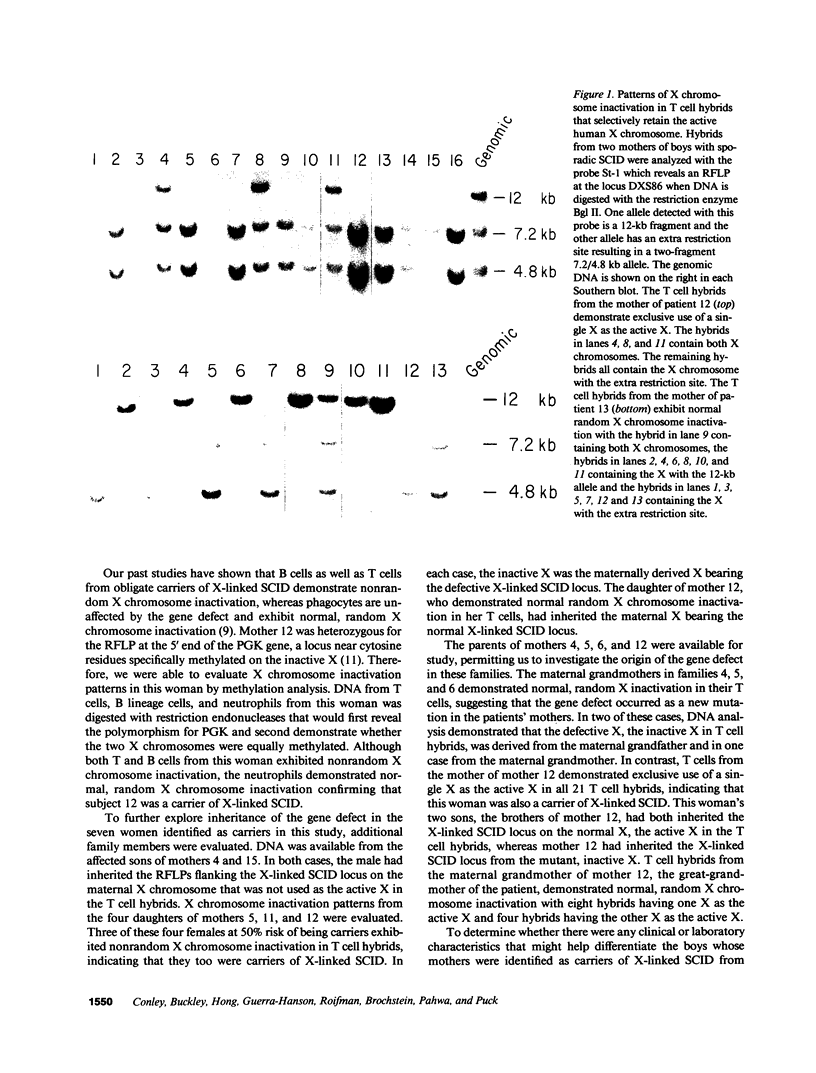
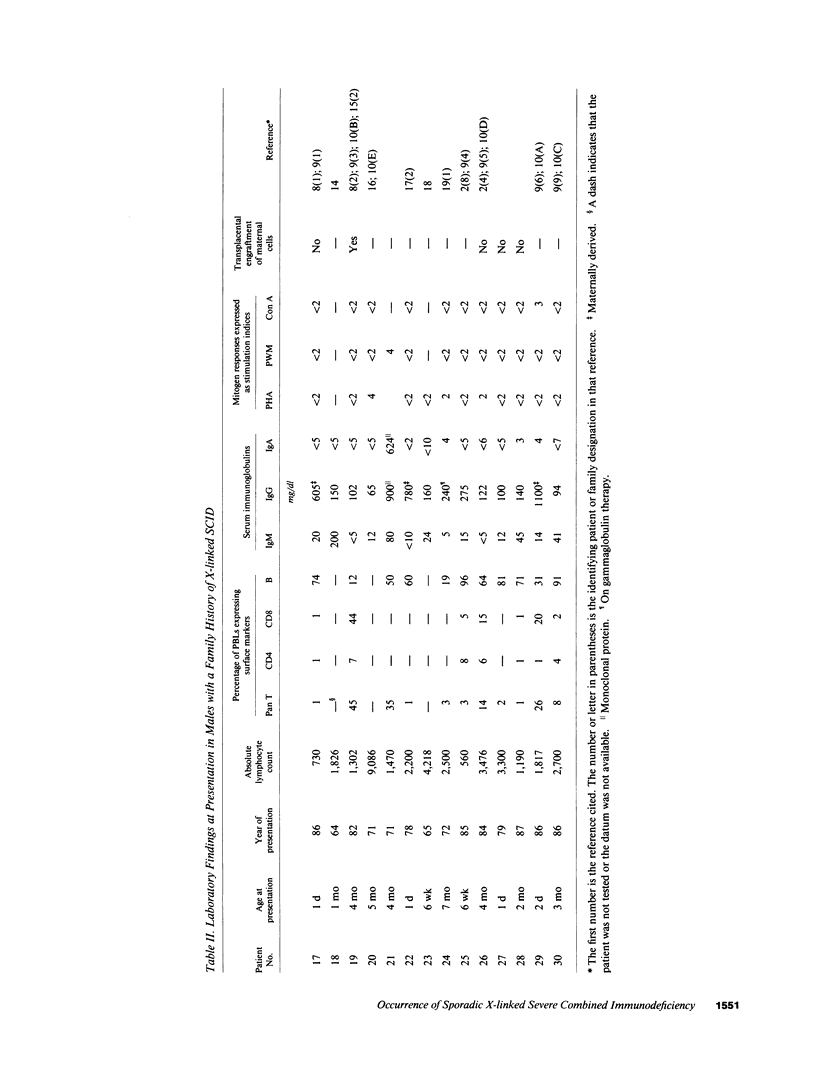
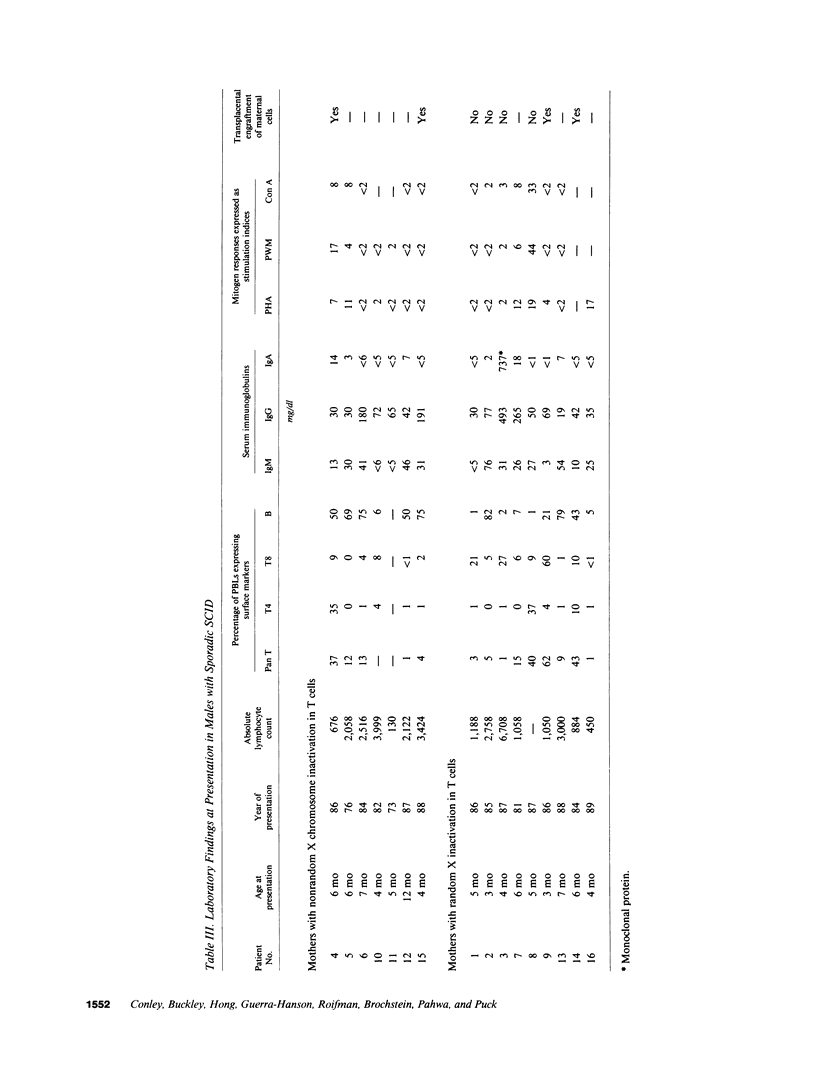
Abstract
Over 80% of infants with severe combined immunodeficiency (SCID) of unknown genetic etiology are males, yet less than a third of these affected males have a family history of X-linked disease. To help identify new mutations of the X-linked SCID gene and to provide genetic counseling, X chromosome inactivation patterns in T cells from 16 women who had sons with sporadic SCID were examined. Between 9 and 35 human/hamster hybrids that selectively retained the active human X chromosome were produced from the T cells of each woman and analyzed with an X-linked restriction fragment length polymorphism for which the woman in question was heterozygous. Exclusive use of a single X as the active X was seen in the T cell hybrids from 7 of the 16 women, identifying these women as carriers of X-linked SCID. Studies on additional family members confirmed the mutant nature of the inactive X and revealed the source of the new mutation in three families. To determine whether there were any laboratory characteristics that might differentiate the boys whose mothers were identified as carriers of X-linked SCID from those whose mothers were not, the clinical records of both groups were compared to each other and to a group of 14 boys with a family history of X-linked SCID. The most consistent finding in the 21 patients with X-linked SCID was an elevated proportion of B cells. These data demonstrate the high incidence of spontaneous mutation for the X-linked SCID gene and help clarify the characteristic presenting features of this disorder.