent bilateral onset in the rats as opposed to the more common unilateral onset in people. In spite of these limitations, the animal data raise the possibility of multifocal seizure onset. Translating this hypothetical construct of a multifocal “origin” back to the clinic and the variable success of surgical resections in which some patients are cured, most are controlled and a small number are only partially benefitted, we can theorize that the cured patients have the more restricted histopathology and epileptogenic zone that is completely removed using standard surgical techniques and others have a more extensive limbic histopathology that is incompletely removed (Fig. 4).79 This scenario could also include patients with dual pathologies in which both generate seizures but only one histopathological region is removed.19
Figure 4. How surgery may fail to control seizures.
Figure depicts adjacent regions of the mesial temporal lobe/limbic system that have been implicated by pathology, seizure recordings, outcome data or animal studies as part of an hypothesized seizure onset zone. Red indicates the seizure onset zone that is likely different in each individual, and the black outline is the line of resection. When the onset zone is entirely within the line of resection, the seizures are controlled, potentially cured. When the onset zone is more extensive relative to the resection, there is increased risk for recurrence.
Where do we go from here?
For the majority of individuals, a standard anterior and mesial temporal lobe resection will lead to seizure freedom in MTLE patients where evaluation suggests good lateralization. Accumulating data, however, show that we are not dealing with a uniform situation and that a “one procedure fits all” surgical approach may be inappropriate in treating certain cases of MTLE. At present we lateralize to the left or right mesial temporal structures and perform a more or less standard operation which may not always be sufficient to remove all responsible tissue that produces seizures (Fig. 4).
For the moment we really can’t say what will help us improve long term seizure control for MTLE surgery, in part we are really not sure why we have failures. Perhaps the most important step might be to examine those patients in whom surgery has been less than absolutely successful. Although it is often said that we learn from our mistakes, there has been no organized review of patients who have failed MTLE surgery to determine all the factors that are associated with less than ideal outcomes. Studies to date have given us some issues to examine (e.g. associated pathology, imaging variants, clinical history) but we don’t know how to use that information at present to define the seizure onset zone preoperatively. Another problem is that there are significant variations from center to center in data collection and surgical approaches so that it may be difficult to draw clear conclusions without controlled mult-center trials. However, without a large scale evaluation of the factors that point to poorer results we can’t determine how to improve on current treatment strategies.
Improved outcomes will require a better classification of candidates including improved definition of clinical subtypes and the associated imaging abnormalities within and beyond mesial temporal structures (Table 2). What biomarkers might predict the extent of the seizure onset zone at this point is unclear. We may have to await further improvements in imaging technology or determine which physiological findings can define the location and extent of the epileptogenic focus more accurately. There is already some evidence that hippocampal subfield atrophy can be detected by 4-Tesla MRI scanners,82 but it is not known how well this approach will work prospectively or how practical this approach will be. Can we improve outcomes by using intracranial recording more extensively to define the extent of the seizure onset zone (and not just, as we do at present, use it to determine which temporal lobe to remove), or can we achieve this goal through interictal biomarkers, using imaging or other new technologies? Electrode implantation might yield a better concept of the extent of tissue requiring removal, but invasive monitoring carries risks, and the procedure is limited by the number of sites that can be sampled and centers that are capable of this technology.
Table 2.
Potential Approaches to Improve Outcome forMTLE Surgery
| Define extent of seizure associated histopathology through enhanced imaging |
| Preoperative identification of type of underlying MTS or dual pathology to identify subtypes |
| More exact correlation of type of MTS histopathology and extent of seizure onset zone |
| Identification of interictal biomarkers that differentiate seizure onset zone from surrounding tissue |
| Determine basis for failed surgeries and surgeries that do not achieve a cure |
| More extensive use of intracranial monitoring or other non-invasive technologies to define limits of seizure onset zone |
Historically, we have considered MTLE a single disorder, but it may be time to view it as a group of closely related syndromes with variable type and extent of histopathology. That recognition may lead to identifying the appropriate subgroups that will require different diagnostic and surgical approaches to improve surgical outcomes. As Professor Einstein suggested, we cannot expect that the same surgical approach to MTLE will change the outcomes for our patients.
