Introduction
It has been suggested that ~10% of pancreatic cancer has a familial basis 1, 2. Individuals with a family history of pancreatic cancer have an increased risk of developing both pancreatic and extrapancreatic malignancies, and an individual’s risk of developing pancreatic cancer can now be quantified based on their family cancer history 1, 3, 4.
While some of the aggregation of pancreatic cancer in families is due to chance, and some to shared environmental exposures such as cigarette smoking, it is now clear that much of this aggregation has a genetic basis 5. Several of the genes responsible for the familial clustering of pancreatic cancer have been discovered. For example, germline mutations in the BRCA2 gene cause familial breast cancer, and individuals with germline BRCA2 gene mutations have an approximately 3.5-fold increased risk of pancreatic cancer 6–12. Germline mutations in the p16/CDKN2A gene cause the Familial Atypical Multiple Mole Melanoma (FAMMM) syndrome, and these individuals have a 13 to 37-fold increased risk of pancreatic cancer 10, 13–23. Inherited mutations in the STK11 gene cause the Peutz-Jeghers syndrome, and individuals with Peutz-Jeghers have a 130-fold increased risk of pancreatic cancer 24–30. The discovery of these familial pancreatic cancer genes has helped identify cellular pathways important for the development of pancreatic cancer, it has provided a basis for genetic counseling of individuals with a family history of pancreatic cancer, and it has established a foundation for prioritizing patients for screening for early pre-invasive disease 21, 29, 31–33. In addition, the discovery of familial pancreatic cancer genes has also lead to the development of gene-specific therapies as demonstrated by the remarkable sensitivity of pancreatic cancers harboring mutations in the BRCA2 gene to Poly[ADP-ribose] polymerase (PARP) inhibitors and to mitomycin C 34–41.
The field of familial pancreatic cancer is getting even more exciting as we enter the era of whole genome sequencing. For example, this year the PALB2 gene was discovered to be a familial pancreatic cancer susceptibility gene through complete, unbiased, sequencing of all of the protein-coding genes in a single patient’s cancer 42, 43. As the speed of “next generation” sequencing technologies rises and the costs fall, we can foresee the discovery of a number of new familial pancreatic cancer genes in the coming years.
The known genetic syndromes account for less than 20% of the observed familial aggregation of pancreatic cancer, and the discovery of additional familial pancreatic cancer genes remains one of the most exciting opportunities in pancreatic cancer research 1, 2. As these genes are discovered, the challenge will be to use these scientific breakthroughs to improve clinical care.
Using Family History to Assess Cancer Risk
As the recognition that pancreatic cancer aggregates in families grows, more and more surgeons are being asked by their patients: “I have a family history of pancreatic cancer, what is my risk of developing cancer?” A body of evidence-based medicine has been developed to answer this question, and it is clear that individuals with a family history of pancreatic cancer have an increased risk of both pancreatic and of extrapancreatic malignancies 4, 44, 45.
Family History and Pancreatic Cancer
Several large epidemiological studies have established that a family history of pancreatic cancer increases one’s risk of developing the disease 4, 44, 46–52. For example, L. Amundadottir and colleagues correlated the risk of a variety of cancers with family cancer history by linking the Iceland Cancer Registry with the deCODE genealogic database 44. A first-degree relationship is a parent-child or sibling-sibling relationship, and Amundadottir and colleagues found that Icelanders with a first-degree family relative with pancreatic cancer had a 2.33-fold increased risk of developing pancreatic cancer themselves 44. Similar observations have been made in a large number of case-control and cohort studies, and it is now clear that having a single close relative with pancreatic cancer doubles one’s risk of developing the disease 4, 44, 46–52.
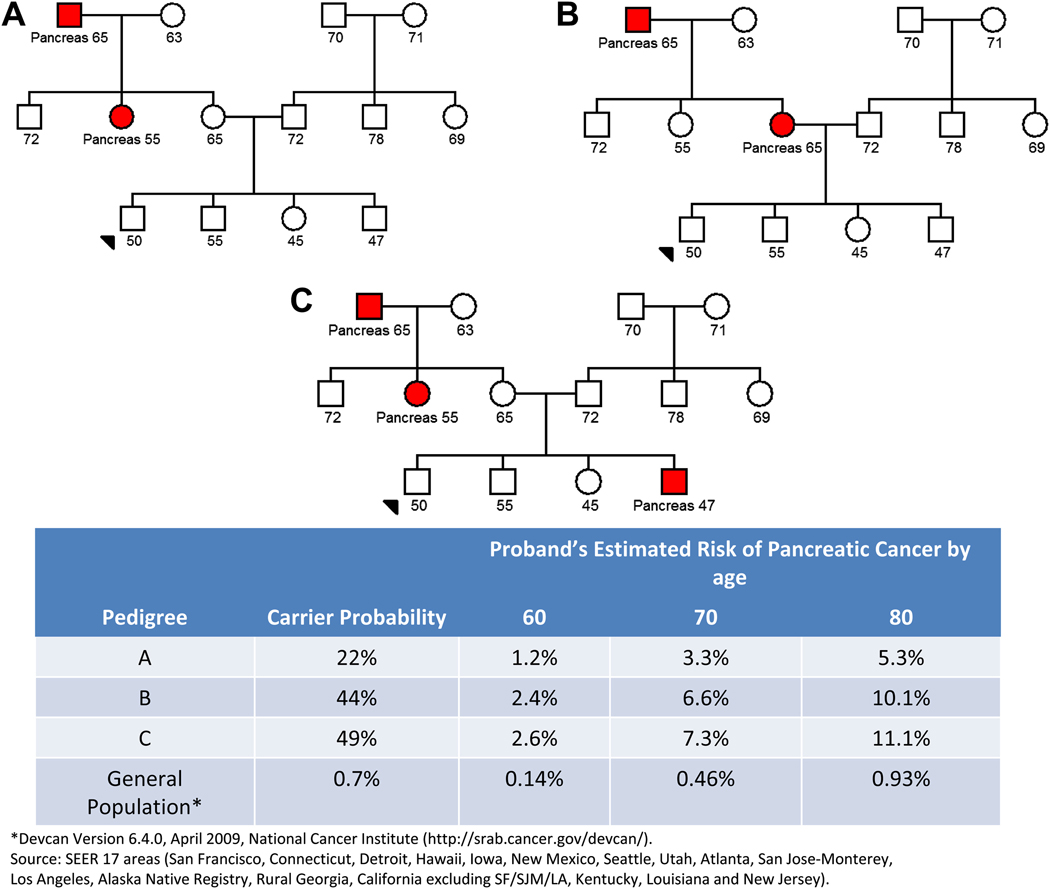
A. Klein and colleagues have extended these analyses by prospectively following thousands of patients with a family history of pancreatic cancer 4. Klein and colleagues found that individuals with two first-degree relatives with pancreatic cancer have a 6-fold increased risk of developing pancreatic cancer, and individuals with three or more first-degree relatives with pancreatic cancer have a 14 to 32-fold increased risk 4. Using these and other data, Klein and colleagues have developed a risk prediction tool, called PancPRO, that can be used to quantify an individual’s risk of developing pancreatic cancer based on their family history of pancreatic cancer 3. PancPRO is available for free on-line (http://astor.som.jhmi.edu/BayesMendel/pancpro.html accessed September 1, 2009) and PancPRO can be used to answer the question posed earlier by our hypothetical patient: “I have a family history of pancreatic cancer, what is my risk of developing cancer?” Figure 1 illustrates three pedigrees of similar structure but differing family histories of pancreatic cancer. The counselee shown with an arrow in Figure 1A is predicted to have a 3.3 % chance of developing pancreatic cancer by the age of 70 years. This risk increases to ~7% if this patients mother rather than their maternal aunt had history pancreatic cancer (Figure 1B) or if their brother also has a history of pancreatic cancer (Figure 1C)3. Without knowing the gene responsible for the aggregation of pancreatic cancer in a family, clinicians can still provide their patients with quantitative estimates of their absolute lifetime pancreatic cancer risks.