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett M. J., Buckley R. H., Schiff S. E., Kidd P. C., Ward F. E. Accelerated development of immunity following transplantation of maternal marrow stem cells into infants with severe combined immunodeficiency and transplacentally acquired lymphoid chimerism. Clin Exp Immunol. 1988 Apr;72(1):118–123. [PMC free article] [PubMed] [Google Scholar]
- Buckley R. H., Gilbertsen R. B., Schiff R. I., Ferreira E., Sanal S. O., Waldmann T. A. Heterogeneity of lymphocyte subpopulations in severe combined immunodeficiency. Evidence against a stem cell defect. J Clin Invest. 1976 Jul;58(1):130–136. doi: 10.1172/JCI108441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. H., Schiff S. E., Sampson H. A., Schiff R. I., Markert M. L., Knutsen A. P., Hershfield M. S., Huang A. T., Mickey G. H., Ward F. E. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986 Apr 1;136(7):2398–2407. [PubMed] [Google Scholar]
- Conley M. E., Lavoie A., Briggs C., Brown P., Guerra C., Puck J. M. Nonrandom X chromosome inactivation in B cells from carriers of X chromosome-linked severe combined immunodeficiency. Proc Natl Acad Sci U S A. 1988 May;85(9):3090–3094. doi: 10.1073/pnas.85.9.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M. E., Nowell P. C., Henle G., Douglas S. D. XX T cells and XY B cells in two patients with severe combined immune deficiency. Clin Immunol Immunopathol. 1984 Apr;31(1):87–95. doi: 10.1016/0090-1229(84)90192-2. [DOI] [PubMed] [Google Scholar]
- Fireman P., Johnson H. A., Gitlin D. Presence of plasma cells and gamma-1-M-globulin synthesis in a patient with thymic alymphoplasia. Pediatrics. 1966 Mar;37(3):485–492. [PubMed] [Google Scholar]
- Gelfand E. W., Dosch H. M. Diagnosis and classification of severe combined immunodeficiency disease. Birth Defects Orig Artic Ser. 1983;19(3):65–72. [PubMed] [Google Scholar]