Acknowledgments
GWM was supported by NIH grants P05 NS02808 and R01 NS38992. EHB is supported in part by NIH grants NS25605 and NS064438.
Footnotes
This review is based on a presentation at the American Epilepsy Society Annual meeting in December 2007.
References
- 1.Chapell R, Reston J, Snyder D. AHRQ Publication No. 03-0028. Vol. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Management of Treatment-Resistant Epilepsy. Evidence Report/Technology Assesment No 77. [Google Scholar]
- 2.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh AM, Kalnins RM, Mitchell LA, Berkovic SF. Early seizures after temporal lobectomy predict subsequent seizure recurrence. Ann Neurol. 2005;57:283–288. doi: 10.1002/ana.20372. [DOI] [PubMed] [Google Scholar]
- 5.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65:912–918. doi: 10.1212/01.wnl.0000176055.45774.71. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh AM, Kalnins RM, Mitchell LA, et al. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 7.Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:751–763. doi: 10.3171/jns.2003.98.4.0751. [DOI] [PubMed] [Google Scholar]
- 8.Elsharkawy AE, Alabbasi AH, Pannek H, et al. Long term outcome of temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009;110:1135–1146. doi: 10.3171/2008.6.JNS17613. [DOI] [PubMed] [Google Scholar]
- 9.Jeong SW, Lee SK, Hong KS, et al. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia. 2005;46:1273–1279. doi: 10.1111/j.1528-1167.2005.33504.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoon HH, Kwon HL, Mattson RH, et al. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology. 2003;61:445–450. doi: 10.1212/01.wnl.0000081226.51886.5b. [DOI] [PubMed] [Google Scholar]
- 11.Janszky J, Janszky I, Schulz R, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain. 2005;128:395–404. doi: 10.1093/brain/awh358. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz TH, Jeha L, Tanner A, et al. Late seizures in patients initially seizure free after epilepsy surgery. Epilepsia. 2006;47:567–573. doi: 10.1111/j.1528-1167.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 13.Siegel AM, Wieser HG, Wichmann W, Yasargil GM. Relationships between MR-imaged total amount of tissue removed, resection scores of specific mediobasal limbic subcompartments and clinical outcome following selective amygdalohippocampectomy. Epilepsy Research. 1990;6:56–65. doi: 10.1016/0920-1211(90)90009-k. [DOI] [PubMed] [Google Scholar]
- 14.Germano IM, Poulin N, Olivier A. Reoperation for recurrent temporal lobe epilepsy. Journal of Neurosurgery. 1994;81:31–6. doi: 10.3171/jns.1994.81.1.0031. [DOI] [PubMed] [Google Scholar]
- 15.Awad IA, Nayel MH, Lüders H. Second operation after the failure of previous resection for epilepsy. Neurosurgery. 1991;28:510–18. doi: 10.1097/00006123-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurg. 1995;37:982–90. doi: 10.1227/00006123-199511000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt D, Baumgartner C, Loscher W. Seizure recurrence after planned discontinuation of antiepileptic drugs in seizure-free patients after epilepsy surgery: a review of current clinical experience. Epilepsia. 2004;45:179–86. doi: 10.1111/j.0013-9580.2004.37803.x. [DOI] [PubMed] [Google Scholar]
- 18.Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 19.Quigg M, Straume M. Dual epileptic foci in a single patient express distinct temporal patterns dependent on limbic versus nonlimbic brain location. Ann Neurol. 2000;48:117–120. doi: 10.1002/1531-8249(200007)48:1<117::aid-ana19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Jackson GD, Duncan JS, Connelly A, et al. Increased signal in the mesial temporal region on T2 Weighted MRI: A quantitative study of hippocampal sclerosis. Neurology. 1991;41 (Suppl 1):170–171. [Google Scholar]