Figure 1. Three Pedigrees with Different Risks of Pancreatic Cancer.
The counselee shown with an arrow in Figure 1A is predicted to have a 3.3 % chance of developing pancreatic cancer by the age of 70 years. This risk increases to ~7% if this patients mother rather than their maternal aunt had history pancreatic cancer (Figure 1B) or if their brother also has a history of pancreatic cancer (Figure 1C).
It is also important to put this risk in perspective, as the average patient may be unduly alarmed by relative risks. Pancreatic cancer is a relatively rare disease, averaging 9 per 100,000 per year in the United States 53. A relative risk of two increases an individual’s risk to ~18 per 100,000 per year, less than a fiftieth of one percent per year. This incidence rate increases with age, particularly above the age of 50 culminating in a lifetime risk of developing pancreatic cancer of 1 percent. Contrast this to the risk of a women developing breast cancer. The National Cancer Institute estimates that 12.7 percent of women born in the United States today will develop breast cancer at some time in their lives. Even having a two-fold increased risk of developing pancreatic cancer, the vast majority of patients with a family history of pancreatic cancer will not develop the disease themselves. For this reason it is important to explain both relative and absolute risks to patients.
Family History and Extrapancreatic Malignancies
The risk of cancer is not confined to one organ in most familial cancer syndromes 45, 54, 55. For example, patients with hereditary nonpolyposis colorectal cancer syndrome have an increased risk of developing cancer of the colorectum, endometrium, ovary, stomach, ureter, renal pelvis, and pancreas 56. Epidemiologic studies have recently shown that the same is true for familial pancreatic cancer; patients with a family history of pancreatic cancer have an increased risk of extrapancreatic malignancies as well 45, 54, 55. Wang and colleagues followed families enrolled in the National Familial Pancreas Tumor Registry (http://pathology.jhu.edu/pancreas/nfptr) and found that overall cancer mortality is increased both in the members of sporadic pancreatic cancer kindreds (defined as at least a single pancreatic cancer in the kindred, but not an affected pair of first-degree relatives, Relative Risk [RR] =1.55: 95%, CI 1.39–1.73) and in familial pancreatic cancer kindreds (defined as a family with at least a pair of first-degree relatives with pancreatic cancer, RR=1.41; 95%CI 1.26–1.58) 45. Relatives of patients with familial pancreatic cancer had an increased risk of dying from breast cancer (RR 1.66, 95% CI=1.15–2.34), ovarian (RR 2.05, 95% CI= 1.10–3.49), and bile duct cancers (RR 2.89, RR= 1.04–6.39) 45.
In addition to the associations identified without knowledge of the gene involved, as noted earlier, several known genetic syndromes increase the risk of both pancreatic and extrapancreatic malignancies (Table 1). For example, individuals with the Peutz-Jeghers syndrome have an increased risk of developing cancers of esophagus, stomach, small intestine, colon, pancreas, breast, lung, ovary and uterus 27, 28. Similarly, a personal history of young-onset breast cancer with or without a family history of pancreatic cancer (particularly in patients with Ashkenazi Jewish ancestry) could suggest the presence of a familial breast-ovarian cancer syndrome involving one of the breast cancer-related genes (BRCA1 or BRCA2). These genes, particularly BRCA2, are associated with a very high risk of breast, ovarian, and prostate cancer and moderate risk for pancreatic cancer 6–12. Surgeons should be aware that their patients with a strong family history of pancreatic cancer are at higher risk for developing extra-pancreatic malignancies.
TABLE 1.
Syndromes Associated with Pancreatic Cancer
| Genetic Syndrome | Gene(s) | Increased Risk of Pancreatic Cancer |
Risk of Pancreatic Cancer by age 70 years |
Other Malignancies |
|---|---|---|---|---|
| No family history | None | RR=1 | 0.5% | None |
| Two First-degree Relatives with Pancreatic Cancer |
Unknown in most cases |
6-fold | 3% | Breast, ovarian and bile duct |
| Hereditary Breast and Ovarian Cancer |
BRCA2, FANC- C, FANC-G, PALB2 |
3.5 to 10-fold | 2–5% | Breast, ovarian |
| Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM) |
p16/CDKN2A | 9 to 47-fold | 5–24% | Melanoma |
| Three or more First-degree Relatives with Pancreatic Cancer |
Unknown in most cases |
14 to 32-fold | 7–16% | Breast, ovarian and bile duct |
| Familial Pancreatitis |
PRSS1, SPINK1 | 50 to 80-fold | 25–40% | None |
| Peutz-Jeghers | STK11 | 132-fold | 60% | Small intestine, lung, esophagus, stomach, , breast, lung, uterus, ovary |
Intraductal Papillary Mucinous Neoplasms and Extrapancreatic Neoplasms
It is well-established that intraductal papillary mucinous neoplasms (IPMNs) are precursors to invasive pancreatic cancer, and that patients with an IPMN have an increased risk of developing invasive pancreatic cancer 53. Patients with a personal history of an IPMN also have an increased risk of developing an extra-pancreatic neoplasm. Excess rates of gastric and colonic epithelial neoplasms have been reported in patients with IPMNs 57–60. This finding suggests the possibility of a common predisposing genetic susceptibility, but no specific hereditary syndrome linking IPMNs with gastric and colonic neoplasms has been established.
It is now clear that individuals with a family history of pancreatic cancer, as well as those with a personal history of an IPMN, have an increased risk of developing pancreatic and selected extra-pancreatic malignancies. These findings have two immediate implications for surgeons. First, a good family cancer history should be obtained from all patients. Don’t just document that a patient has a “family history of cancer,” instead carefully document which family members have had cancer, which types of cancer they have had, and how each affected individual is related to the patient. Second, as will be discussed in detail later in this review, knowledge of these increased risks can help guide clinical management.
Familial Pancreatic Cancer Genes
We have handled the question of risk assessment using family cancer history, but it is clear that in most instances an individual’s family cancer history is, at best, just a surrogate for gene status, and that determining the specific gene responsible for a given patient’s cancer can have significant clinical implications. Our second patient therefore comes to the office and asks “I have a family history of pancreatic cancer, can I undergo genetic testing, and if so, for which genes?”
While we believe that this question is often best answered by a trained cancer genetic counselor (see www.nsgc.org to find a local genetic counselor), it is important for surgeons to know the major genes responsible for the familial aggregation of pancreatic cancer (Table 1).