- Goldblum R. M., Lord R. A., Dupree E., Weinberg A. G., Goldman A. S. Transfer factor induced delayed hypersensitivity in X-linked combined immunodeficiency. Cell Immunol. 1973 Nov;9(2):297–305. doi: 10.1016/0008-8749(73)90081-6. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Durandy A., Virelizier J. L., Ballet J. J., Daguillard F. Selective defect of precursor T cells associated with apparently normal B lymphocytes in severe combined immunodeficiency disease. J Pediatr. 1978 Sep;93(3):404–411. doi: 10.1016/s0022-3476(78)81146-9. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. Genetic deficiencies of adenosine deaminase and purine nucleoside phosphorylase: overview, genetic heterogeneity and therapy. Birth Defects Orig Artic Ser. 1983;19(3):73–81. [PubMed] [Google Scholar]
- Lederman H. M., Winkelstein J. A. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 1985 May;64(3):145–156. [PubMed] [Google Scholar]
- Mandel J. L., Willard H. F., Nussbaum R. L., Romeo G., Puck J. M., Davies K. E. Report of the committee on the genetic constitution of the X chromosome. Cytogenet Cell Genet. 1989;51(1-4):384–437. doi: 10.1159/000132801. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Schiff R. I., Buckley R. H. Adenosine deaminase and purine nucleoside phosphorylase deficiencies: evaluation of therapeutic interventions in eight patients. J Clin Immunol. 1987 Sep;7(5):389–399. doi: 10.1007/BF00917017. [DOI] [PubMed] [Google Scholar]
- Miller M. E. Thymic dysplasia ("Swiss agammaglobulinemia"). I. Graft versus host reaction following bone-marrow transfusion. J Pediatr. 1967 May;70(5):730–736. doi: 10.1016/s0022-3476(67)80323-8. [DOI] [PubMed] [Google Scholar]