- 21.Jackson GD, Berkovic SF, Tress BM, et al. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869–1875. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- 22.Berkovic SF, Andermann F, Oliver A, et al. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol. 1991;29:175–182. doi: 10.1002/ana.410290210. [DOI] [PubMed] [Google Scholar]
- 23.Heinz ER, Crain BJ, Radtke RA, et al. MR imaging in patients with temporal lobe seizures: correlation of results with pathologic findings. AJNR. 1990;11:827–832. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DH, Gao FQ, Rogers JM, et al. MR in temporal lobe epilepsy: analysis with pathologic confirmation. AJNR. 1998;19:19–27. [PMC free article] [PubMed] [Google Scholar]
- 25.Van Paesschen W, Sisodiya S, Connelly A, et al. Quantitative hippocampal MRI and intractable temporal lobe epilepsy. Neurology. 1995;45:2233–2240. doi: 10.1212/wnl.45.12.2233. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Sharbrough FW, Cascino GD, et al. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992;31:138–146. doi: 10.1002/ana.410310204. [DOI] [PubMed] [Google Scholar]
- 27.Lin JJ, Salamon N, Dutton RA, et al. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1094–1097. doi: 10.1212/01.wnl.0000179003.95838.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson GD, Connelly A, Duncan JS, et al. Detection of hippocampal pathology in intractable partial epilepsy: Increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology. 1993;43:1793–1799. doi: 10.1212/wnl.43.9.1793. [DOI] [PubMed] [Google Scholar]
- 29.Scott RC, Gadian DG, Cross JH, et al. Quantitative magnetic resonance characterisation of mesial temporal sclerosis in childhood. Neurology. 2001;56:1659–1665. doi: 10.1212/wnl.56.12.1659. [DOI] [PubMed] [Google Scholar]
- 30.Van Paesschen W, Connelly A, Johnson CL, et al. The amygdala and intractable tempopral lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology. 1996;47:1021–1031. doi: 10.1212/wnl.47.4.1021. [DOI] [PubMed] [Google Scholar]
- 31.Cross JH, Connelly A, Jackson GD, et al. Proton magnetic resonance spectroscopy in children with temporal lobe epilepsy. Ann Neurol. 1996;39:107–113. doi: 10.1002/ana.410390116. [DOI] [PubMed] [Google Scholar]
- 32.Cendes F, Andermann F, Preul MC, et al. Lateralisation of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol. 1994;35:211–216. doi: 10.1002/ana.410350213. [DOI] [PubMed] [Google Scholar]
- 33.Kuzniecky R, Hugg J, Hetherington H, et al. Predictive value of 1H MRSI for outcome in temporal lobectomy. Neurology. 1999;53:694–698. doi: 10.1212/wnl.53.4.694. [DOI] [PubMed] [Google Scholar]
- 34.Li LM, Cendes F, Antel SB, et al. Prognostic value of proton magnetic resonance spectroscopic imaging for surgical outcome in patients with intractable temporal lobe epilepsy and bilateral hippocampal atrophy. Ann Neurol. 2003;47:195–200. [PubMed] [Google Scholar]
- 35.Diehl B, LaPresto E, Najm I, et al. Neocortical temporal FDG-PET hypometabolism correlates with temporal lobe atrophy in hippocampal sclerosis associated with microscopic cortical dysplasia. Epilepsia. 2003;44:559–564. doi: 10.1046/j.1528-1157.2003.36202.x. [DOI] [PubMed] [Google Scholar]
- 36.Vinton AB, Carne R, Hicks RJ, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain. 2007;130:548–560. doi: 10.1093/brain/awl232. [DOI] [PubMed] [Google Scholar]
- 37.Ho S, Newton MR, McIntosh AM, et al. Perfusion patterns during temporal lobe seizures: relationship to surgical outcome. Brain. 1997;120:1921–1928. doi: 10.1093/brain/120.11.1921. [DOI] [PubMed] [Google Scholar]
- 38.Kazemi NJ, Worrell GA, Stead SM, et al. Ictal SPECT statistical parametric mapping in temporal lobe epilepsy surgery. Neurology. 74:70–6. doi: 10.1212/WNL.0b013e3181c7da20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho SS, Consalvo D, Gilliam F, et al. Amygdala atrophy and seizure outcome after temporal lobe epilepsy surgery. Neurology. 1998;51:1502–1504. doi: 10.1212/wnl.51.5.1502. [DOI] [PubMed] [Google Scholar]
- 40.Raymond AA, Fish DR, Stevens JM, et al. Association of hippocampal sclerosis with cortical dysgenesis in patients with epilepsy. Neurology. 1994;44:1841–1845. doi: 10.1212/wnl.44.10.1841. [DOI] [PubMed] [Google Scholar]