BRCA2 and Other Fanconi Anemia Pathway Genes
BRCA2 is probably the best characterized of all of the familial pancreatic cancer genes. Germline (inherited) mutations in the BRCA2 gene increase the risk of breast and ovarian cancer, and increase the risk of pancreatic cancer 3.5 to 10-fold 6–12. While breast cancer develops in most families with a BRCA2 gene mutation, the absence of a breast cancer in a family should not be used to exclude germline BRCA2 mutations, as Goggins and colleagues reported that pancreatic cancer can run in BRCA2 gene mutation-carrying families without an apparent association with breast cancer 8. Germline BRCA2 mutations are particularly common in individuals of Ashkenazi Jewish heritage 61–65. It has been calculated that 1% of the Ashkenazi Jewish population carries a germline BRCA2 gene mutation, the 6174delT mutation, and these individuals, in addition to an increased risk of breast and ovarian cancer, have a 10-fold increased risk of developing pancreatic cancer 61–65. Individuals without a Jewish heritage can also carry a germline BRCA2 gene mutation, but these mutations are more widely distributed throughout the gene and overall it is estimated that only 1 in every 400 to 800 individuals carries a mutation in BRCA2 66.
Clinical genetic testing for germline BRCA2 gene mutations is commercially available through Myriad Genetics (http://www.myriad.com/products/bracanalysis.php). Testing should be considered in patients with a strong family history of pancreatic cancer, especially if the patient or other family members have been diagnosed with bilateral or young age of onset breast or ovarian cancer, and if the individual is of Ashkenazi Jewish heritage. A clinical tool is available to help clinicians identify who would best benefit from genetic testing for BRCA2 gene mutations (http://astor.som.jhmi.edu/BayesMendel/brcapro.html), but it should be noted that this model does not include pancreatic or prostate cancers as a risk criterion.
The protein product of the BRCA2 gene functions in the same DNA repair pathway as the Fanconi’s anemia proteins to repair DNA cross-linking damage 67. It should therefore not be surprising that germline mutations in genes coding for other members of the pathway, including FANC-C and FANC-G, have also been linked to the familial clustering of pancreatic cancer 67, 68. Germline BRCA1 gene mutations have been reported in only a few patients with familial pancreatic cancer 69–72.
Most recently, the PALB2 gene has been discovered to be a familial pancreatic cancer gene 42. The PALB2 gene codes for a protein that binds to the Brca2 protein and helps to localize Brca2, and possibly also Brca1, to the nucleus 73. Indeed, “PALB2” stands for “partner and localizer of BRCA2.” Jones and colleagues discovered that PALB2 is a familial pancreatic cancer gene by sequencing all of the genes in a pancreatic cancer from a single patient with familial pancreatic cancer 42. This remarkable achievement highlights the potential of whole genome sequencing to discover the causes of inherited diseases. The PALB2 gene finding has been confirmed, and PALB2 appears to account for 1 to 3% of familial pancreatic cancer 42, 43.
We can take several important lessons from the BRCA2 gene story. First, as noted earlier, many familial cancer genes do not increase the risk of just one cancer type. Germline BRCA2 gene mutations increase the risk of breast, ovarian, prostate and pancreatic cancer 6, 66, 74– 77. Once the gene is found in a family, lives can be saved by screening gene carriers for these extrapancreatic neoplasms and, in selected cases, by prophylactic surgery 6, 66, 74–77. Second, BRCA2 nicely demonstrates that once a gene is found and its function determined, then genes coding for other members of the same pathway can be screened to see if they also contribute to familial pancreatic cancer. Germline mutations in four members of the Fanconi anemia pathway, BRCA2, FANC-C, FANC-G and PALB2, can cause familial pancreatic cancer 42, 43, 67, 68. Finally, as will be discussed in greater detail later, BRCA2 gene mutations are a great example of the potential power of gene-specific therapies 34–41.
P16/CDKN2A and FAMMM
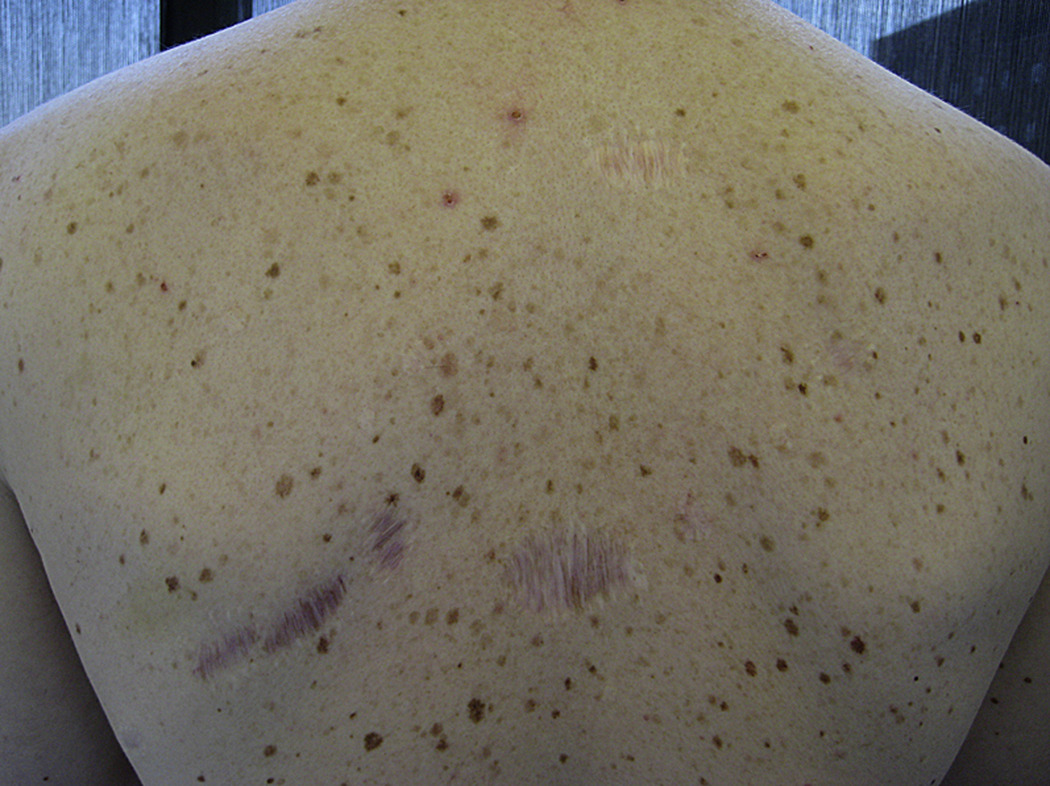
Germline mutations in the p16/CDKN2A gene cause about 30 to 40% of FAMMM syndrome, a syndrome characterized my multiple nevi, multiple atypical nevi, and an increased risk of melanoma (Figure 2). Patients with FAMMM due to p16/CDKN2A gene mutations also have a 9 to 47-fold increased risk of developing pancreatic cancer 10, 13–23, 78. For example, de Snoo and colleagues studied 22 families with the p16-Leiden founder mutation who had attended a surveillance clinic and found that carriers of the mutation have a 47-fold increased risk of developing pancreatic cancer (RR, 46.6; 95% CI, 24.7–76.4) 17. Similarly, H. Lynch and colleagues followed eight families with the FAMMM and pancreatic carcinoma in concert with a germline p16/CDKN2A mutation and reported four incidences of melanoma and pancreatic carcinoma as double primaries in the same individuals 21. The FAMMM syndrome is important to recognize because lives can be saved by screening at-risk individuals for extrapancreatic neoplasms, in this case atypical nevi and early curable melanomas 79.
Figure 2. Familial Atypical Multiple Mole Melanoma Syndrome.