- Moen R. C., Horowitz S. D., Sondel P. M., Borcherding W. R., Trigg M. E., Billing R., Hong R. Immunologic reconstitution after haploidentical bone marrow transplantation for immune deficiency disorders: treatment of bone marrow cells with monoclonal antibody CT-2 and complement. Blood. 1987 Sep;70(3):664–669. [PubMed] [Google Scholar]
- Puck J. M., Nussbaum R. L., Conley M. E. Carrier detection in X-linked severe combined immunodeficiency based on patterns of X chromosome inactivation. J Clin Invest. 1987 May;79(5):1395–1400. doi: 10.1172/JCI112967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck J. M., Nussbaum R. L., Smead D. L., Conley M. E. X-linked severe combined immunodeficiency: localization within the region Xq13.1-q21.1 by linkage and deletion analysis. Am J Hum Genet. 1989 May;44(5):724–730. [PMC free article] [PubMed] [Google Scholar]
- Reisner Y., Kapoor N., Kirkpatrick D., Pollack M. S., Cunningham-Rundles S., Dupont B., Hodes M. Z., Good R. A., O'Reilly R. J. Transplantation for severe combined immunodeficiency with HLA-A,B,D,DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983 Feb;61(2):341–348. [PubMed] [Google Scholar]
- Reith W., Satola S., Sanchez C. H., Amaldi I., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Congenital immunodeficiency with a regulatory defect in MHC class II gene expression lacks a specific HLA-DR promoter binding protein, RF-X. Cell. 1988 Jun 17;53(6):897–906. doi: 10.1016/s0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- de Saint Basile G., Arveiler B., Oberlé I., Malcolm S., Levinsky R. J., Lau Y. L., Hofker M., Debre M., Fischer A., Griscelli C. Close linkage of the locus for X chromosome-linked severe combined immunodeficiency to polymorphic DNA markers in Xq11-q13. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7576–7579. doi: 10.1073/pnas.84.21.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]