- 41.Sisodiya SM, Moran N, Free SL, et al. Correlation of widespread preoperative magnetic resonance imaging changes with unsuccessful surgery for hippocampal sclerosis. Ann Neurol. 1997;41:490–496. doi: 10.1002/ana.410410412. [DOI] [PubMed] [Google Scholar]
- 42.Bernasconi N, Duchesne S, Janke A, et al. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. NeuroImage. 2004;23:717–723. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Keller SS, Cresswell P, Denby C, et al. Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res. 2007;74:131–139. doi: 10.1016/j.eplepsyres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Cormack F, Gadian DG, Vargha-Khadem F, et al. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. NeuroImage. 2005;27:635–643. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Blumcke I. Neuropathology of focal epilepsies: a critical review. Epilepsy Behav. 2009;15:34–9. doi: 10.1016/j.yebeh.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 46.Cavanagh JB, Meyer A. Aetiological aspects of Ammon’s horn sclerosis associated with temporal lobe epilepsy. Br Med J. 1956;2:1403–7. doi: 10.1136/bmj.2.5006.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruton CJ University of London. Institute of Psychiatry. The neuropathology of temporal lobe epilepsy. Oxford: Oxford University Press; 1988. [Google Scholar]
- 48.Meldrum BS. First Alfred Meyer Memorial Lecture. Epileptic brain damage: a consequence and a cause of seizures. Neuropathol Appl Neurobiol. 1997;23:185–201. discussion 201–2. [PubMed] [Google Scholar]
- 49.Blumcke I, Pauli E, Clusmann H, et al. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol. 2007;113:235–244. doi: 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987;22:334–340. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- 51.Van Paesschen W, Revesz T, Duncan JS, et al. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Ann Neurol. 1997;42:756–766. doi: 10.1002/ana.410420512. [DOI] [PubMed] [Google Scholar]
- 52.Thom M, Sisodiya SM, Beckett A, et al. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002;61:510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- 53.de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, et al. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–87. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- 54.Babb TL, Brown WJ, Pretorius J, et al. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25:729–740. doi: 10.1111/j.1528-1157.1984.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 55.Quigg M, Bertram EH, Jackson T. Longitudinal distribution of hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsy Res. 1997;27:101–110. doi: 10.1016/s0920-1211(97)01026-7. [DOI] [PubMed] [Google Scholar]
- 56.Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 57.Hudson LP, Munoz DG, Miller L, et al. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33:622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- 58.Yilmazer-Hanke DM, Wolf HK, Schramm J, et al. Subregional pathology of the amygdala complex and entorhinal region in surgical specimens from patients with pharmacoresistant temporal lobe epilepsy. J Neuropathol Exp Neurol. 2000;59:907–20. doi: 10.1093/jnen/59.10.907. [DOI] [PubMed] [Google Scholar]
- 59.Du F, Whetsell WO, Jr, Abou-Khalil B, et al. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- 60.Bernasconi N, Bernasconi A, Caramanos Z, et al. Entorhinal cortex atrophy in epilepsy patients exhibiting normal hippocampal volumes. Neurology. 2001;56:1335–1339. doi: 10.1212/wnl.56.10.1335. [DOI] [PubMed] [Google Scholar]
- 61.Dawodu S, Thom M. Quantitative neuropathology of the entorhinal cortex region in patients with hippocampal sclerosis and temporal lobe epilepsy. Epilepsia. 2005;46:23–30. doi: 10.1111/j.0013-9580.2005.21804.x. [DOI] [PubMed] [Google Scholar]