This patient has multiple melanocytic nevi, some of which were atypical. Note the surgical scars. (Kindly provided by Dr. Rhoda M. Alani)
STK11 and the Peutz-Jeghers Syndrome
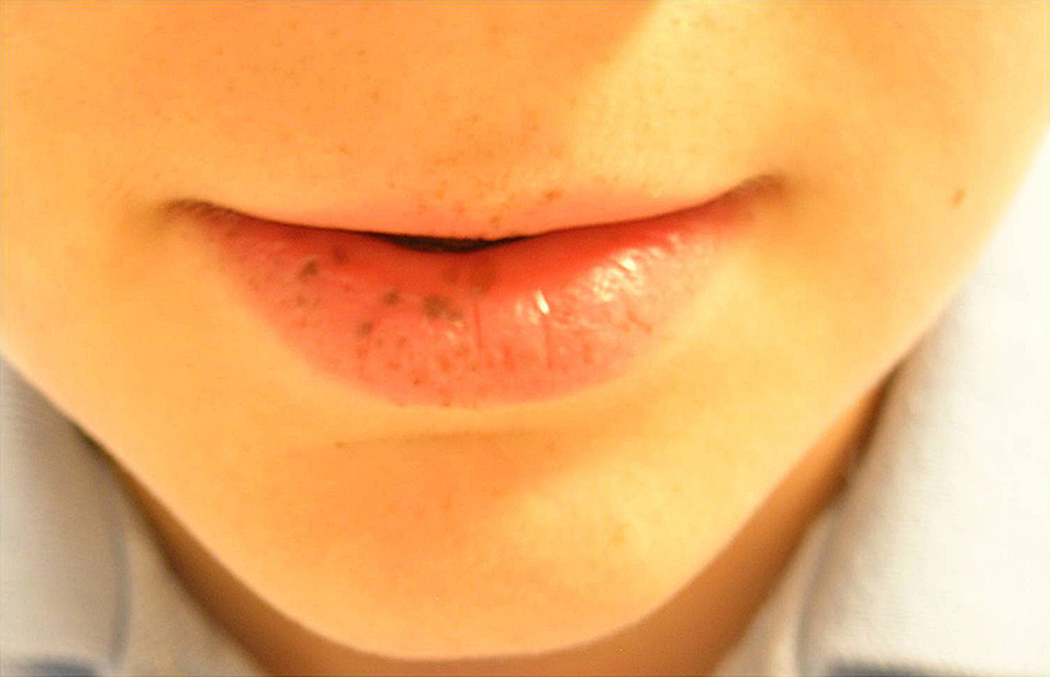
The Peutz-Jeghers syndrome is an autosomal dominant syndrome characterized by melanocytic macules on the lips and buccal mucosa, and hamartomatous polyps of the gastrointestinal tract (Figure 3) 24–30. In addition to gastrointestinal and breast cancer, patients with the Peutz-Jeghers syndrome have a very high risk of developing pancreatic cancer 24–30. Indeed, an obligate carrier of the gene developed pancreatic cancer in the kindred first described by Jeghers and colleagues 80. Hearle and colleagues reported that 80% of STK11 mutation carriers develop cancer by the age of 60 years 28, and Giardiello and colleagues reported that patients with the syndrome have a remarkable 132-fold increased risk of developing pancreatic cancer (CI= 44, 261) 26, 27. The very high risk of pancreatic cancer in patients with the Peutz-Jeghers syndrome highlights the clinical need to develop effective screening tests for early curable pancreatic neoplasia in at-risk patients 24–30.
Figure 3. Peutz-Jeghers Syndrome.
This young patient has multiple freckles on his lips. These may fade with age. (Kindly provided by Dr. Francis Giardiello)
A first step in screening patients with the Peutz-Jeghers syndrome for curable disease will be to characterize the lesions that precede the development of invasive cancer in these patients. Preliminary analyses correlating histopathology with genetics have shown that some patients with Peutz-Jeghers develop intraductal papillary mucinous neoplasms (IPMNs) as the result of their genetic defect 30, 81. Since IPMNs typically grow to several centimeters in size before they invade, these data suggest that some curable precursor lesions should be detectable and treatable in patients with Peutz-Jeghers.
PRSS1, SPINK1 and Familial Pancreatitis
Familial pancreatitis, also known as hereditary pancreatitis, is characterized by recurrent episodes of severe acute pancreatitis starting at a young age 82. Most patients ultimately develop chronic pancreatitis. Germline mutations in the PRSS1 and SPINK1 genes have both been shown to cause familial pancreatitis 83–85. Germline mutations in PRSS1 lead to an autosomal dominant form of inheritance 85. Germline mutations in SPINK1 increase the risk of developing pancreatitis, but the relative risk is small (2–5 fold) and most patients with SPINK1 mutations never develop pancreatitis 86. Patients with familial pancreatitis have as high as a 40% lifetime risk of developing pancreatic cancer 87, 88. As will be discussed in greater detail in the section on therapy, some of these patients elect to have prophylactic pancreatectomy.
Other Genes
Linkage analyses have suggested that chromosome 4q may harbor a pancreatic cancer susceptibility gene, and Pogue-Geile and colleagues have suggested that this gene is palladin (PALLD) 89. Follow-up studies on PALLD, have, however, failed to confirm that it is a significant familial pancreatic cancer gene 90–94.
The familial adenomatous polyposis (FAP) syndrome is characterized by the development of greater than 100 adenomatous polyps of the colon 95. The small bowel can also be affected. It has been suggested that patients with FAP have an increased risk of pancreatic cancer, but some of the apparent increased risk may simple be the result of misclassification of duodenal adenocarcinomas as pancreatic primaries 95.
The hereditary nonpolyposis colorectal cancer syndrome (HNPCC) is characterized by early onset colon cancer, and an increased risk of carcinomas of the endometrium, ovary, bile duct, kidney, bladder, ureter and skin 96. HNPCC is caused by inherited mutations in one of the DNA mismatch repair genes, including hMSH2, hMLH1, hPMS1, hPMS2 and hMSH6/GTBP 1. There have been several case reports of patients with HNPCC developing pancreatic cancer, but the exact contribution of HNPCC to the familial clustering of pancreatic cancer is poorly defined 1, 97.
While genetic testing may be of benefit to many families, the genetic basis of >80% of the clustering of pancreatic cancer in families remains unknown. Many families with an aggregation of pancreatic cancer may harbor mutations in yet to be identified genes and they will not be found to carry mutations in the above mentioned genes. Mutations in the BRCA2 gene account for 6–12% of families with at least two pancreatic cancers, PALB2 1–3% and the remaining genes account for <1% of familial pancreatic cancer.
Therapeutic Implications
So far in this review we have quantified the risk of pancreatic cancer and we have identified some of the genes responsible for familial pancreatic cancer. We are now ready for a third question from our hypothetical patient: “I have a germline mutation in a cancer predisposition gene, can this knowledge be used to guide my treatment?” The answer depends on the patient’s specific gene mutation.
Targeting BRCA2 Gene Mutations
BRCA2-targeted therapies demonstrate that the discovery of a familial pancreatic cancer gene can lead to the development of gene-specific therapies. Pancreatic cancer cell lines harboring biallelic mutations in the BRCA2 gene are exquisitely sensitive to mitomycin C and to PARP (Poly[ADP-ribose] polymerase) inhibitors in the laboratory 34–41. These drugs target the very pathway, the repair of DNA cross-linking damage, that is inactivated in BRCA2 mutant cells 34–41. Normal cells, with a functional copy of the BRCA2 gene, can repair the DNA injury caused by these agents, while cancer cells with biallelic inactivating mutations in the BRCA2 gene do not produce functional Brca2 protein and cannot repair the damage from these agents 34–41. The cancer cells are killed, while the normal cells survive. These results in the laboratory are now being translated to the clinic and there are already several reports of significant clinical responses in patients with BRCA2 mutant cancers 34–41. These findings suggest a scenario in which a patient’s genotype can be used to identify the most effective therapy for that patient.
Prophylactic Surgery in Patients with Familial Pancreatitis
The increased risk of cancer in patients with familial pancreatitis is confined to the pancreas, and many patients with familial pancreatitis have severe exocrine and endocrine pancreatic insufficiency 87, 88. Some of these patients therefore consider prophylactic total pancreatectomy 98–100. While this surgery will eliminate the patient’s very significant risk of developing pancreatic cancer, the benefit of prophylactic surgery has to be weighed against the real risks of total pancreatectomy 101. The main complication of total pancreatectomy is brittle diabetes and although there is now increasing experience in managing diabetes after total pancreatectomy, there is an increased risk of morbidity and mortality associated with this surgery 98, 99, 101–103. Some have considered the option of islet autotransplantation, but the “cell of origin” for pancreatic cancer is not known, and the risk of autotransplanting a potential neoplastic cell remains a theoretical concern 98, 99, 101–103.
As more familial pancreatic cancer genes are discovered, we can envision a future in which genetic testing will be used routinely to both determine an individual’s risk and to guide therapy should they develop disease.
Screening for Early Neoplasia
Our hypothetical patient, recognizing that an ounce of prevention is worth a pound of cure, next asks: “I have seen several of my family members die of pancreatic cancer, and I do not want to suffer the same fate. Are any screening tests available?” The short answer to this question is that, unfortunately there are no clinically proven effective screening tests available for the early detection of pancreatic cancer at this time. Serum CA19-9 levels have been suggested as a possible test, but the assay lacks the sensitivity and specificity needed to screen for pancreatic cancer 104. There are, however, a number of screening tests being evaluated in clinical trials, and several approaches hold promise.
Recently M. Canto and colleagues studied endoscopic ultrasound (EUS) as a screening test for asymptomatic members of at-risk families 31, 105. In this trial, called “Cancer of the Pancreas Screening (CAPS),” Canto and colleagues screened asymptomatic patients with a strong family history of pancreatic cancer, as well as asymptomatic patients with the Peutz-Jeghers syndrome 31, 105. Close to 10% of the asymptomatic individuals screened were found to have a lesion in their pancreas that resulted in surgery 31, 105. Most of these lesions were IPMNs, and one-fourth of the precursors discovered on screening had significant dysplasia (carcinoma in situ), demonstrating that curable precancerous lesions can be detected and treated in asymptomatic at-risk individuals 31, 105–107. Other groups using either EUS-based or abdominal magnetic resonance imaging (MRI) to screen individuals with multiple affected family members or germline mutation carriers have also detected and treated IPMNs, pancreatic intraepithelial neoplasia (PanIN), and invasive pancreatic ductal adenocarcinomas 108, 109. One group in The Netherlands recently reported a low diagnostic yield for screening for pancreatic neoplasia, but the study included subjects in a lower risk population with only two affected relatives 110. Clearly, identifying the correct group to screen is a critical first step in developing an effective screening test.
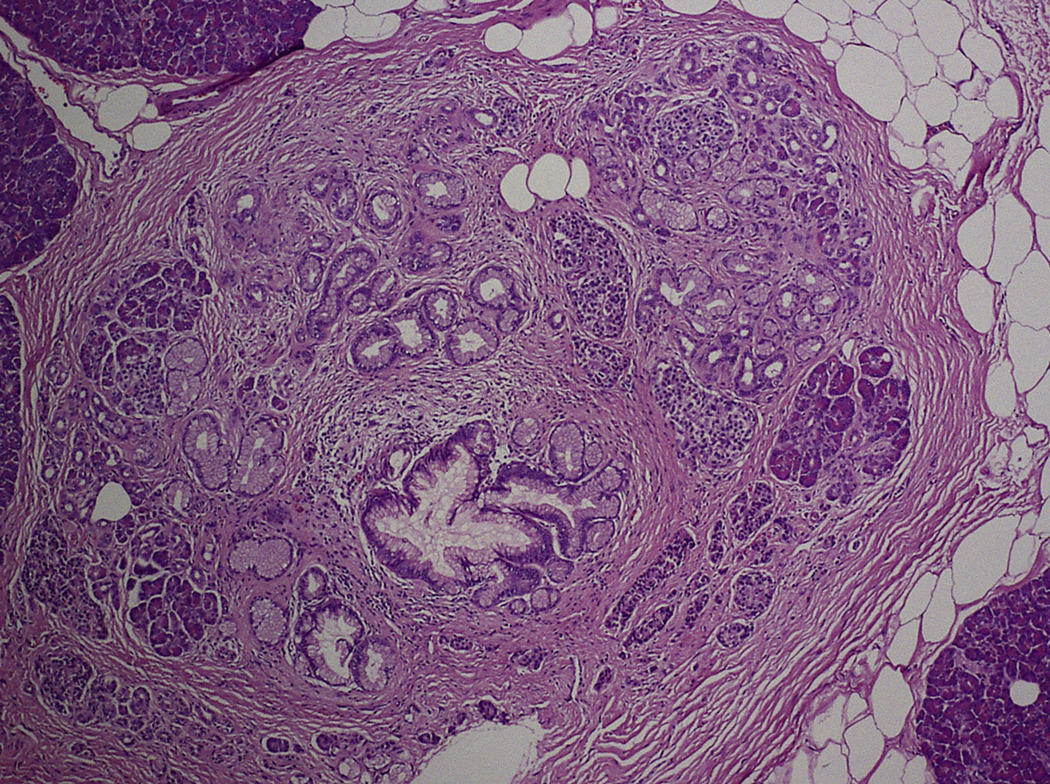
The screening and surgical resection of early curable neoplasms in at-risk individuals in the CAPS and other similar trials has also provided a unique opportunity for pathologists to study the morphology of unadulterated precursor lesions in individuals with a strong family history of pancreatic cancer 107, 111. Three observations can be drawn from these morphological studies. First, PanINs are often associated with lobulocentric atrophy (Figure 4) 111. Although PanIN lesions are small, most are associated with larger areas of lobulocentric atrophy and fibrosis 111. Second, PanINs in patients with a strong family history of pancreatic cancer are often multifocal 111. As many as 20% of the smaller ducts in some patients contain PanIN lesions 111. Third, the combination of lobulocentric atrophy and multifocality of PanIN often produces grossly appreciable changes in the pancreas, and these changes can be detected by EUS 111–113. While single PanIN lesions are almost always too small to be appreciated grossly, larger PanINs (2–5 mm) can be seen by EUS as anechoic nonseptated lesions, often indistinguishable from saccular dilatations of branch ducts along the main duct or small branch duct IPMNs. Multifocal PanINs together with their multiple foci of associated lobulocentric atrophy produce a mosaic of fibrosis, atrophy and uninvolved parenchyma, changes very similar to chronic pancreatitis 111. These changes are often detectable by EUS using standard criteria for the diagnosis of chronic pancreatitis, such as heterogeneous parenchyma, multifocal lobularity and dilated main and branch pancreatic ducts 111, 113.
Figure 4. Lobulocentric Atrophy Associated with Pancreatic Intraepithelial Neoplasia lesion.
The lobule of pancreatic parenchyma surrounding this small PanIN lesion is remarkable for fibrosis and acinar drop-out.