- 62.Meencke HJ, Veith G, Lund S. Bilateral hippocampal sclerosis and secondary epileptogenesis. Epilepsy Res Suppl. 1996;12:335–42. [PubMed] [Google Scholar]
- 63.Quigg MS, Bertram EH, Jackson T, Laws ER. Evidence for bilateral atrophy in unilateral mesial temporal lobe epilepsy. Epilepsia. 1997;38:588–595. doi: 10.1111/j.1528-1157.1997.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 64.Thom M, Martinian L, Catarino C, et al. Bilateral reorganization of the dentate gyrus in hippocampal sclerosis. A postmortem study. Neurology. 2009;73:1033–1040. doi: 10.1212/WNL.0b013e3181b99a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Araujo D, Santos AC, Velasco TR, et al. Volumetric evidence of bilateral damage in unilateral mesial temporal lobe epilepsy. Epilepsia. 2006;47:1354–1359. doi: 10.1111/j.1528-1167.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- 66.Urbach H, Siebenhaar G, Koenig R, et al. Limbic system abnormalities associated with Ammon’s horn sclerosis do not alter seizure outcome after amygdalohippocampectomy. Epilepsia. 2005;46:549–555. doi: 10.1111/j.0013-9580.2005.29104.x. [DOI] [PubMed] [Google Scholar]
- 67.Thom M, Eriksson S, Martinian L, et al. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J Neuropathol Exp Neurol. 2009;68:928–38. doi: 10.1097/NEN.0b013e3181b05d67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JH, Guimaraes PO, Shen MY, et al. Hippocampal neuronal density in temporal lobe epilepsy with and without gliomas. Acta Neuropathologica. 1990;80:41–45. doi: 10.1007/BF00294220. [DOI] [PubMed] [Google Scholar]
- 69.Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–51. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- 70.Mathern GW, Bertram EH, Babb TL, et al. In contrast to kindled seizures, the frequency of spontaneous epilepsy in the limbic status model correlates with greater fascia dentata excitatory and inhibitory axon sprouting and increased staining for NMDA, AMPA and GABA-A receptors. Neuroscience. 1997;77:1003–1019. doi: 10.1016/s0306-4522(96)00516-7. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5:515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- 72.Cavalheiro EA, Leite JP, Bortolotto ZA, et al. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 73.Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu DG. Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Research. 2002;51:93–107. doi: 10.1016/s0920-1211(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 74.Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–13. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitkanen A, Tuunanen J, Kalviainen R, et al. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–53. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 76.Benini R, Avoli M. Altered inhibition in lateral amygdala networks in a rat model of temporal lobe epilepsy. Journal of Neurophysiology. 2006;95:2143–54. doi: 10.1152/jn.01217.2005. [DOI] [PubMed] [Google Scholar]
- 77.Bragin A, Engel J, Jr, Wilson CL, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. Epilepsia. 1999;40:127–37. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 78.Bear J, Fountain NB, Lothman EW. Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. Jour Neurophysiol. 1996;76:2928–40. doi: 10.1152/jn.1996.76.5.2928. [DOI] [PubMed] [Google Scholar]
- 79.Bertram EH, Zhang DX, Mangan P, et al. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Research. 1998;32:194–205. doi: 10.1016/s0920-1211(98)00051-5. [DOI] [PubMed] [Google Scholar]
- 80.Bertram EH. The functional anatomy of spontaneous seizures in a rat model of chronic limbic epilepsy. Epilepsia. 1997;38:95–105. doi: 10.1111/j.1528-1157.1997.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 81.Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 82.Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-resolution MRI at 4 Tesla: preliminary results. Epilepsia. 2009;50:1474–83. doi: 10.1111/j.1528-1167.2009.02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]