Thus, although there are no clinically proven effective methods to screen at-risk individuals for early pancreatic neoplasia, several EUS-based studies have established that it is possible, in principle, to detect curable pancreatic neoplasms in asymptomatic at-risk patients. As the resolution of imaging improves and as our knowledge of precursor lesions grows, we believe that multifocal PanIN lesions will be detectable in clinical practice.
Future
The coming year will see an explosion in our understanding of familial pancreatic cancer. Next generation sequencing will allow researchers to sequence candidate familial pancreatic cancer genes on a scale unimaginable just a few years ago. In fact, investigators at Johns Hopkins University are planning to sequence the entire coding genomes of a series of patients with familial pancreatic cancer. The resultant flood of information will offer unparalleled opportunities to improve patient care. Investigators at Johns Hopkins have also formed an international screening and surveillance consortium involving 25 countries from North America, Europe, Australia, and Asia. It is hoped that this consortium will define the best methods to assess pancreatic cancer risk, increase our understanding of the natural history of apparently benign precancerous neoplasms, and define the survival benefit, if any, of treating premalignant neoplasms in high-risk individuals.
Surgeons will be at the forefront translating these advances to patient care. Surgical management will not be simply operating to resect a well-defined, but incurable carcinoma. Instead, an integration of clinical history, family cancer history, gene status, imaging and surgical skill will be needed to identify and treat early curable pancreatic neoplasia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009 March;133(3):365–374. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8(2):109–117. doi: 10.1007/s10689-008-9214-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. PancPRO: risk assessment in individuals with a family history of pancreatic cancer. J Clin Oncol. 2007;25(11):1417–1422. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64(7):2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 5.Klein AP, Beaty TH, Bailey-Wilson JE, Brune KA, Hruban RH, Petersen GM. Evidence for a major gene influencing risk of pancreatic cancer. Genetic Epidemiology. 2002 August 23;23(2):133–149. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- 6.Cancer risks in BRCA2 mutation carriers.The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999 August 4;91(15):1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 7.Couch FJ, Johnson MR, Rabe KG, et al. The Prevalence of BRCA2 Mutations in Familial Pancreatic Cancer. Cancer Epidemiol Biomarkers Prev. 2007 February;16(2):342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 8.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 9.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003 February 5;95(3):214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 10.Lal G, Liu G, Schmocker B, et al. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Res. 2000 January 15;60:409–416. [PubMed] [Google Scholar]
- 11.Murphy KM, Brune KA, Griffin CA, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002 July 1;62(13):3789–3793. [PubMed] [Google Scholar]
- 12.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005 September;42(9):711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruis NA, Sandkuiji LA, van der Velden PA, Bergman W, Franto RR. CDKN2 explains part of the clinical phenotype in Dutch familial atypical multiple-mole melanoma (FAMMM) syndrome families. Melanoma Res. 1995;5:169–177. doi: 10.1097/00008390-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lynch HT, Fusaro RM. Pancreatic cancer and the familial atypical multiple mole melanoma (FAMMM) syndrome. Pancreas. 1991;6:127–131. doi: 10.1097/00006676-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch DK, Sina-Frey M, Lang S, et al. CDKN2A germline mutations in familial pancreatic cancer. Ann Surg. 2002 December;236(6):730–737. doi: 10.1097/00000658-200212000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borg A, Sandberg T, Nilsson K, et al. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst. 2000 August 2;92(15):1260–1266. doi: 10.1093/jnci/92.15.1260. [DOI] [PubMed] [Google Scholar]
- 17.De Snoo FA, Bishop DT, Bergman W, et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin Cancer Res. 2008 November 1;14(21):7151–7157. doi: 10.1158/1078-0432.CCR-08-0403. [DOI] [PubMed] [Google Scholar]
- 18.de vos tot Nederveen Cappel WH, Offerhaus GJ, van Puijenbroek M, et al. Pancreatic carcinoma in carriers of a specific 19 base pair deletion of CDKN2A/p16 (p16-leiden) Clin Cancer Res. 2003 September 1;9(10 Pt 1):3598–3605. [PubMed] [Google Scholar]
- 19.Goldstein AM, Fraser MC, Struewing JP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333(15):970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 20.Lynch HT, Brand RE, Lynch JF, et al. Genetic counseling and testing for germline p16 mutations in two pancreatic cancer-prone families. Gastroenterology. 2000 December;119(6):1756–1760. doi: 10.1053/gast.2000.20335. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HT, Brand RE, Hogg D, et al. Phenotypic variation in eight extended CDKN2A germline mutation familial atypical multiple mole melanoma-pancreatic carcinoma-prone families: the familial atypical mole melanoma-pancreatic carcinoma syndrome. Cancer. 2002 January 1;94(1):84–96. doi: 10.1002/cncr.10159. [DOI] [PubMed] [Google Scholar]
- 22.Moskaluk CA, Hruban RH, Lietman AS, et al. Novel germline p16(INK4) allele (Asp145Cys) in a family with multiple pancreatic carcinomas. Hum Mutat. 1998;12(1):70–73. doi: 10.1002/(SICI)1098-1004(1998)12:1<70::AID-HUMU13>3.0.CO;2-G. Mutations in brief no. 148. Online. [DOI] [PubMed] [Google Scholar]
- 23.Vasen HF, Gruis NA, Frants RR, van der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000 September 15;87(6):809–811. [PubMed] [Google Scholar]
- 24.Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers Syndrome. Ann Intern Med. 1998;128:896–899. doi: 10.7326/0003-4819-128-11-199806010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bowlby LS. Pancreatic adenocarcinoma in an adolescent male with Peutz-Jeghers syndrome. Hum Pathol. 1986;17:97–99. doi: 10.1016/s0046-8177(86)80163-0. [DOI] [PubMed] [Google Scholar]
- 26.Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316(24):1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 27.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers Syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 28.Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006 May 15;12(10):3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 29.Latchford A, Greenhalf W, Vitone LJ, Neoptolemos JP, Lancaster GA, Phillips RK. Peutz-Jeghers syndrome and screening for pancreatic cancer. Br J Surg. 2006 December;93(12):1446–1455. doi: 10.1002/bjs.5609. [DOI] [PubMed] [Google Scholar]
- 30.Su GH, Hruban RH, Bova GS, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154(6):1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4(6):766–781. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Axilbund JE, Brune KA, Canto MI, Brehon BC, Wroblewski LD, Griffin CA. Patient perspective on the values of genetic counseling for familial pancreatic cancer. Hereditary Cancer in Clinical Practice. 2005;3(3):115–122. doi: 10.1186/1897-4287-3-3-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kluijt I, Cats A, Fockens P, Nio Y, Gouma DJ, Bruno MJ. Atypical Familial Presentation of FAMMM Syndrome With a High Incidence of Pancreatic Cancer: Case Finding of Asymptomatic Individuals by EUS Surveillance. J Clin Gastroenterol. 2009 May 4; doi: 10.1097/MCG.0b013e3181981123. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo Therapeutic Responses Contingent on Fanconi Anemia/BRCA2 Status of the Tumor. Clin Cancer Res. 2005 October 15;11(20):7508–7515. doi: 10.1158/1078-0432.CCR-05-1048. [DOI] [PubMed] [Google Scholar]
- 35.Helleday T, Bryant HE, Schultz N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle. 2005 September;4(9):1176–1178. doi: 10.4161/cc.4.9.2031. [DOI] [PubMed] [Google Scholar]
- 36.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005 April 14;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 37.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007 May 1;13(9):2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 38.Abbott DW, Freeman ML, Holt JT. Double-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cells. J Natl Cancer Inst. 1998;90(13):978–985. doi: 10.1093/jnci/90.13.978. [DOI] [PubMed] [Google Scholar]
- 39.Hay T, Jenkins H, Sansom OJ, Martin NM, Smith GC, Clarke AR. Efficient deletion of normal Brca2-deficient intestinal epithelium by poly(ADP-ribose) polymerase inhibition models potential prophylactic therapy. Cancer Res. 2005 November 15;65(22):10145–10148. doi: 10.1158/0008-5472.CAN-05-1186. [DOI] [PubMed] [Google Scholar]
- 40.James E, Waldron-Lynch MG, Saif MW. Prolonged survival in a patient with BRCA2 associated metastatic pancreatic cancer after exposure to camptothecin: a case report and review of literature. Anticancer Drugs. 2009 May 8; doi: 10.1097/CAD.0b013e32832b511e. [DOI] [PubMed] [Google Scholar]
- 41.McCabe N, Lord CJ, Tutt AN, Martin NM, Smith GC, Ashworth A. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of Poly (ADP-Ribose) polymerase: an issue of potency. Cancer Biol Ther. 2005 September;4(9):934–936. doi: 10.4161/cbt.4.9.2141. [DOI] [PubMed] [Google Scholar]
- 42.Jones S, Hruban RH, Kamiyama M, et al. Exomic Sequencing Identifies PALB2 as a Pancreatic Cancer Susceptibility Gene. Science. 2009 March 5; doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tischkowitz M, Sabbaghian N, Hamel N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 2004 December;1(3):e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Brune KA, Visvanathan K, et al. Elevated cancer mortality in the relatives of pancreatic cancer patients. Clinical Cancer Research. 2009 doi: 10.1158/1055-9965.EPI-09-0557. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SS, Calle EE, Patel AV, Thun MJ. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000 December;11(10):915–923. doi: 10.1023/a:1026580131793. [DOI] [PubMed] [Google Scholar]
- 47.Falk RT, Pickle LW, Fontham ET, Correa P, Fraumeni JF. Life-style risk factors for pancreatic cancer in Louisana: a case-control study. Am J Epidemiol. 1988;128(2):324–336. doi: 10.1093/oxfordjournals.aje.a114972. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez E, La Vecchia C, D'Avanzo B, Negri E, Franceschi S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:209–212. [PubMed] [Google Scholar]
- 49.Ghadirian P, Boyle P, Simard A, Baillargeon J, Maisonneuve P, Perret C. Reported family aggregation of pancreatic cancer within a population-based case-control study in the Francophone community in Montreal, Canada. Int J Pancreatol. 1991;10:183–196. doi: 10.1007/BF02924156. [DOI] [PubMed] [Google Scholar]
- 50.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007 December;102(12):2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemminki K, Li X. Familial and second primary pancreatic cancers: a nationwide epidemiologic study from Sweden. Int J Cancer. 2003 February 10;103(4):525–530. doi: 10.1002/ijc.10863. [DOI] [PubMed] [Google Scholar]
- 52.Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999 August;80(11):1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. Atlas of tumor pathology. Fourth Series, Fascicle 6 ed. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. [Google Scholar]
- 54.Lumadue JA, Griffin CA, Osman M, Hruban RH. Familial pancreatic cancer and the genetics of pancreatic cancer. Surg Clin North Am. 1995;75:845–855. doi: 10.1016/s0039-6109(16)46731-9. [DOI] [PubMed] [Google Scholar]
- 55.McWilliams RR, Rabe KG, Olswold C, de Andrade M, Petersen GM. Risk of malignancy in first-degree relatives of patients with pancreatic carcinoma. Cancer. 2005 July 15;104(2):388–394. doi: 10.1002/cncr.21166. [DOI] [PubMed] [Google Scholar]
- 56.Lynch HT, Lanspa SJ, Smyrk T, Boman B, Watson P, Lynch J. Hereditary nonpolyposis colorectal cancer (Lynch Syndromes I & II). Genetics, pathology, natural history, and cancer control, part 1. Cancer Genet Cytogenet. 1991;53:143–160. doi: 10.1016/0165-4608(91)90093-a. [DOI] [PubMed] [Google Scholar]
- 57.Baumgaertner I, Corcos O, Couvelard A, et al. Prevalence of extrapancreatic cancers in patients with histologically proven intraductal papillary mucinous neoplasms of the pancreas: a case-control study. Am J Gastroenterol. 2008 November;103(11):2878–2882. doi: 10.1111/j.1572-0241.2008.02142.x. [DOI] [PubMed] [Google Scholar]
- 58.Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999 February;94(2):470–473. doi: 10.1111/j.1572-0241.1999.879_h.x. [DOI] [PubMed] [Google Scholar]
- 59.Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol. 2005 September 28;11(36):5688–5690. doi: 10.3748/wjg.v11.i36.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eguchi H, Ishikawa O, Ohigashi H, et al. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006 June;139(6):749–754. doi: 10.1016/j.surg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Figer A, Irmin L, Geva R, et al. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer. 2001 February;84(4):478–481. doi: 10.1054/bjoc.2000.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClain MR, Nathanson KL, Palomaki GE, Haddow JE. An evaluation of BRCA1 and BRCA2 founder mutations penetrance estimates for breast cancer among Ashkenazi Jewish women. Genet Med. 2005 January;7(1):34–39. doi: 10.1097/01.gim.0000151156.14983.08. [DOI] [PubMed] [Google Scholar]
- 63.Neuhausen S, Gilewski T, Norton L, et al. Recurrent BRCA2 6174deIT mutations in Ashkenazi Jewish women affected by breast cancer. Nat Genet. 1996;13:126–128. doi: 10.1038/ng0596-126. [DOI] [PubMed] [Google Scholar]
- 64.Oddoux C, Struewing JP, Clayton MC, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1% Nat Genet. 1996;14:188–190. doi: 10.1038/ng1096-188. [DOI] [PubMed] [Google Scholar]
- 65.Ozcelik H, Schmocker B, DiNicola N, et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet. 1997 May;16:17–18. doi: 10.1038/ng0597-17. [DOI] [PubMed] [Google Scholar]
- 66.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004 February 15;22(4):735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 67.van der Heijden MS, Yeo CJ, Hruban RH, Kern SE. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003 May 15;63(10):2585–2588. [PubMed] [Google Scholar]
- 68.Couch FJ, Johnson MR, Rabe K, et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005;65(2):383–386. [PubMed] [Google Scholar]
- 69.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002 September 18;94(18):1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 70.Al Sukhni W, Rothenmund H, Eppel BA, et al. Germline BRCA1 mutations predispose to pancreatic adenocarcinoma. Hum Genet. 2008 September 2; doi: 10.1007/s00439-008-0554-0. [DOI] [PubMed] [Google Scholar]
- 71.Axilbund JE, Argani P, Kamiyama M, et al. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Biol Ther. 2009 February 4;8(2) doi: 10.4161/cbt.8.2.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 March 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 73.Zhang F, Ma J, Wu J, et al. PALB2 Links BRCA1 and BRCA2 in the DNA-Damage Response. Curr Biol. 2009 March 4; doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antoniou AC, Pharoah PD, Narod S, et al. Breast and ovarian cancer risks to carriers of the BRCA1 5382insC and 185delAG and BRCA2 6174delT mutations: a combined analysis of 22 population based studies. J Med Genet. 2005 July;42(7):602–603. doi: 10.1136/jmg.2004.024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satagopan JM, Offit K, Foulkes W, et al. The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2001 May;10(5):467–473. [PubMed] [Google Scholar]
- 76.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 77.Tulinius H, Olafsdottir GH, Sigvaldason H, et al. The effect of a single BRCA2 mutation on cancer in Iceland. J Med Genet. 2002 July;39(7):457–462. doi: 10.1136/jmg.39.7.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldstein AM, Chan M, Harland M, et al. High-risk Melanoma Susceptibility Genes and Pancreatic Cancer, Neural System Tumors, and Uveal Melanoma across GenoMEL. Cancer Res. 2006 October 15;66(20):9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. [DOI] [PubMed] [Google Scholar]
- 79.Mesters I, Jonkman L, Vasen H, de Vries H. Skin self-examination of persons from families with familial atypical multiple mole melanoma (FAMMM) Patient Educ Couns. 2009 May;75(2):251–255. doi: 10.1016/j.pec.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 80.Jeghers H, McKusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits. N Engl J Med. 1949;241(25):992–1005. doi: 10.1056/NEJM194912222412501. [DOI] [PubMed] [Google Scholar]
- 81.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001 December;159(6):2017–2022. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorry MC, Gabbaizedeh D, Furey W, et al. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997 October;113(4):1063–1068. doi: 10.1053/gast.1997.v113.pm9322498. [DOI] [PubMed] [Google Scholar]
- 83.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000 June;25(2):213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 84.las Heras-Castano G, Castro-Senosiain B, Fontalba A, Lopez-Hoyos M, Sanchez-Juan P. Hereditary pancreatitis: clinical features and inheritance characteristics of the R122C mutation in the cationic trypsinogen gene (PRSS1) in six Spanish families. JOP. 2009;10(3):249–255. [PubMed] [Google Scholar]
- 85.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 86.Schneider A, Suman A, Rossi L, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology. 2002 October;123(4):1026–1030. doi: 10.1053/gast.2002.36059. [DOI] [PubMed] [Google Scholar]
- 87.Lowenfels AB, Maisonneuve P, Cavallini G, et al. International Hereditary Pancreatitis Study Group. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 88.Lowenfels AB, Maisonneuve EP, Dimagno YE, et al. International Hereditary Pancreatitis Study Group. Hereditary pancreatitis and the risk of pancreatic cancer. J Natl Cancer Inst. 1997 March 19;89(6):442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 89.Pogue-Geile KL, Chen R, Bronner MP, et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Medicine. 2006;3(12):e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Earl J, Yan L, Vitone LJ, et al. Evaluation of the 4q32–34 locus in European familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2006 October;15(10):1948–1955. doi: 10.1158/1055-9965.EPI-06-0376. [DOI] [PubMed] [Google Scholar]
- 91.Klein AP, Borges M, Griffith M, et al. Absence of deleterious palladin mutations in patients with familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009 April;18(4):1328–1330. doi: 10.1158/1055-9965.EPI-09-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salaria SN, Illei PB, Walter KM, et al. Palladin is overexpressed in the non-neoplastic stroma of infiltrating ductal adenocarcinomas of the pancreas, but is only rarely overexpressed. Cancer Biol Ther. 2007;6(3):324–328. doi: 10.4161/cbt.6.3.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slater E, Amrillaeva V, Fendrich V, et al. Palladin mutation causes familial pancreatic cancer: absence in European families. PLoS Med. 2007 April;4(4):e164. doi: 10.1371/journal.pmed.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zogopoulous G, Rothenmund H, Eppel A, et al. The P239S palladin variant does not account for a significant fraction of hereditary or early onset pancreas cancer. Hum Genet. 2007 June;121(5):635–637. doi: 10.1007/s00439-007-0361-z. [DOI] [PubMed] [Google Scholar]
- 95.Giardiello FM, Offerhaus GJ, Lee DH, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: An updated review. Gastroenterology. 1993;104:1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- 97.Lynch HT, Voorhees GJ, Lanspa SJ, McGreevy PS, Lynch J. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br J Cancer. 1985;52:271–273. doi: 10.1038/bjc.1985.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keim V. Identification of patients with genetic risk factors of pancreatitis: impact on treatment and cancer prevention. Dig Dis. 2003;21(4):346–350. doi: 10.1159/000075358. [DOI] [PubMed] [Google Scholar]
- 99.Davis B, Lowy AM. Surgical management of hereditary pancreatic cancer. Med Clin North Am. 2000 May;84(3):749–759. doi: 10.1016/s0025-7125(05)70256-x. [DOI] [PubMed] [Google Scholar]
- 100.Charpentier KP, Brentnall TA, Bronner MP, Byrd D, Marsh C. A new indication for pancreas transplantation: high grade pancreatic dysplasia. Clin Transplant. 2004 February;18(1):105–107. doi: 10.1111/j.1399-0012.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 101.Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007 December;87(6):1477–1501. doi: 10.1016/j.suc.2007.08.014. x. [DOI] [PubMed] [Google Scholar]
- 102.Jethwa P, Sodergren M, Lala A, et al. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006 June;38(6):415–419. doi: 10.1016/j.dld.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 103.Billings BJ, Christein JD, Harmsen WS, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005 November;9(8):1059–1066. doi: 10.1016/j.gassur.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 104.Homma T, Tsuchiya R. The study of the mass screening of persons without symptoms and of the screening of outpatients with gastrointestinal complaints or icterus for pancreatic cancer in Japan, using CA19-9 and elastase-1 or ultrasonography. Int J Pancreatol. 1991;9:119–124. doi: 10.1007/BF02925587. [DOI] [PubMed] [Google Scholar]
- 105.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: An EUS-based approach. Clinical Gastroenterology and Hepatology. 2004;2(7):606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 106.Rulyak SJ, Kimmey MB, Veenstra DL, Brentnall TA. Cost-effectiveness of pancreatic cancer screening in familial pancreatic cancer kindreds. Gastrointestinal Endoscopy. 2003 January;57(1):23–29. doi: 10.1067/mge.2003.28. [DOI] [PubMed] [Google Scholar]
- 107.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999 August 17;131(4):247–255. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 108.Kurtz RC, Simon J, Ludwig E, et al. A pancreatic cancer screening program for familial high-risk individuals. Gastroenterology. 2007;132(4) suppl 2:A-199. Ref Type: Abstract. [Google Scholar]
- 109.Poley JW, Kluijt I, Gouma DJ, et al. The Yield of First-Time Endoscopic Ultrasonography in Screening Individuals at a High Risk of Developing Pancreatic Cancer. Am J Gastroenterol. 2009 June 2; doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 110.Langer P, Kann PH, Fendrich V, et al. 5 Years of Prospective Screening of High Risk Individuals from Familial Pancreatic Cancer - Families. Gut. 2009 May 25; doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 111.Brune KA, Abe T, Canto MI, et al. Multifocal neoplastic precusor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. American Journal of Surgical Pathology. 2006;30(9):1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 112.Takaori K, Matsusue S, Fujikawa T, et al. Carcinoma in situ of the pancreas associated with localized fibrosis: a clue to early detection of neoplastic lesions arising from pancreatic ducts. Pancreas. 1998 July;17(1):102–105. doi: 10.1097/00006676-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 113.Aimoto T, Uchida E, Nakamura Y, et al. Multicentric pancreatic intraepithelial neoplasias (PanINs) presenting with the clinical features of chronic pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15(5):549–553. doi: 10.1007/s00534-007-1269-7. [DOI] [PubMed] [Google Scholar]